Bioassay-Guided Separation of Centipeda minima Using Comprehensive Linear Gradient Centrifugal Partition Chromatography
Abstract
1. Introduction
2. Results and Discussion
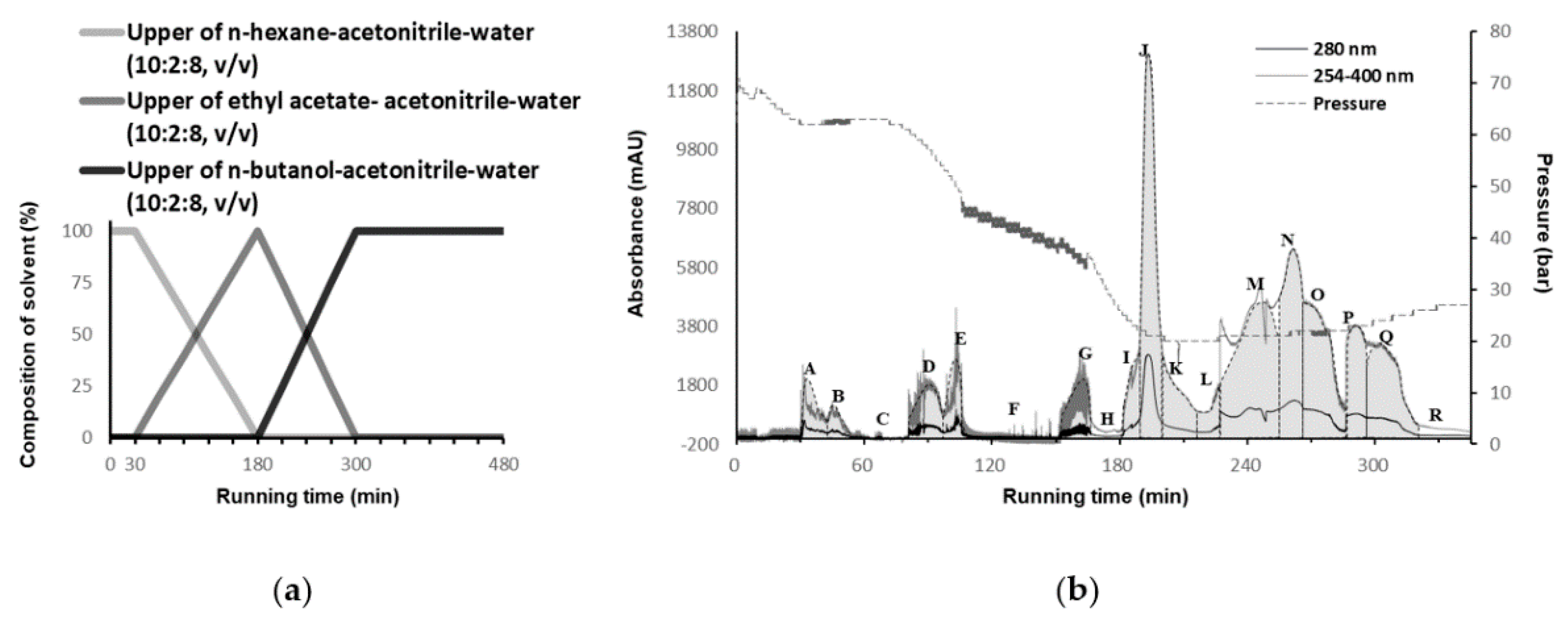
2.1. Gradient Biphasic Solvent System Selection
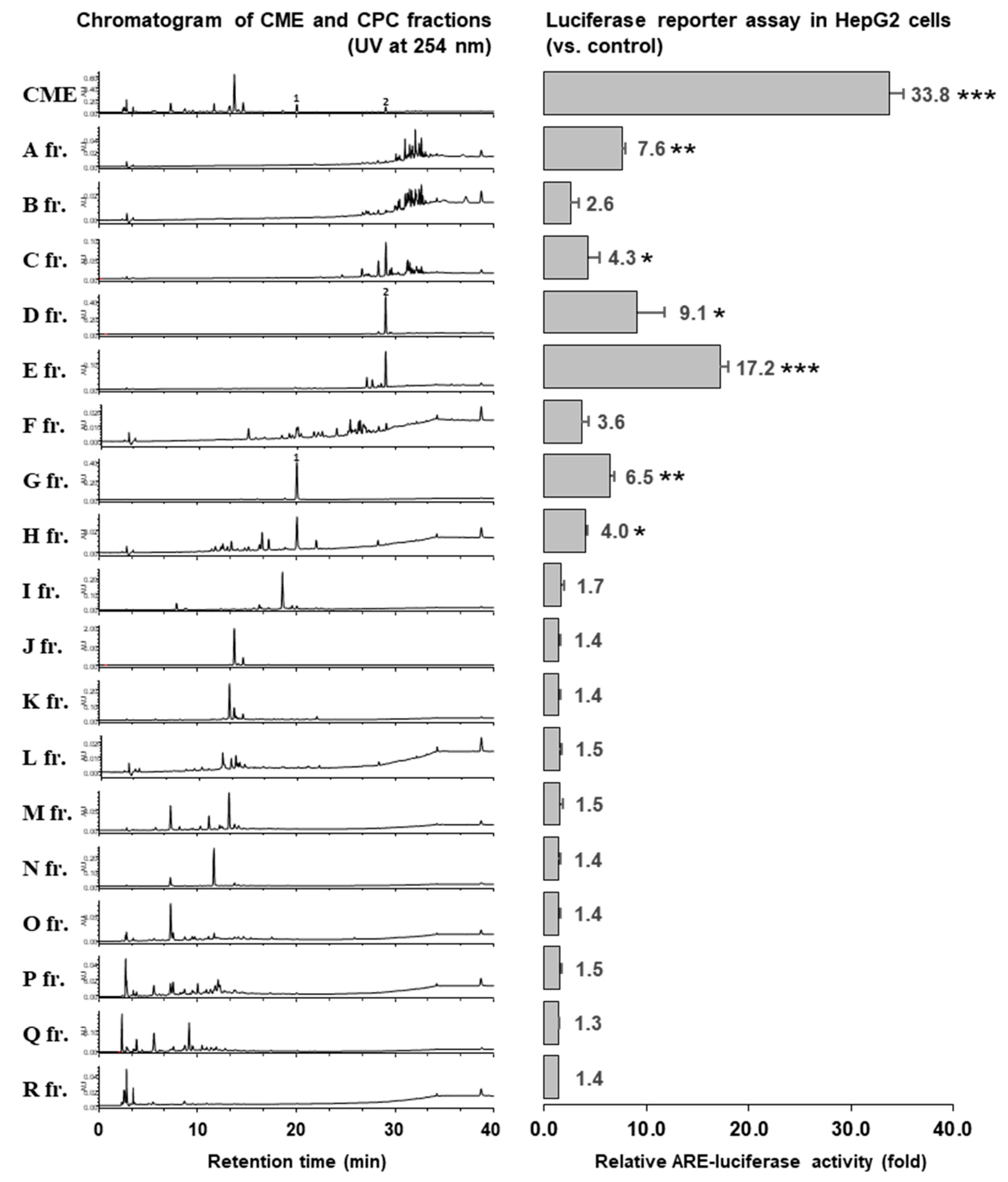
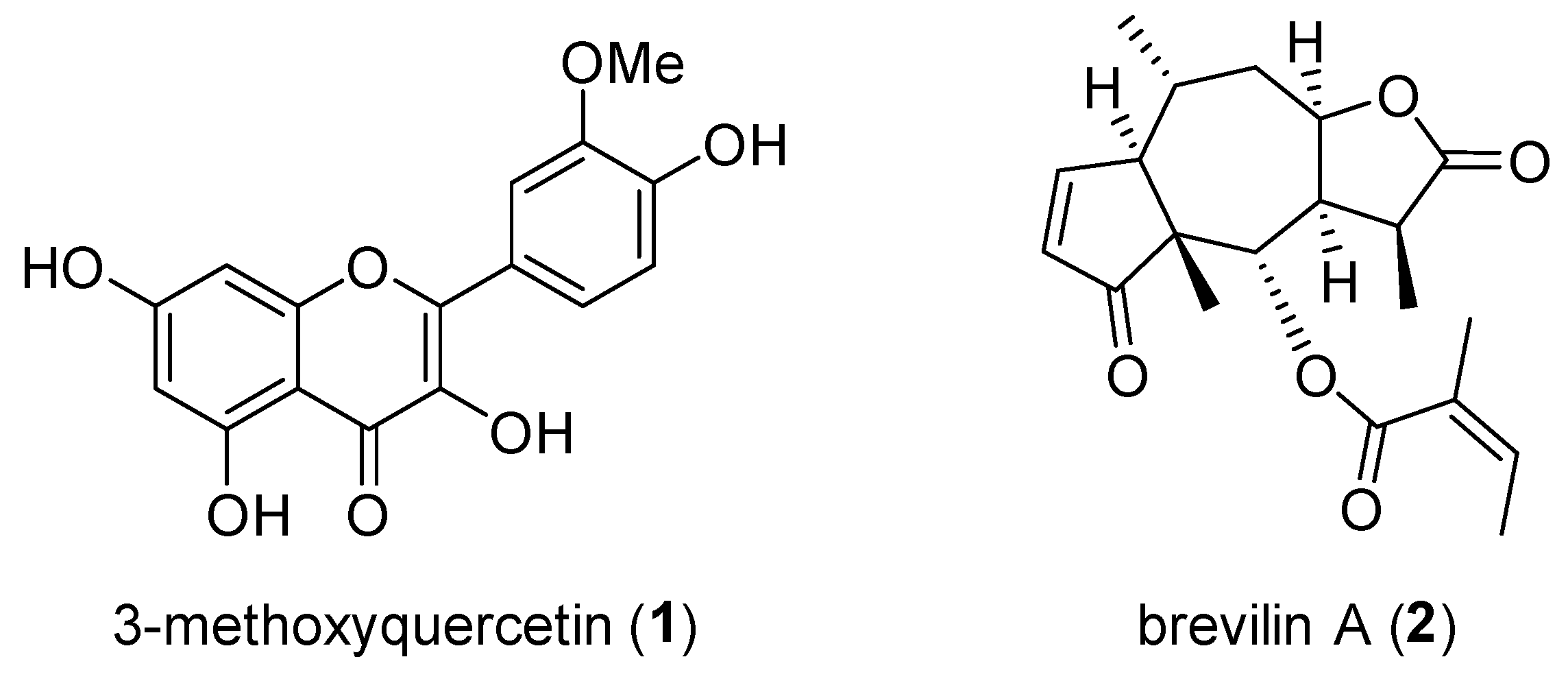
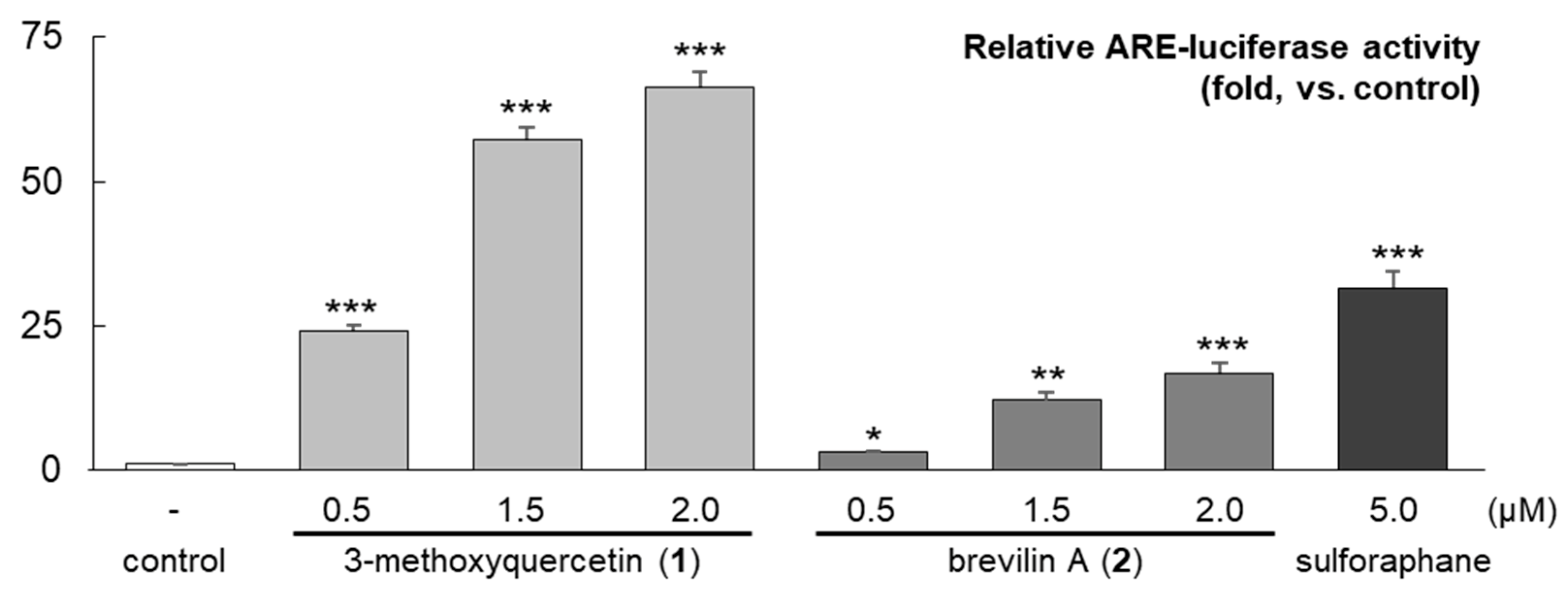
2.2. Separation of the CPC Gradient Elution and ARE Induction Activity
3. Materials and Methods
3.1. Reagents and Materials
3.2. C. minima Extract Preparation
3.3. Ternary Biphasic Solvent System Preparation
3.4. Settling Time and Phase Ratio Measurement
3.5. HPLC Analysis
3.6. CPC Procedure
3.7. Cell Culture and Viability Assay
3.8. ARE-Inducing Activity Assay
3.9. Protein Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-based molecular networking for the discovery of drug leads in natural product bioassay-guided fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.J.; Todd, D.A.; Egan, J.M.; Raja, H.A.; Oberlies, N.H.; Kvalheim, O.M.; Cech, N.B. Biochemometrics for natural products research: Comparison of data analysis approaches and application to identification of bioactive compounds. J. Nat. Prod. 2016, 79, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Alvi, K.A. Screening natural products: Bioassay-directed isolation of active components by dual-mode CCC. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1765–1773. [Google Scholar] [CrossRef]
- Ignatova, S.; Sumner, N.; Colclough, N.; Sutherland, I. Gradient elution in counter-current chromatography: A new layout for an old path. J. Chromatogr. A 2011, 1218, 6053–6060. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Luo, J.G.; Han, C.; Xu, J.F.; Kong, L.Y. Bioassay-guided preparative separation of angiotensin-converting enzyme inhibitory C-flavone glycosides from Desmodium styracifolium by recycling complexation high-speed counter-current chromatography. J. Pharm. Biomed. Anal. 2015, 102, 276–281. [Google Scholar] [CrossRef]
- Wang, M.; Gu, D.; Guo, X.; Li, H.; Wang, Y.; Guo, H.; Yang, Y.; Tian, J. Bioassay-guided isolation of an active compound with protein tyrosine phosphatase 1B inhibitory activity from Sargassum fusiforme by high-speed counter-current chromatography. J. Sep. Sci. 2016, 39, 4408–4414. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Q.; Chen, M.; Huang, X.; Chen, X. Large-scale separation of acetylcholinesterase inhibitors from Zanthoxylum nitidum by pH-zone-refining counter-current chromatography target-guided by ultrafiltration high-performance liquid chromatography with ultraviolet and mass spectrometry screening. J. Sep. Sci. 2019, 42, 1194–1201. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, Z.; Wang, J.; Wu, G.; Li, S. Comprehensive separation and identification of chemical constituents from Apocynum venetum leaves by high-performance counter-current chromatography and high performance liquid chromatography coupled with mass spectrometry. J. Chromatogr. B 2010, 878, 3149–3155. [Google Scholar] [CrossRef]
- Ying, H.; Jiang, H.; Liu, H.; Chen, F.; Du, Q. Ethyl acetate–n-butanol gradient solvent system for high-speed countercurrent chromatography to screen bioactive substances in okra. J. Chromatogr. A 2014, 1359, 117–123. [Google Scholar] [CrossRef]
- Leitão, G.G.; Costa, F.D.N. Gradient elution in countercurrent chromatography. Planta Med. 2015, 81, 1592–1596. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.H.; Lim, S.S. Comprehensive profiling of minor tyrosinase inhibitors from Gastrodia elata using an off-line hyphenation of ultrafiltration, high-speed countercurrent chromatography, and high-performance liquid chromatography. J. Chromatogr. A 2017, 1529, 63–71. [Google Scholar] [CrossRef]
- Chan, C.O.; Jin, D.P.; Dong, N.P.; Chen, S.B.; Mok, D.K.W. Qualitative and quantitative analysis of chemical constituents of Centipeda minima by HPLC-QTOF-MS & HPLC-DAD. J. Pharm. Biomed. Anal. 2016, 125, 400–407. [Google Scholar]
- Nguyen, N.Y.T.; Nguyen, T.H.; Dang, P.H.; Le Tran, Q. Three terpenoid glycosides of Centipeda minima. Phytochem. Lett. 2017, 21, 21–24. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, X.Y.; Hao, X.Y.; Yan, Y.M.; Hong, M.; Wei, S.F.; Zhou, Y.L.; Wang, Q.; Cheng, Y.X.; Liu, Y.Q. Ethanol Extract of Centipeda minima Exerts Antioxidant and Neuroprotective Effects via Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, J. Preparative isolation and purification of geniposide from gardenia fruits by centrifugal partition chromatography. Phytochem. Anal. 2007, 18, 115–117. [Google Scholar] [CrossRef]
- Yeon, S.H.; Jeon, J.S.; Um, K.A.; Kim, C.Y.; Ahn, Y.J. Large-scale purification of unstable, water-soluble secologanic acid using centrifugal partition chromatography. Phytochem. Anal. 2018, 29, 487–492. [Google Scholar] [CrossRef]
- Hubert, D.J.; Dawe, A.; Florence, N.T.; Gilbert, K.D.; Angele, T.N.; Buonocore, D.; Vita Finzi, P.; Vidari, G.; Bonaventure, N.T.; Marzatico, F.; et al. In vitro hepatoprotective and antioxidant activities of crude extract and isolated compounds from Ficus gnaphalocarpa. Inflammopharmacology 2011, 19, 35–43. [Google Scholar] [CrossRef]
- Jeon, J.S.; Park, C.L.; Syed, A.S.; Kim, Y.M.; Cho, I.J.; Kim, C.Y. Preparative separation of sesamin and sesamolin from defatted sesame meal via centrifugal partition chromatography with consecutive sample injection. J. Chromatogr. B 2016, 1011, 108–113. [Google Scholar] [CrossRef]
Sample Availability: Samples of the 3-methoxyquercetin and brevilin A are available from the authors. |
| Less Polar Solvent Systems | Medium Polar Solvent Systems | Polar Solvent Systems | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent System | Volume Ratios (v/v/v) | Phase Ratios | Settling Time | Solvent System | Volume Ratios (v/v/v) | Phase Ratios | Settling Time | Solvent System | Volume Ratios (v/v/v) | Phase Ratios | Settling Time |
| H/M/W | 10:1:9 | 50/50 | 12 s | EA/M/W | 10:1:9 | 58/42 | 19 s | B/M/W | 10:1:9 | 50/50 | 26 s |
| 10:2:8 | 50/50 | 12 s | 10:2:8 | 58/42 | 20 s | 10:2:8 | 56/44 | 3 m 17 s | |||
| H/E/W | 10:1:9 | 50/50 | >4 m | EA/E/W | 10:1:9 | 48/52 | 13 s | B/E/W | 10:1:9 | 48/52 | 1 m 30 s |
| 10:2:8 | 49/51 | 50 s | 10:2:8 | 52/48 | 19 s | 10:2:8 | 57/43 | >4 m | |||
| H/I/W | 10:1:9 | 50/50 | >4 m | EA/I/W | 10:1:9 | 50/50 | 14 s | B/I/W | 10:1:9 | 50/50 | 15 s |
| 10:2:8 | 51/49 | 24 s | 10:2:8 | 54/46 | 19 s | 10:2:8 | 58/42 | 24 s | |||
| 10:3:7 | 52/48 | 20 s | 10:3:7 | 62/38 | 22 s | 10:3:7 | 70/30 | 37 s | |||
| 10:4:6 | 54/46 | 17 s | 10:4:6 | 74/26 | 27 s | 10:4:6 | 96/4 | 1 m 40 s | |||
| H/A/W | 10:1:9 | 50/50 | 14 s | EA/A/W | 10:1:9 | 50/50 | 10 s | B/A/W | 10:1:9 | 50/50 | 36 s |
| 10:2:8 | 50/50 | 11 s | 10:2:8 | 56/44 | 11 s | 10:2:8 | 60/40 | 43 s | |||
| Fraction | A | B | C | D | E | F | G | H | I |
| Weight (mg) | 173.7 | 44.8 | 48.6 | 76.7 | 119.2 | 51.5 | 65.4 | 25.7 | 55.6 |
| Weight ratio (%) | 4.4 | 1.1 | 1.2 | 2 | 3.1 | 1.3 | 1.7 | 0.7 | 1.4 |
| Calculated conc. (μg/mL) | 1.33 | 0.34 | 0.37 | 0.59 | 0.92 | 0.4 | 0.5 | 0.2 | 0.43 |
| Fraction | J | K | L | M | N | O | P | Q | R |
| Weight (mg) | 100.8 | 106.7 | 75.8 | 243.9 | 116.6 | 97.2 | 33.8 | 82.4 | 2387.5 |
| Weight ratio (%) | 2.6 | 2.7 | 1.9 | 6.2 | 3 | 2.5 | 0.9 | 2.1 | 61.1 |
| Calculated conc. (μg/mL) | 0.77 | 0.82 | 0.58 | 1.87 | 0.9 | 0.75 | 0.26 | 0.63 | 18.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Jung, E.J.; Lee, Y.J.; Gao, E.M.; Syed, A.S.; Kim, C.Y. Bioassay-Guided Separation of Centipeda minima Using Comprehensive Linear Gradient Centrifugal Partition Chromatography. Molecules 2020, 25, 3077. https://doi.org/10.3390/molecules25133077
Kim JH, Jung EJ, Lee YJ, Gao EM, Syed AS, Kim CY. Bioassay-Guided Separation of Centipeda minima Using Comprehensive Linear Gradient Centrifugal Partition Chromatography. Molecules. 2020; 25(13):3077. https://doi.org/10.3390/molecules25133077
Chicago/Turabian StyleKim, Ji Hoon, Eun Ju Jung, Yun Jung Lee, En Mei Gao, Ahmed Shah Syed, and Chul Young Kim. 2020. "Bioassay-Guided Separation of Centipeda minima Using Comprehensive Linear Gradient Centrifugal Partition Chromatography" Molecules 25, no. 13: 3077. https://doi.org/10.3390/molecules25133077
APA StyleKim, J. H., Jung, E. J., Lee, Y. J., Gao, E. M., Syed, A. S., & Kim, C. Y. (2020). Bioassay-Guided Separation of Centipeda minima Using Comprehensive Linear Gradient Centrifugal Partition Chromatography. Molecules, 25(13), 3077. https://doi.org/10.3390/molecules25133077





