A New Validated Potentiometric Method for Sulfite Assay in Beverages Using Cobalt(II) Phthalocyanine as a Sensory Recognition Element
Abstract
:1. Introduction
2. Results and Discussions
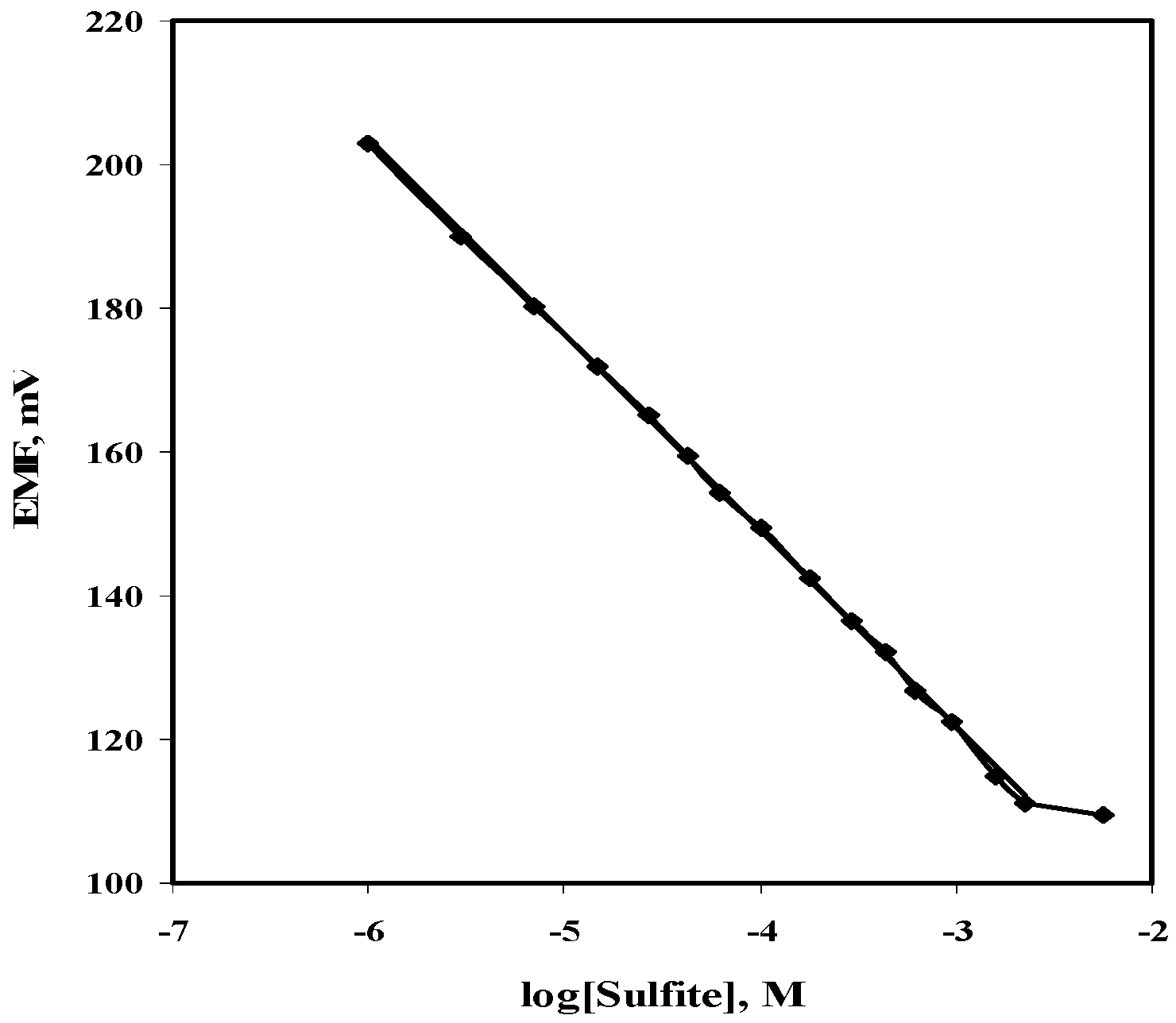
2.1. Potentiometric Recognition of Sulfite
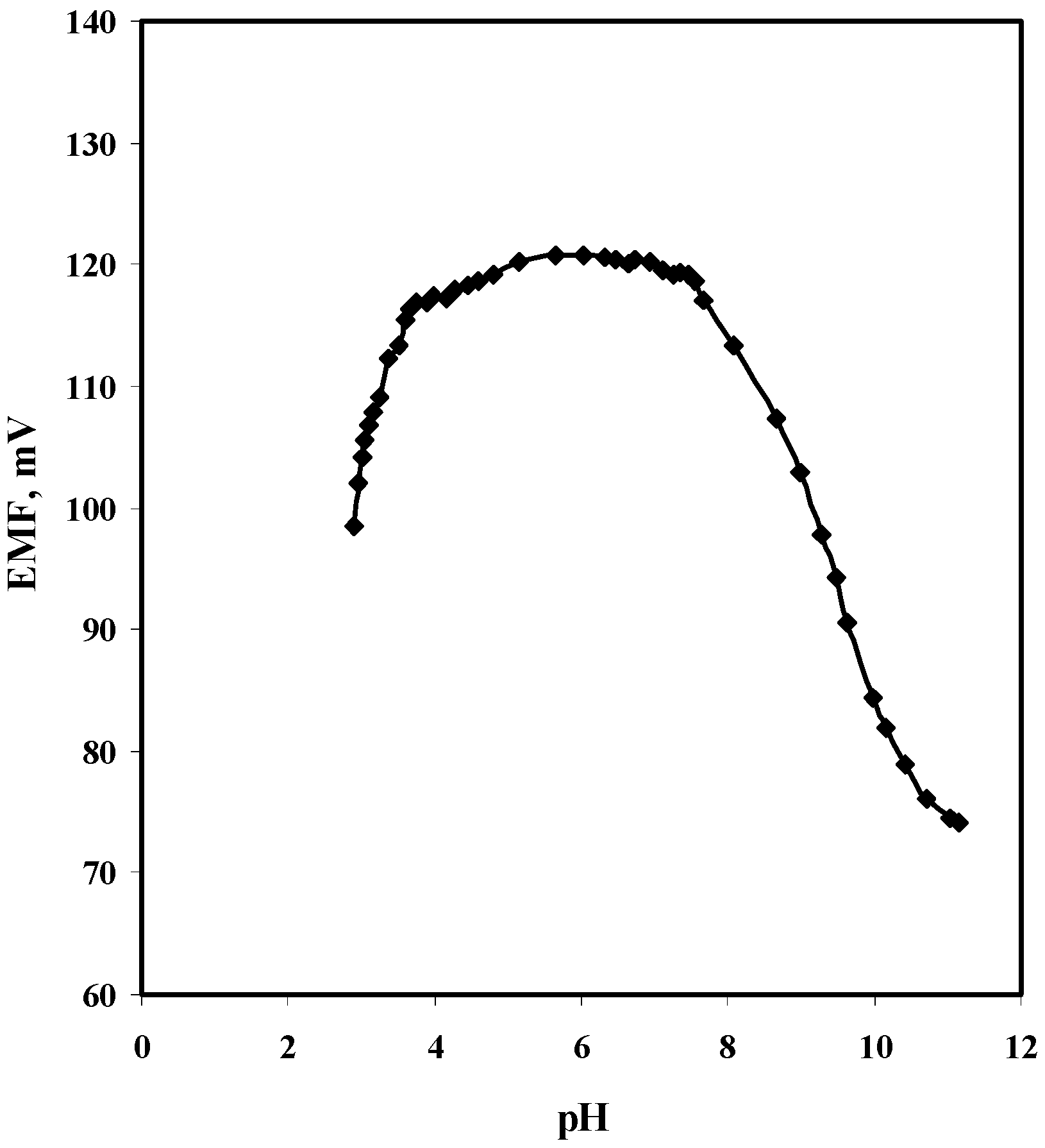
2.2. Effect of pH
2.3. Potentiometric Selectivity of CoPC/TDMAC-based Sulfite Sensor
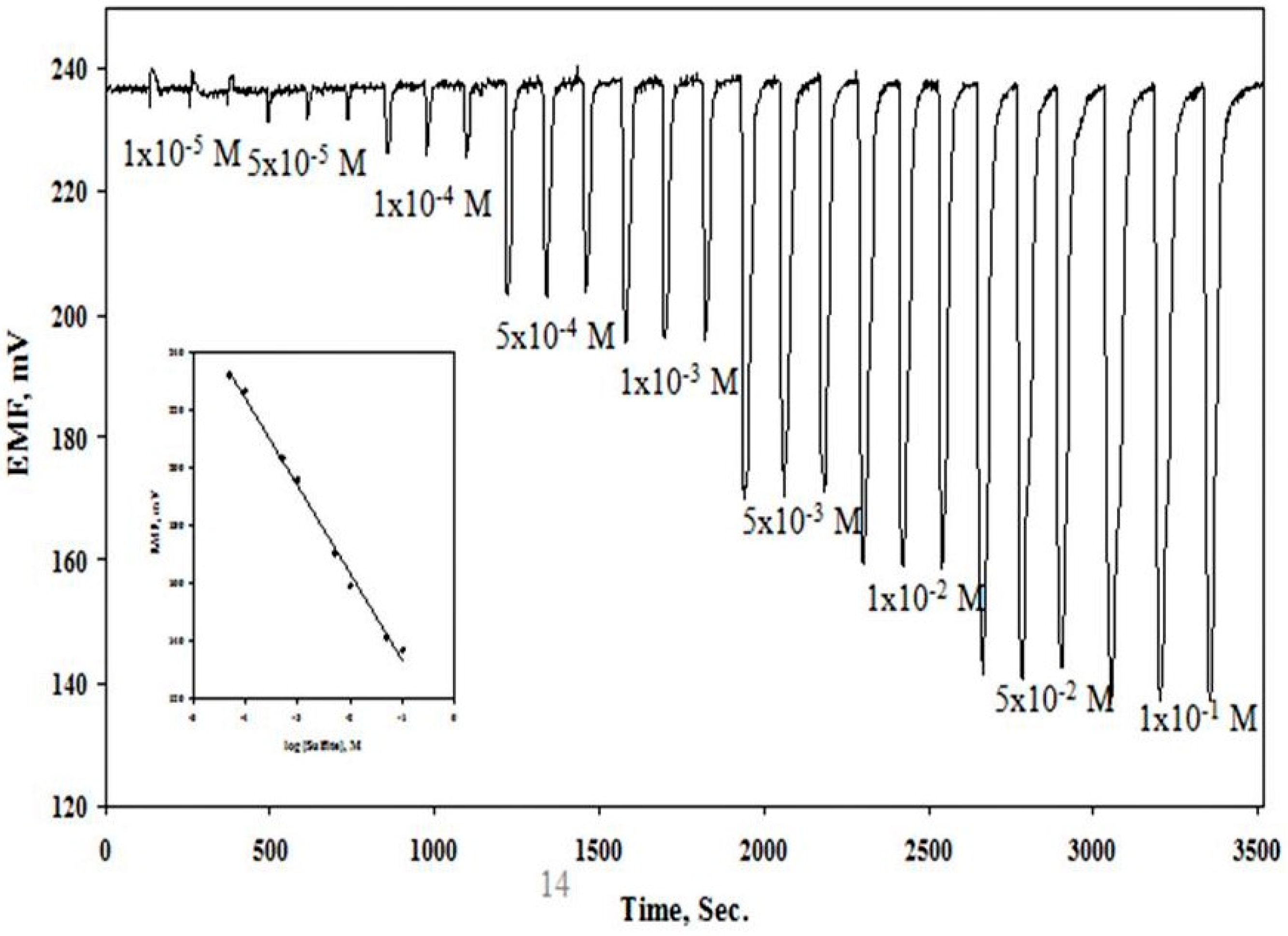
2.4. Potentiometric Batch and Flow Injection Analysis (FIA) of Sulfite
2.5. Method Validation
2.5.1. Data Precision and Accuracy
2.5.2. Data Trueness and Bias
2.5.3. Data Repeatability and Reproducibility
2.5.4. Dynamic Measurement Range and Limit of Detection
2.5.5. X-bar and R-Charts
2.6. Potentiometric Determination of Sulfite in Beverages
3. Materials and Methods
3.1. Equipment
3.2. Materials and Chemicals
3.3. Preparation of Sulfite PVC Membrane Sensor
3.4. Sensor Calibration
3.5. Potentiometric Batch Determination of Sulfite in Beverages
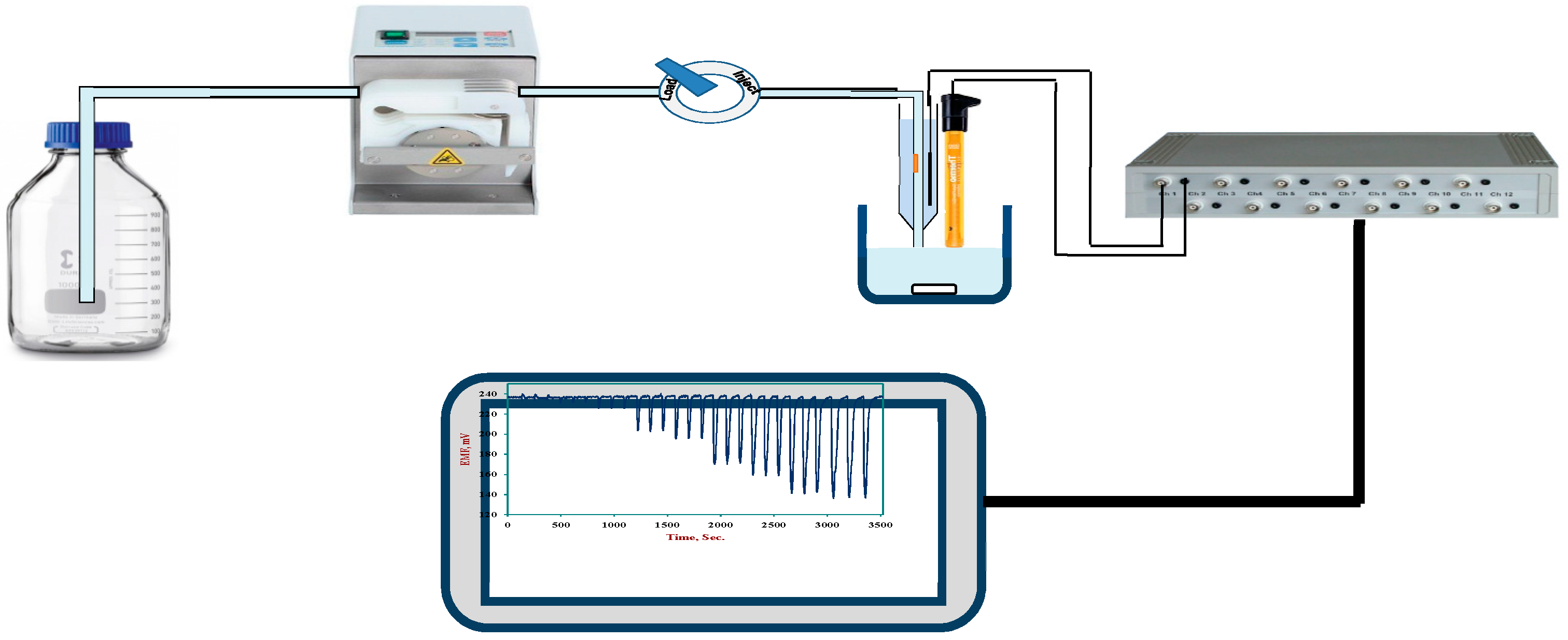
3.6. Potentiometric Flow Injection Analysis (FIA) of Sulfite in Beverages
3.7. Iodometric Determination of Sulfite in Beverages
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gunnison, A.F. Sulphite toxicity: A critical review of in vitro and in vivo data. Food Cosmet. Toxicol. 1981, 19, 666–682. [Google Scholar] [CrossRef]
- Taylor, S.L.; Bush, R.K. Sulfites as food ingredients. Food Technol. 1986, 6, 47–52. [Google Scholar]
- Walker, R. Sulphiting agents in foods: Some risk/benefit considerations. Food Addit. Contam. 1985, 2, 5–24. [Google Scholar] [CrossRef]
- Smith, V.J. Determination of sulfite using a sulfite oxidase enzyme electrode. Anal. Chem. 1987, 59, 2256–2259. [Google Scholar] [CrossRef]
- Langdon, T.T. Preventing of browning in fresh prepared potatoes without the use of sulfiting agents. Food Technol. 1987, 5, 64–67. [Google Scholar]
- Yongjie, L.; Li, Y.; Zhao, M. Simple methods for rapid determination of sulfite in food products. Food Control 2006, 17, 975–980. [Google Scholar]
- Geetha, K.; Balasubramania, N. An indirect method for the determination of sulfur dioxide using Methyl Red. Microchem. J. 2000, 65, 45–49. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Hamza, M.S.; Mohamed, A.H.K. A novel spectrophotometric method for batch and flow injection determination of sulfite in beverages. Anal. Chim. Acta 2006, 570, 232–239. [Google Scholar] [CrossRef]
- Zhan, X.; Dong-hui, L.; Hong, Z. Jin-gou, X. Fluorimetric determination of sulfite by the co-quenching effect of formaldehyde and sulfite on the fluorescence of tetra-substituted amino aluminum phthalocyanine. Anal. Chim. Acta 2001, 448, 71–77. [Google Scholar]
- Yang, X.; Guo, X.; Zhao, Y. Novel spectrofluorimetric method for the determination of sulfite with rhodamine B hydrazide in a micellar medium. Anal. Chim. Acta 2002, 456, 121–128. [Google Scholar] [CrossRef]
- Fujii, S.; Tokuyama, T.; Abo, M.; Okuba, A. Fluorometric determination of sulfite and nitrite in aqueous samples using a novel detection unit of a microfluidic device. Anal. Sci. 2004, 20, 209–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbari, A.; Shamsipur, M. Kinetic Spectrophotometric Determination of Traces of Sulfite Based on Its Additional Reaction with Methyl Green. Microchem. J. 1993, 48, 349–355. [Google Scholar] [CrossRef]
- Afkhami, A.; Sarlak, N.; Zarei, A.R.; Madrakian, T. Simultaneous Kinetic Spectrophotometric Determination of Sulfite and Sulfide Using Partial Least Squares (PLS) Regression. Bull. Korean Chem. Soc. 2006, 27, 863–868. [Google Scholar]
- Yamada, M.; Nakada, M.; Suzuki, S. The determination of sulfite in a flow injection system with chemiluminescence detection. Anal. Chim. Acta 1983, 147, 401–404. [Google Scholar] [CrossRef]
- Huang, Y.L.; Kim, J.M.; Schmid, R.D. Determination of sulphite in wine through flow-injection analysis based on the suppression of luminol chemiluminescence. Anal. Chim. Acta 1992, 266, 317–323. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, Z.; Zhang, C. Reagentless chemiluminescence flow sensor for sulfite. Anal. Chim. Acta 1998, 361, 201–203. [Google Scholar] [CrossRef]
- Papkovsky, D.; Uskova, M.A.; Ponomarve, G.V.; Korpela, T.; Kulmale, S.; Guilbault, G.G. Optical sensing of sulfite with a phosphorescent probe. Anal. Chim. Acta 1998, 374, 1–9. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Uekusa, Y.; Sakuragawa, A. Determination of sulphite in wines using suppressed ion chromatography. Food Chem. 2015, 174, 387–391. [Google Scholar] [CrossRef]
- Aberi, A.; Coelhan, M. Determination of sulfur dioxide in wine using headspace gas chromatography and electron capture detection. Food Add. Contam. A 2013, 30, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Rethmeier, J.; Robenstein, A.; Langer, M.; Fischer, U. Detection of traces of oxidized and reduced sulfur compounds in small samples by combination of different high-performance liquid chromatography methods. J. Chromatog. A 1997, 760, 295–302. [Google Scholar] [CrossRef]
- Abrahamsson, V.; Hoff, S.; Nielsen, N.J.; Lund, M.N.; Andersen, M.L. Determination of sulfite in beer based on fluorescent derivatives and liquid chromatographic separation. J. Am. Soc. Brew. Chem. 2012, 70, 296–302. [Google Scholar] [CrossRef]
- Masár, M.; Danková, M.; Ölvecká, E.; Stachurová, A.; Kaniansky, D.; Stanislawski, B. Determination of free sulfite in wine by zone electrophoresis with isotachophoresis sample pretreatment on a column-coupling chip. J. Chromatog. 2004, 1026, 31–39. [Google Scholar]
- Kim, H.J. Determination of sulfite in foods and beverages by ion exclusion chromatography with electrochemical detection: Collaborative study. J. Assoc. Off. Anal. Chem. 1990, 73, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.; Chawla, S.; Pundir, C.S. An electrochemical sulfite biosensor based on gold coated magnetic nanoparticles modified gold electrode. Biosens. Bioelectron. 2012, 31, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Cipriani, P.; Martini, T.M.; Sammartino, M.P.; Tomasetti, M. New enzyme sensor for sulfite analysis in sea and river water samples. Anal. Chim Acta 1995, 305, 32–41. [Google Scholar] [CrossRef]
- Abass, A.K.; Hart, J.P.; Cowell, D. Development of an amperometric sulfite biosensor based on sulfite oxidase with cytochrome c, as electron acceptor, and a screen-printed transducer. Sens. Actuators 2000, 62, 148–153. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis. Open Chem. 2018, 16, 1248–1256. [Google Scholar] [CrossRef]
- Norouzi, B.; Parsa, Z. Determination of Sulfite in Real Sample by an Electrochemical Sensor Based on Ni/Poly (4-Aminobenzoic Acid)/Sodium Dodecylsulfate/Carbon Paste Electrode. Russ. J. Electrochem. 2018, 54, 613–622. [Google Scholar] [CrossRef]
- Silva, E.M.; Takeuchi, R.M.; Santos, A.L. Carbon nanotubes for voltammetric determination of sulphite in some beverages. Food Chem. 2015, 173, 763–769. [Google Scholar] [CrossRef]
- Molinero-Abad, B.; Alonso-Lomillo, M.A.; Dominguez-Renedo, O.; Arcos-Martinez, M.J. Amperometric determination of sulfite using screen-printed electrodes modified with metallic nanoparticles. Mikrochim. Acta. 2013, 180, 1351–1355. [Google Scholar] [CrossRef]
- Isaac, A.; Livingstone, C.; Wain, A.J.; Compton, R.G.; Davis, J. Electroanalytical methods for the determination of sulfite in food and beverages. TrAc-Trends Anal. Chem. 2006, 25, 589–598. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid Contact Potentiometric Sensors Based on Host–Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef] [PubMed]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2, 4-dichlorophenol as a food Taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper–based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- Kamel, A.H.; Soror, T.Y.; Al-Romian, F.M. Graphite Solid-Contact Mepiquat Potentiometric Sensors Based on Molecularly Imprinted Polymers and Their Application to Flow Through Analysis. Anal. Meth. 2012, 4, 3007–3012. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Badr, I.H.A.; Kamel, A.H.; Mohamed, M.S. A Novel Poly (Vinyl Chloride) Matrix Membrane Sensor for Batch and Flow–injection Determination of Thiocyanate, Cyanide and Some Metal Ions. Anal. Sci. 2009, 25, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Badr, I.H.A.; Meyerhoff, M.E.; Hassan, S.S.M. Novel response mechanism and application of sulfite sensitive polymeric membrane electrode based on dithiocarbamate complexes of mercury(II). Anal. Chim. Acta 1995, 310, 211–221. [Google Scholar] [CrossRef]
- Hutchins, R.S.; Molina, P.; Alajarín, M.; Vidal, A.; Bachas, L.G. Use of a guanidinium ionophore in a hydrogen sulfite-selective electrode. Anal. Chem. 1994, 66, 3188–3192. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Marei, S.A.; Badr, I.H.; Arida, H.A. Flow injection analysis of sulfite ion with a potentiometric titanium phosphate–epoxy based membrane sensor. Talanta 2001, 54, 773–782. [Google Scholar] [CrossRef]
- Zagal, J.H. Metallophthalocyanines as catalysts in electrochemical reactions. Coord. Chem. Rev. 1992, 119, 89–136. [Google Scholar] [CrossRef]
- Thamae, M.; Nyokong, T. Interaction of sulfur dioxide and cyanide with cobalt(II) tetrasulfophthalocyanine in aqueous media. Polyhedron 2002, 21, 133–140. [Google Scholar] [CrossRef]
- IUPAC. Analytical Chemistry Division Commission on Analytical Nomenclature. Pure Appl. Chem. 2000, 72, 185. [Google Scholar]
- FDA. Guidance for Industry: Analytical Procedures and Method Validation, Chemistry, Manufacturing, and Controls Documentation; U.S. Department of Health and Human Services: Washington, DC, USA, 2015 July.
- Montgomery, D.C. Introduction to Statistical Quality Control, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- IUPAC. Analytical Chemistry Division, Commission on Analytical Nomenclature, Recommendations forNomenclature of Ion-Selective Electrodes. Pure Appl. Chem. 1976, 48, 127–132. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Waters and Wastewaters, 23th ed.; American Public Health Association: Baltimore, Maryland, 2017. [Google Scholar]
- Hassan, S.S.M.; El-Nashar, R.M.; El-Tantawy, A.S.M. A new validated potentiometric method for batch and continuous quality control monitoring of oseltamivir phosphate (Taminil) in drug formulations and biological fluids. Electroanalysis 2013, 25, 408–416. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Parameter | Value | |
|---|---|---|
| Batch | FIA | |
| Calibration range, M | 1.1 ± 0.1 × 10−6–2.2 ± 0.1 × 10−3 | 5.0 ± 0.5 × 10−5–1.0 ± 2 × 10−1 |
| Slope, mV/log [SO3] | −27.4 ± 0.3 | −23.7 ± 0.6 |
| Low detection limit, M | 1.1 ± 0.1 × 10−6 | 1.1 ± 0.3 × 10−5 |
| Correlation coefficient, r2 | 0.9994 | 0.9965 |
| Response time for 10−5 M, s | 5 ± 2 | 50 ± 5 |
| Recovery time for 10−5 M, s | 10 ± 3 | 55 ± 5 |
| Working acidity range, pH | 5–7 | 5–7 |
| Accuracy, % | 98.1 ± 0.7 | 97.3 ± 1.1 |
| Trueness, % | 98.0 ± 0.8 | 97.1 ± 1.2 |
| Bias, % | 2.0 ± 0.2 | 3.2 ± 0.8 |
| Within-day Repeatability, CVw% | 0.7 ± 0.2 | 1.1 ± 0.4 |
| Between days- variation, CVb% | 0.9 ± 0.1 | 1.3 ± 0.5 |
| Relative Standard deviation, % | 0.9 ± 0.1 | 1.2 ± 0.2 |
| Precision, % | 0.7 ± 0.2 | 0.8 ± 0.4 |
| Sample | Sulfite, µg/ mL | |||
|---|---|---|---|---|
| Potentiometry, (Sulfite Sensor) | Spectro- Photometry | Iodometry, [47] (Standard Methods) | ||
| Batch | FIA | |||
| Beer,(Barq’s), USA | 10.9 ± 0.6 | 10.4 ± 0.8 | - | 11.1 ± 0.7 |
| Non-alcoholic Malt Beverage, (Birell), Egypt | 8.1 ± 0.7 | 7.9 ± 0.8 | - | 8.2 ± 0.8 |
| Non-alcoholic Malt Beverage, (Barbican), USA | 9.1 ± 0.6 | 9.6 ± 0.8 | - | 9.1 ± 0.8 |
| White Sparkling Grape Drink, (Carl Yong), Germany | 277.2 ± 0.6 | 281.1 ± 0.9 | 275.3 ± 0.7 | 278.4 ± 2.3 |
| White Vinegar, (Heinz), Egypt | 87.0 ± 0.6 | 89.2 ± 1.7 | 86.9 ± 1.9 | 88.3 ± 2.3 |
| Sugar Lump, (National Sugar Co.), Egypt | 244.2 ± 0.6 | 250.3 ± 1.3 | 252.2 ± 0.8 | 247.2 ± 2.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.S.M.; H. Kamel, A.; Amr, A.E.-G.E.; Abd-Rabboh, H.S.M.; Al-Omar, M.A.; Elsayed, E.A. A New Validated Potentiometric Method for Sulfite Assay in Beverages Using Cobalt(II) Phthalocyanine as a Sensory Recognition Element. Molecules 2020, 25, 3076. https://doi.org/10.3390/molecules25133076
Hassan SSM, H. Kamel A, Amr AE-GE, Abd-Rabboh HSM, Al-Omar MA, Elsayed EA. A New Validated Potentiometric Method for Sulfite Assay in Beverages Using Cobalt(II) Phthalocyanine as a Sensory Recognition Element. Molecules. 2020; 25(13):3076. https://doi.org/10.3390/molecules25133076
Chicago/Turabian StyleHassan, Saad S. M., Ayman H. Kamel, Abd El-Galil E. Amr, Hisham S. M. Abd-Rabboh, Mohamed A. Al-Omar, and Elsayed A. Elsayed. 2020. "A New Validated Potentiometric Method for Sulfite Assay in Beverages Using Cobalt(II) Phthalocyanine as a Sensory Recognition Element" Molecules 25, no. 13: 3076. https://doi.org/10.3390/molecules25133076








