Droplet Rolling and Spinning in V-Shaped Hydrophobic Surfaces for Environmental Dust Mitigation
Abstract
1. Introduction
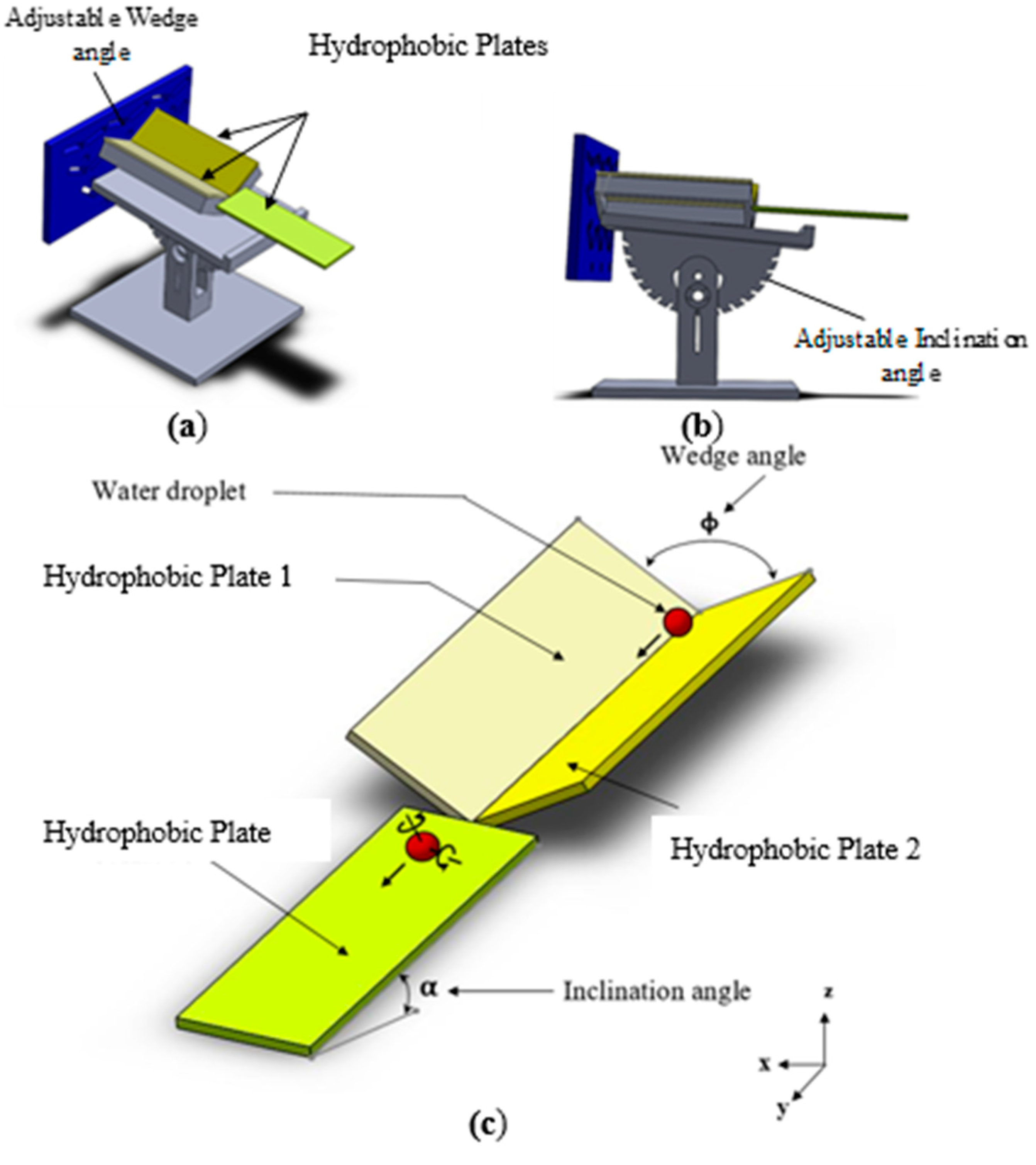
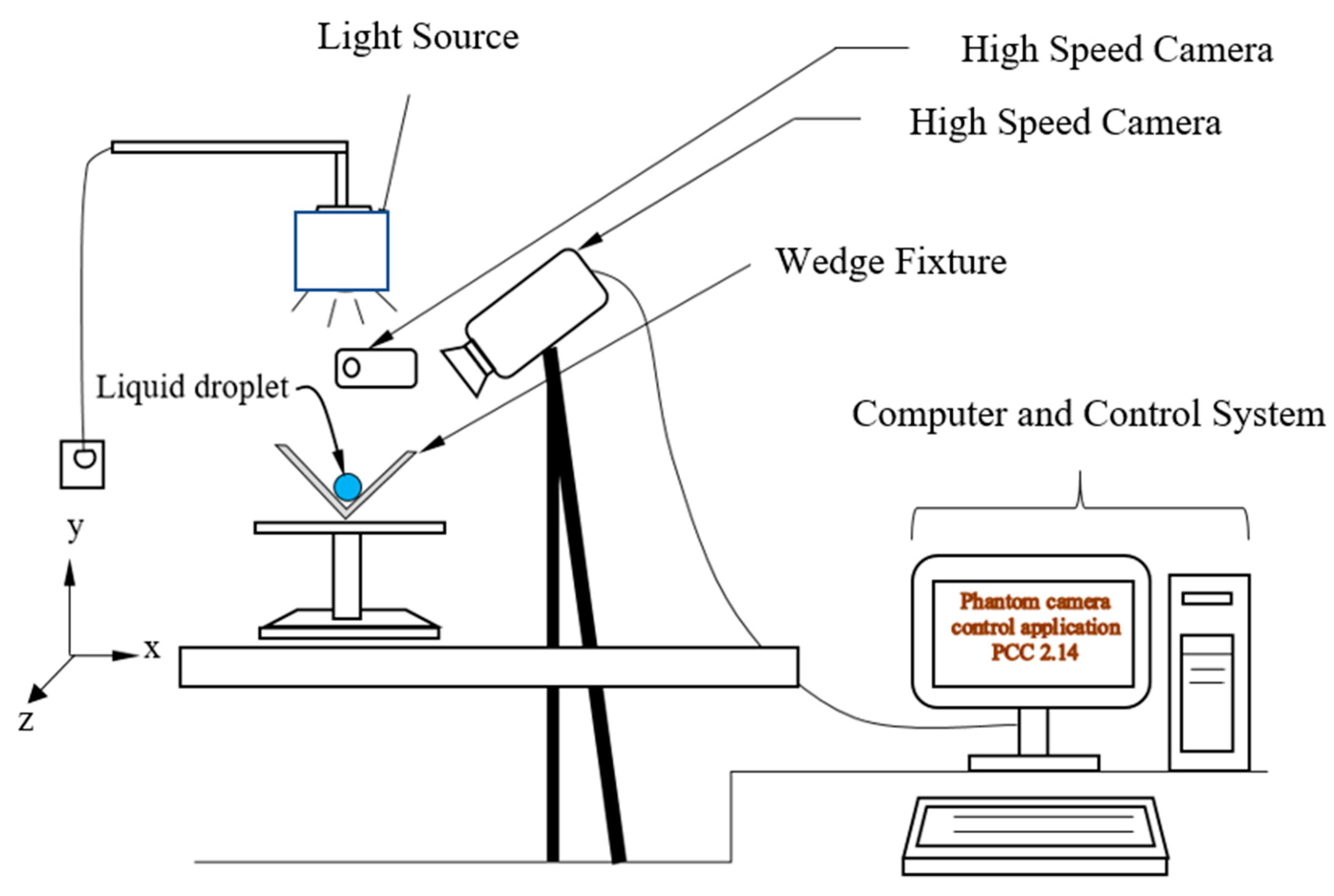
2. Experimental
3. Results and Discussion
3.1. Hydrophobizing of Wedge Surfaces and Dust Properties
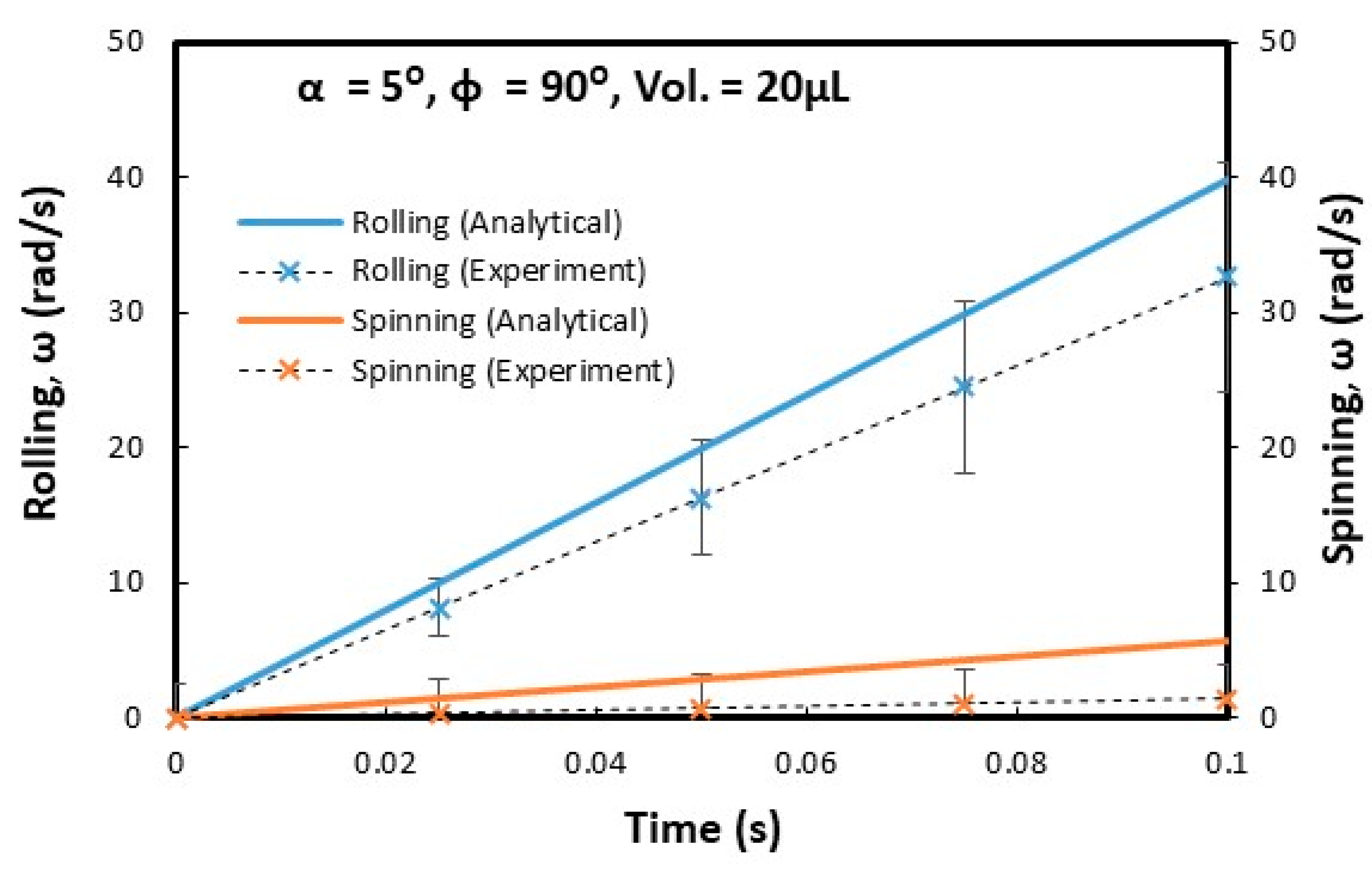
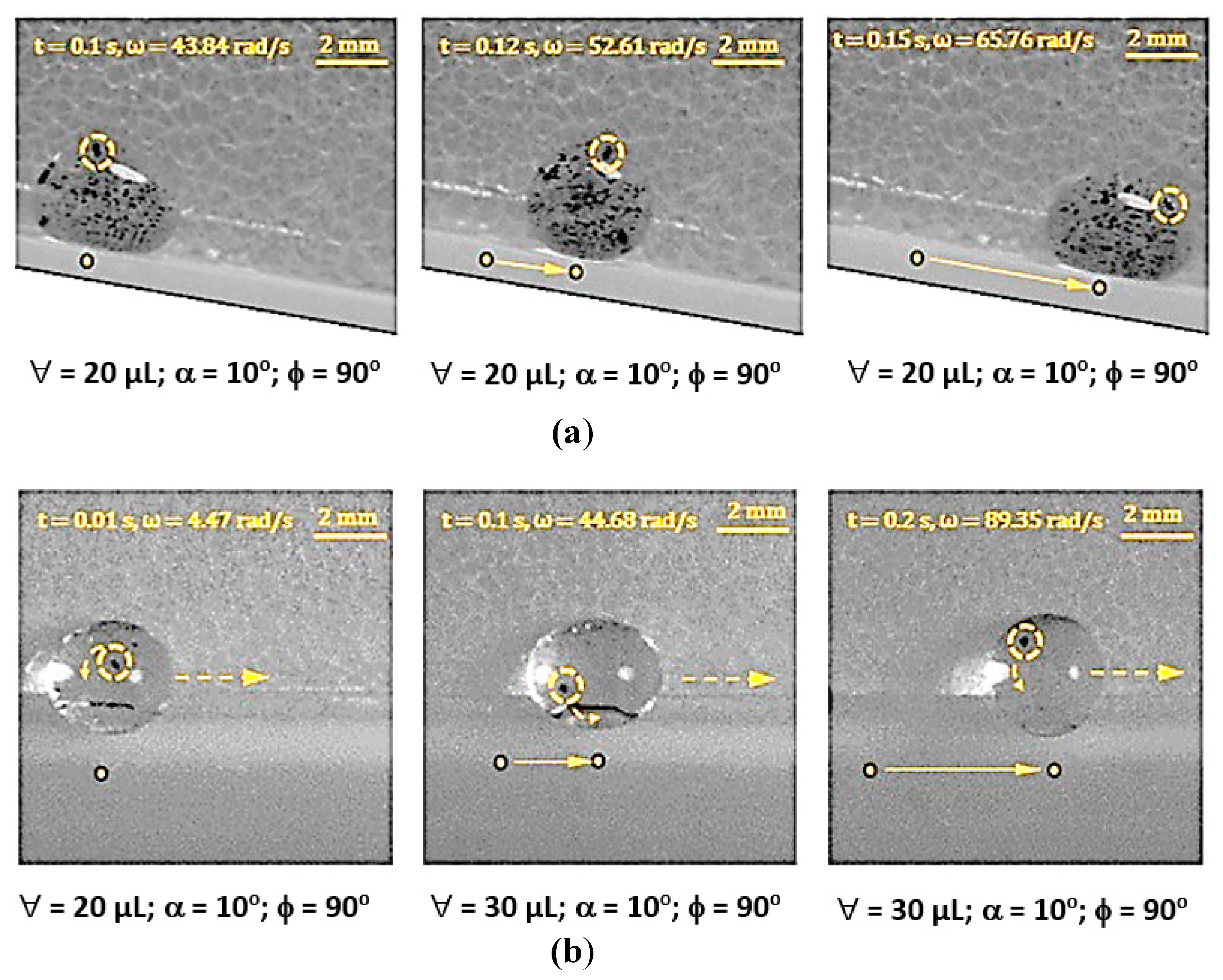
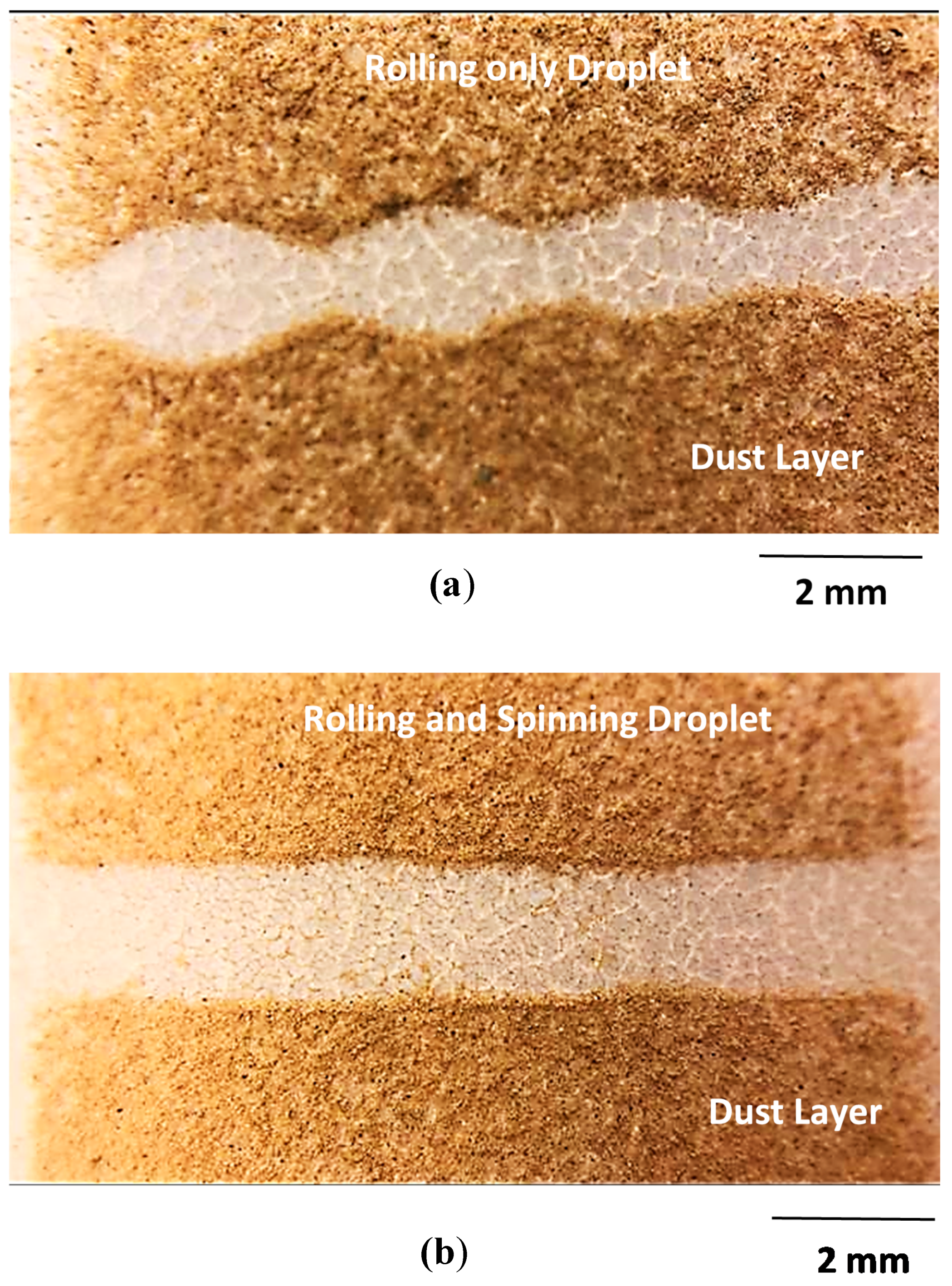
3.2. Droplet Rolling and Spinning Dynamics
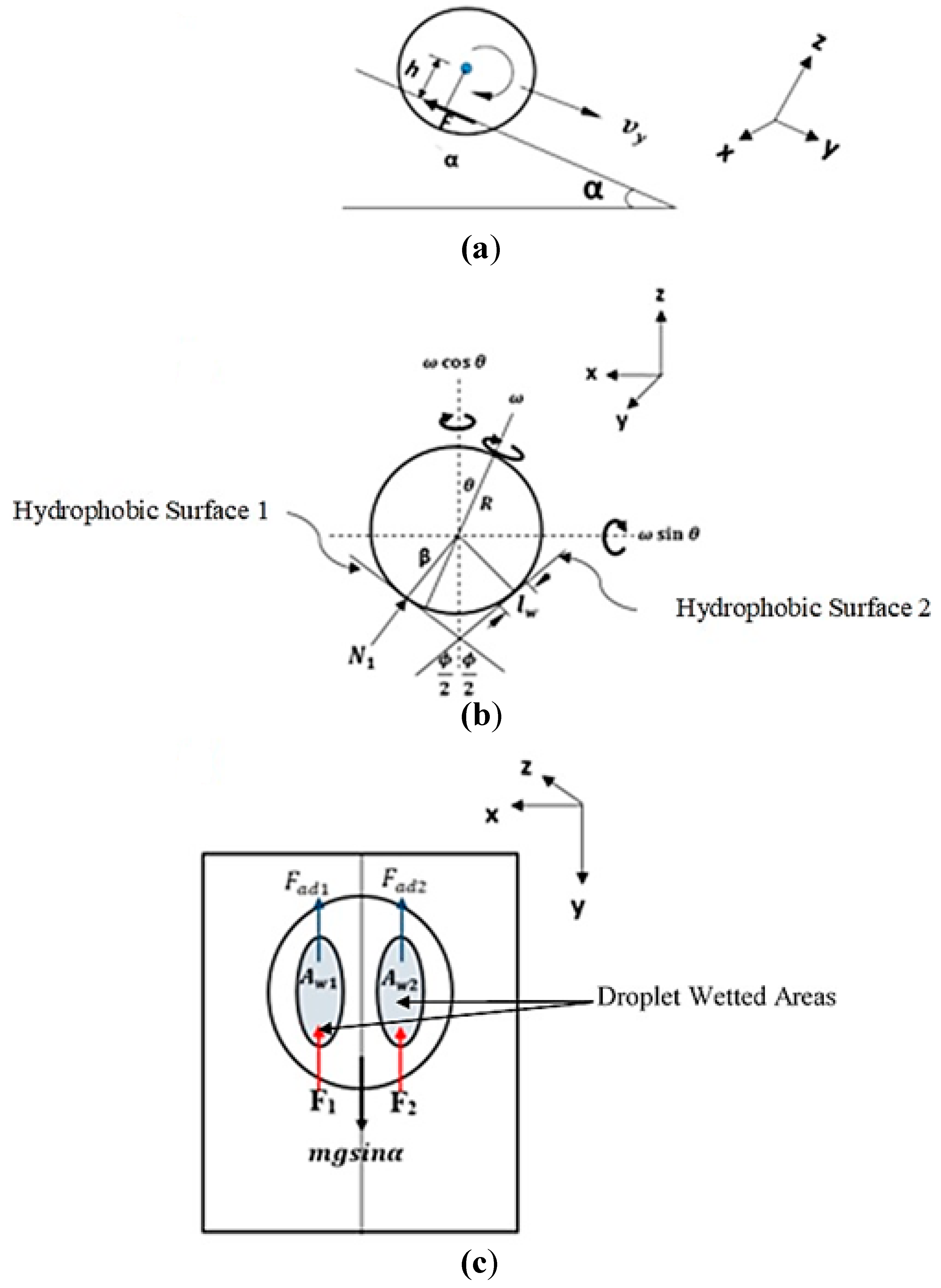
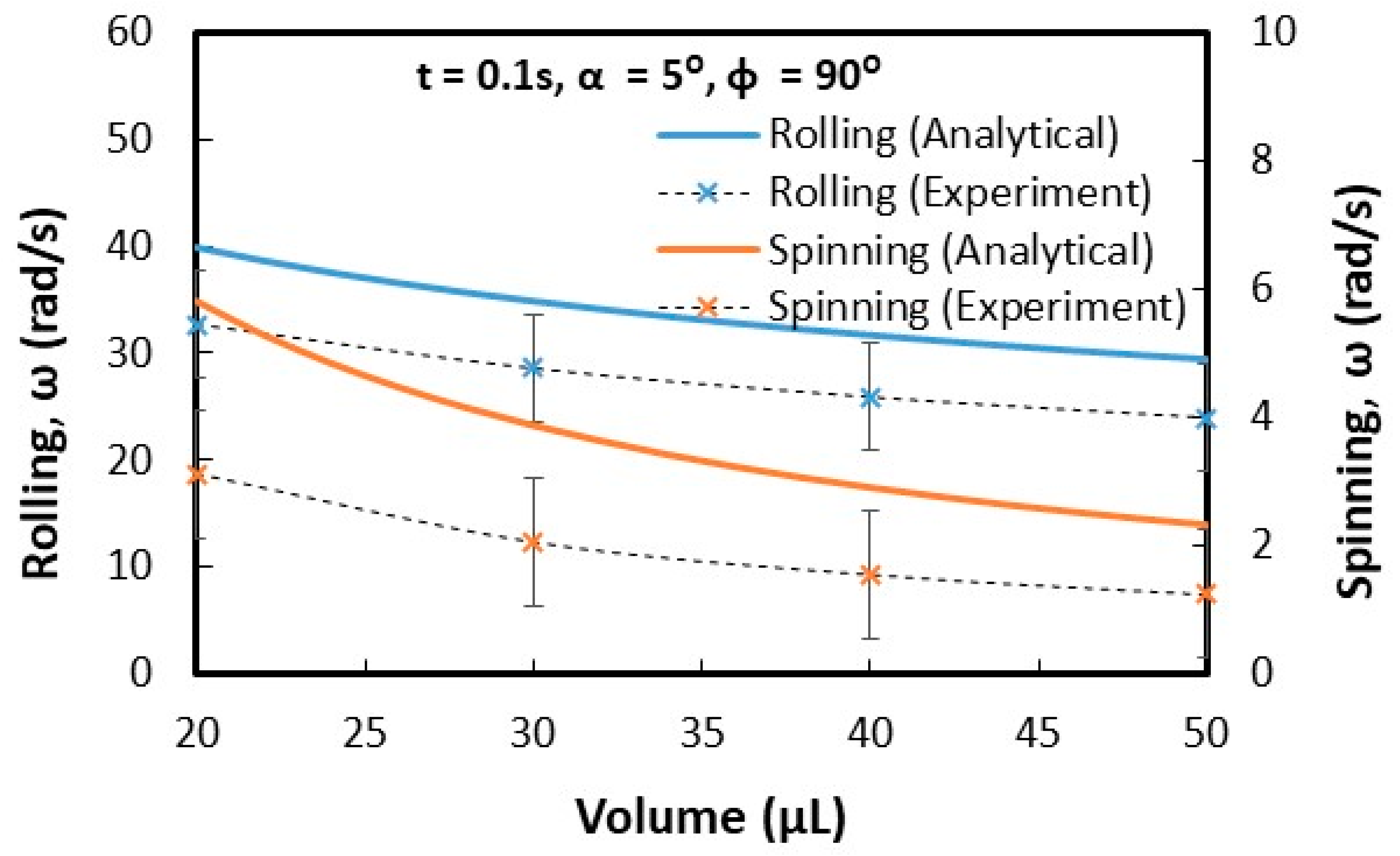
4. Analytical Modeling of Rolling and Spinning Droplet on Inclined Wedge Fixture
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Analysis of Droplet Motion in Inclined Wedge with Hydrophobic Flat Surfaces
References
- Yilbas, B.S.; Al-Qahtani, H.; Al-Sharafi, A.; Bahattab, S.; Hassan, G.; Al-Aqeeli, N.; Kassas, M. Environmental dust particles repelling from a hydrophobic surface under electrostatic influence. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.K.; Bansal, K. A brief history and future aspects in automatic cleaning systems for solar photovoltaic panels. Adv. Robot. 2015, 29, 515–524. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Hassan, G.; Al-Sharafi, A.; Ali, H.; Al-Aqeeli, N.; Al-Sarkhi, A. Water droplet dynamics on a hydrophobic surface in relation to the self-cleaning of environmental dust. Sci. Rep. 2018, 8, 2984. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, H.; Xu, D.; He, Z.; Pan, X.; Gui, S.; Dai, X.; Fan, J. Screening of nanocellulose from different biomass resources and its integration for hydrophobic transparent nanopaper. Molecules 2020, 25, 227. [Google Scholar] [CrossRef] [PubMed]
- Yilbas, B.S.; Al-Sharafi, A.; Ali, H. Self-Cleaning of Surfaces and Water Droplet Mobility; Elsevier: New York, NY, USA, 2019; ISBN 0128147776. [Google Scholar]
- Hassan, G.; Yilbas, B.S.; Said, S.A.M.; Al-Aqeeli, N.; Matin, A. Chemo-mechanical characteristics of mud formed from environmental dust particles in humid ambient air. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Xu, L.; Li, S.; Jiang, J.; Liu, T.; Wu, H.; Wang, J.; Li, X. The influence of dust deposition on the temperature of soiling photovoltaic glass under lighting and windy conditions. Sol. Energy 2020, 199, 491–496. [Google Scholar] [CrossRef]
- Ilse, K.K.; Figgis, B.W.; Naumann, V.; Hagendorf, C.; Bagdahn, J. Fundamentals of soiling processes on photovoltaic modules. Renew. Sustain. Energy Rev. 2018, 98, 239–254. [Google Scholar] [CrossRef]
- Hassan, G.; Yilbas, B.S.; Samad, M.A.; Ali, H.; Al-Sulaiman, F.A.; Al-Aqeeli, N. Analysis of environmental dust and mud adhesion on aluminum surface in relation to solar energy harvesting. Sol. Energy 2017, 153, 590–599. [Google Scholar] [CrossRef]
- Xia, M.; Yang, T.; Chen, S.; Yuan, G. Fabrication of superhydrophobic Eucalyptus wood surface with self-cleaning performance in air and oil environment and high durability. Colloid Interface Sci. Commun. 2020, 36, 100264. [Google Scholar] [CrossRef]
- Chi, F.; Zeng, Y.; Liu, C.; Pan, N.; Ding, C.; Yi, F. Highly stable self-cleaning antireflection coatings from fluoropolymer brush grafted silica nanoparticles. Appl. Surf. Sci. 2020, 507, 144836. [Google Scholar] [CrossRef]
- Sarkın, A.S.; Ekren, N.; Sağlam, Ş. A review of anti-reflection and self-cleaning coatings on photovoltaic panels. Sol. Energy 2020, 199, 63–73. [Google Scholar] [CrossRef]
- Hassan, G.; Yilbas, B.S.; Al-Sharafi, A.; Sahin, A.Z.; Al-Qahtani, H. Solar energy harvesting and self-cleaning of surfaces by an impacting water droplet. Int. J. Energy Res. 2020, 44, 388–401. [Google Scholar] [CrossRef]
- Xie, J.; Xu, J.; Shang, W.; Zhang, K. Mode selection between sliding and rolling for droplet on inclined surface: Effect of surface wettability. Int. J. Heat Mass Transf. 2018, 122, 45–58. [Google Scholar] [CrossRef]
- Hassan, G.; Yilbas, B.S.; Al-Sharafi, A.; Al-Qahtani, H. Self-cleaning of a hydrophobic surface by a rolling water droplet. Sci. Rep. 2019, 9, 5744. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wan, J.; Han, H.; Wang, Y.; Li, K.; Wang, Q. Sliding and rolling behavior of water droplets on an ordered nanoball matrix fluorocarbon polymer layer under simulated weather conditions. Surf. Sci. 2018, 675, 91–98. [Google Scholar] [CrossRef]
- Nagato, K.; Takahashi, N.; Shimura, T.; Nakao, M. Droplet sliding behaviour on textured and fluorinated surface. CIRP Ann. 2019, 68, 587–590. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Ali, H.; Al-Sharafi, A.; Al-Aqeeli, N. Reversible exchange of wetting state of a hydrophobic surface via phase change material coating. RSC Adv. 2018, 8, 938–947. [Google Scholar] [CrossRef]
- Al-Sharafi, A.; Yilbas, B.S.; Ali, H. Water droplet mobility on a hydrophobic surface under a thermal radiative heating. Appl. Therm. Eng. 2018, 128, 92–106. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Hassan, G.; Al-Qahtani, H.; Al-Aqeeli, N.; Al-Sharafi, A.; Al-Merbati, A.S.; Baroud, T.N.; Adukwu, J.A.E. Stretchable hydrophobic surfaces and self-cleaning applications. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Ali, H.; Al-Aqeeli, N.; Khaled, M.; Abu-Dheir, N.; Varanasi, K.K. Solvent-induced crystallization of a polycarbonate surface and texture copying by polydimethylsiloxane for improved surface hydrophobicity. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Yong, W.Y.D.; Zhang, Z.; Cristobal, G.; Chin, W.S. One-pot synthesis of surface functionalized spherical silica particles. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 460, 151–157. [Google Scholar] [CrossRef]
- Heib, F.; Schmitt, M. Statistical contact angle analyses with the high-precision drop shape analysis (HPDSA) approach: Bsic principles and applications. Coatings 2016, 6, 57. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Charonko, J.J.; Vlachos, P.P. Particle image velocimetry (PIV) uncertainty quantification using moment of correlation (MC) plane. Meas. Sci. Technol. 2018, 29, 115301. [Google Scholar] [CrossRef]
- Cui, Y.; Paxson, A.T.; Smyth, K.M.; Varanasi, K.K. Hierarchical polymeric textures via solvent-induced phase transformation: A single-step production of large-area superhydrophobic surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 394, 8–13. [Google Scholar] [CrossRef]
- Turska, E.; Benecki, W. Studies of liquid-induced crystallization of bisphenol a polycarbonate. J. Appl. Polym. Sci. 1979, 23, 3489–3500. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook; John & Wiley Sons. Inc.: New York, NY, USA, 1999; pp. 171–186. [Google Scholar]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure: Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: New York, NY, USA, 2009; ISBN 0080915108. [Google Scholar]
- Alfrey, T., Jr.; Gurnee, E.F.; Lloyd, W.G. Diffusion in Glassy Polymers. In Journal of Polymer Science Part C: Polymer Symposia; Wiley Online Library: New York, NY, USA, 1966; Volume 12, pp. 249–261. [Google Scholar]
- Sanopoulou, M.; Stamatialis, D.F. Study of the transition from Fickian to Case II sorption kinetics in the system poly (ethyl methacrylate)–liquid methyl alcohol. Polymer (Guildf) 2001, 42, 1429–1439. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Al-Sharafi, A.; Ali, H.; Al-Aqeeli, N. Dynamics of a water droplet on a hydrophobic inclined surface: Influence of droplet size and surface inclination angle on droplet rolling. Rsc Adv. 2017, 7, 48806–48818. [Google Scholar] [CrossRef]
- Brochard-Wyart, F.; Hervet, H.; Redon, C.; Rondelez, F. Spreading of “heavy” droplets: I. Theory. J. Colloid Interface Sci. 1991, 142, 518–527. [Google Scholar] [CrossRef]
- Elkins-Tanton, L.T.; Aussillous, P.; Bico, J.; Quere, D.; Bush, J.W.M. A laboratory model of splash-form tektites. Meteorit. Planet. Sci. 2003, 38, 1331–1340. [Google Scholar] [CrossRef]
- Mahadevan, L.; Pomeau, Y. Rolling droplets. Phys. fluids 1999, 11, 2449–2453. [Google Scholar] [CrossRef]
- Karpitschka, S.; Das, S.; Van Gorcum, M.; Perrin, H.; Andreotti, B.; Snoeijer, J.H. Droplets move over viscoelastic substrates by surfing a ridge. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| S/N | Property | Name | Value | Unit |
|---|---|---|---|---|
| 1 | Density | 997.1 | Kg/m3 | |
| 2 | Surfaces tension | 7.2 × 10−2 | N/m | |
| 3 | Roughness parameter | 0.6 | - | |
| 4 | Advancing angle, surface 1 | 158 | Degree | |
| 5 | Receding angle, surface 1 | 132 | Degree | |
| 6 | Roughness parameter | 0.4 | - | |
| 7 | Advancing angle, surface 2 | 161 | Degree | |
| 8 | Receding angle, surface 2 | 159 | Degree |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakubu, M.; Yilbas, B.S.; Abubakr, A.A.; Al-Qahtani, H. Droplet Rolling and Spinning in V-Shaped Hydrophobic Surfaces for Environmental Dust Mitigation . Molecules 2020, 25, 3039. https://doi.org/10.3390/molecules25133039
Yakubu M, Yilbas BS, Abubakr AA, Al-Qahtani H. Droplet Rolling and Spinning in V-Shaped Hydrophobic Surfaces for Environmental Dust Mitigation . Molecules. 2020; 25(13):3039. https://doi.org/10.3390/molecules25133039
Chicago/Turabian StyleYakubu, Mubarak, Bekir Sami Yilbas, Abba A. Abubakr, and Hussain Al-Qahtani. 2020. "Droplet Rolling and Spinning in V-Shaped Hydrophobic Surfaces for Environmental Dust Mitigation " Molecules 25, no. 13: 3039. https://doi.org/10.3390/molecules25133039
APA StyleYakubu, M., Yilbas, B. S., Abubakr, A. A., & Al-Qahtani, H. (2020). Droplet Rolling and Spinning in V-Shaped Hydrophobic Surfaces for Environmental Dust Mitigation . Molecules, 25(13), 3039. https://doi.org/10.3390/molecules25133039





