In Vitro Anthelmintic Evaluation of Gliricidia sepium, Leucaena leucocephala, and Pithecellobium dulce: Fingerprint Analysis of Extracts by UHPLC-Orbitrap Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. Identified Compounds with Reported Anthelmintic Activity
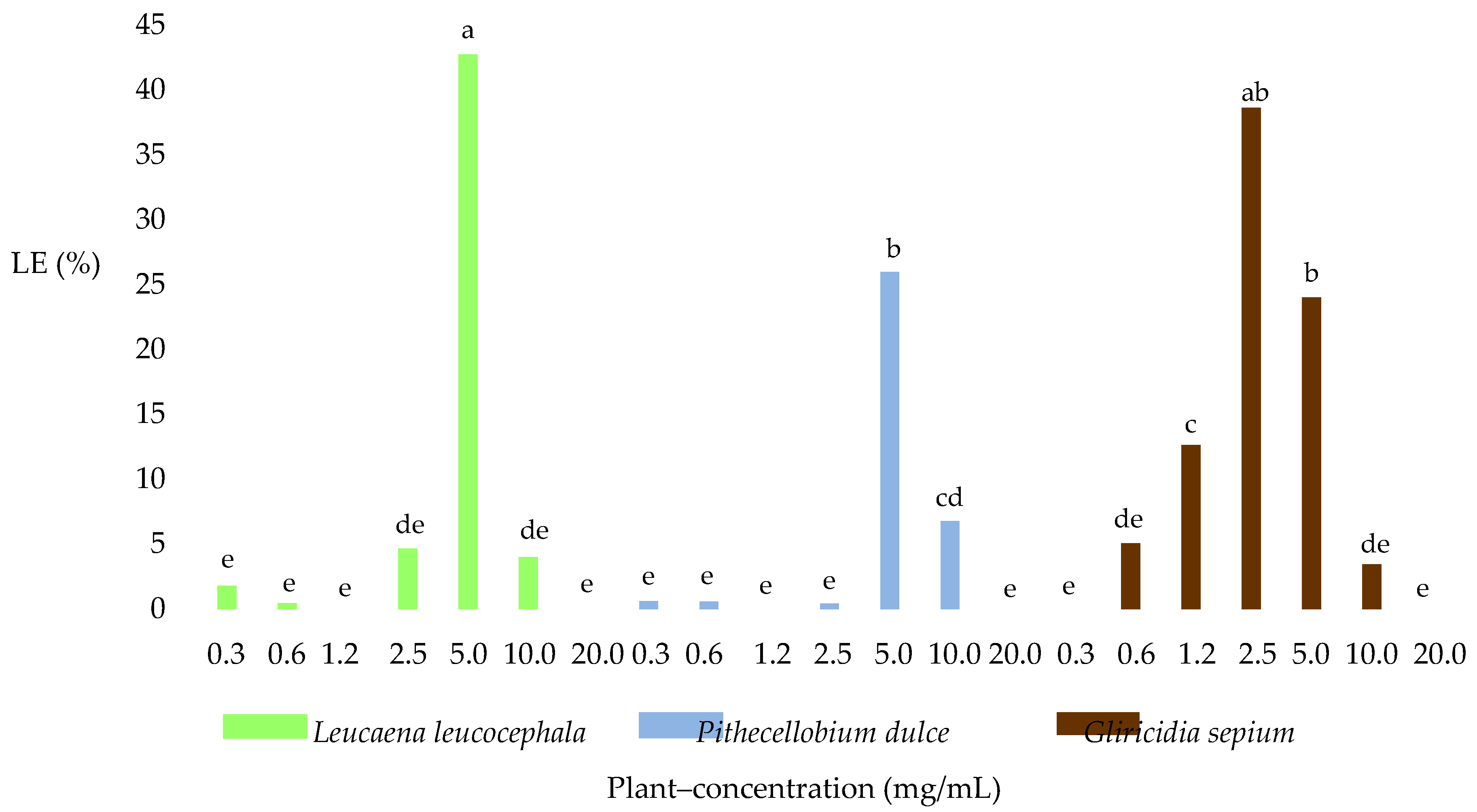
2.2. Larval Exsheathment Inhibition
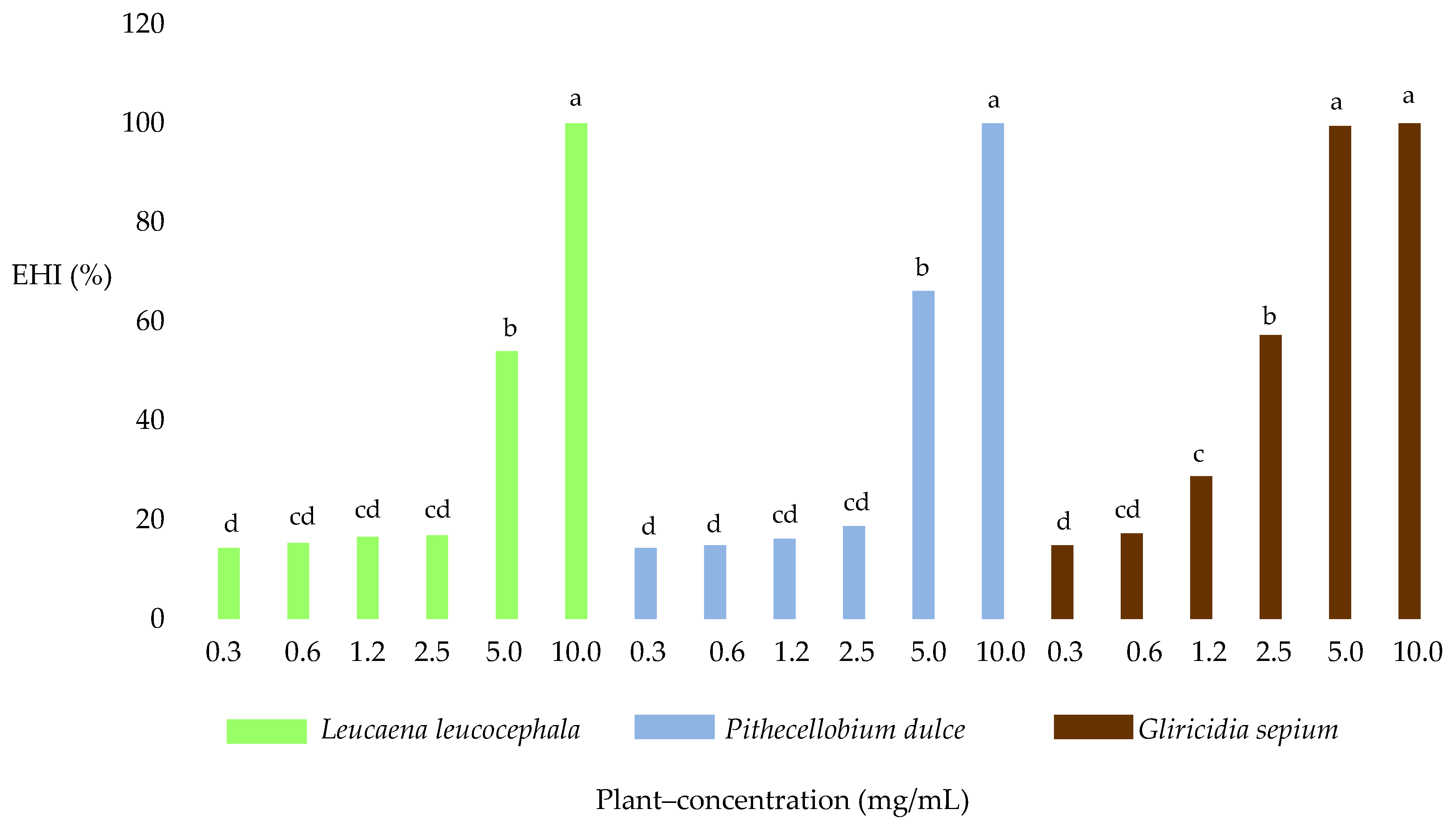
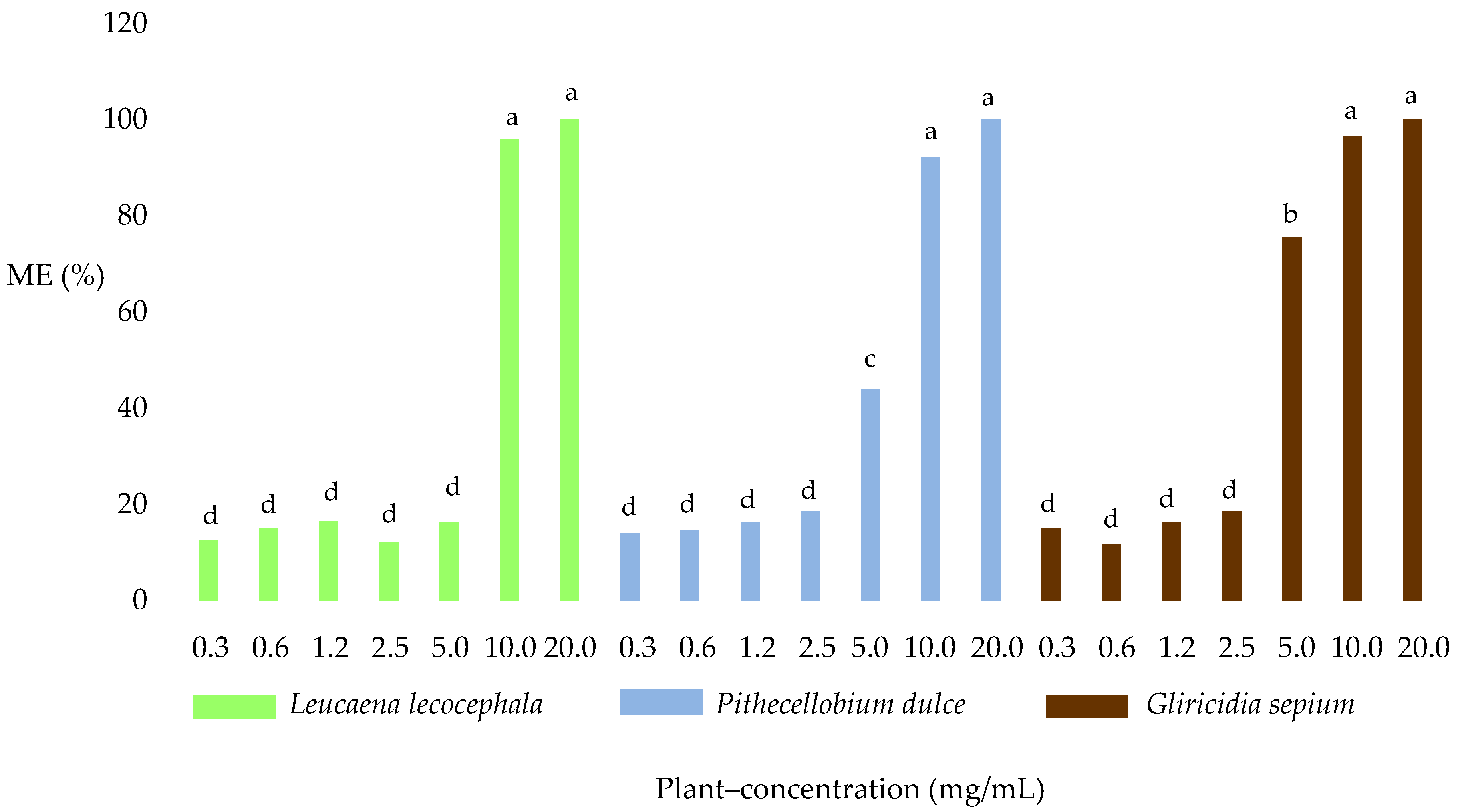
2.3. Egg Hatch Test
2.3.1. Morulated Eggs (ME)
2.3.2. Larvated Eggs (LE)
2.4. Inhibitory Concentrations 50 and 99 (IC50, IC99)
2.4.1. Larval Exsheathment Inhibition (LEI)
2.4.2. Egg Hatching Inhibition (EHI)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Instrumentation
4.4.1. Liquid Chromatography Parameters
4.4.2. Mass Spectrometry Parameters
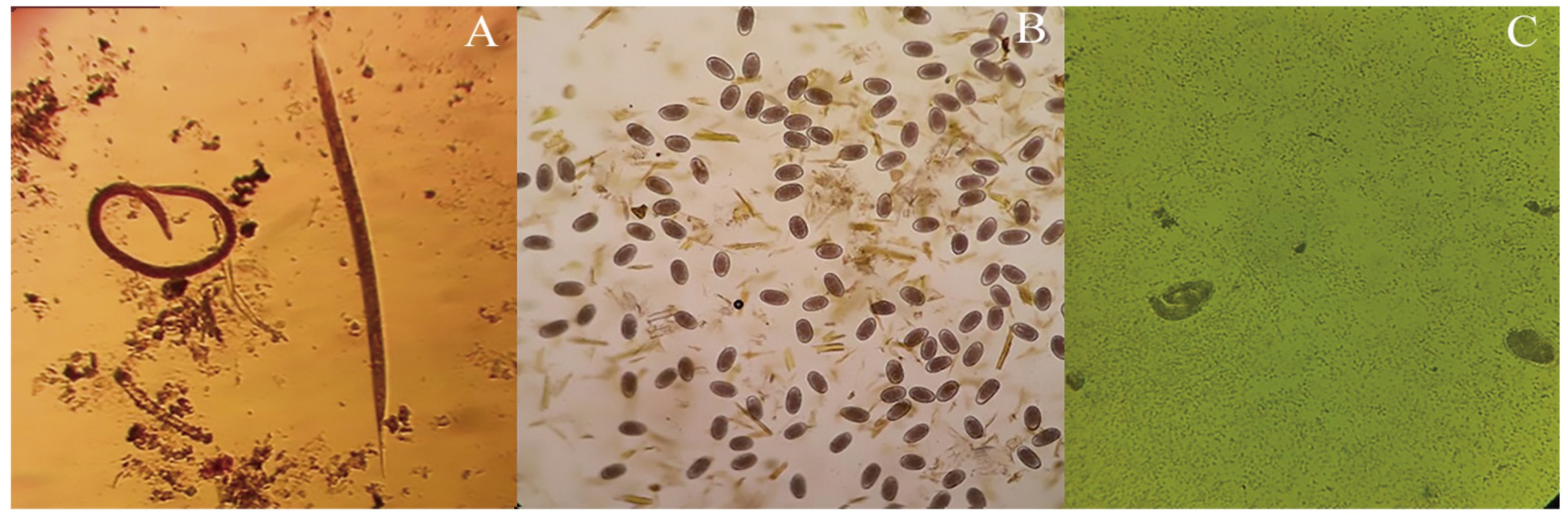
4.5. Anthelmintic Activity
4.5.1. Larval Exsheathment Inhibition Test
4.5.2. Egg Hatch Test
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed rumiants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mavrot, F. Livestock Nematode Infection in a Changing World: Investigating the European Situation. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2016. [Google Scholar]
- Herrera, L.; Ríos, L.; Zapata, R. Frequency of infection with gastrointestinal nematodes in sheep and goats in five municipalities of Antioquia. Rev. MVZ Cordoba 2013, 18, 3851–3860. [Google Scholar]
- Mavrot, F.; Hertzberg, H.; Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: A systematic review and meta-analysis. Parasit. Vectors 2015, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Anaya, A.M.; Chavarro-Tulcán, G.I.; Pulido-Medellín, M.O.; García-Corredor, D.; Vargas-Avella, J.C. Coproparasitological study in sheep grazing in Boyacá, Colombia. Rev. Salud Anim. 2017, 391, 1–8. [Google Scholar]
- Parra, R.I.; Magaña, M.A.; Duarte, J.H.; Téllez, G. Technical characterization and profitability of sheep farms with entrepreneurial vision of the department of Tolima. Rev. Colomb. Cienc. Anim. 2014, 7, 64–72. [Google Scholar]
- Laviano, H.D. Prevalence of Gastrointestinal Parasites in Sheep in the Department of Tolima; Graduate work; University of Tolima: Ibagué, Colombia, 2017. [Google Scholar]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 2016, 93, 95–143. [Google Scholar]
- Enejoh, O.S.; Suleiman, M.M. Anthelmintics and their application in veterinary medicine. Res. Med. Eng. Sci. 2017, 2, 000536. [Google Scholar]
- Lara, D. Anthelmintic resistance: Origin, development and control. Cienc. Tecnol. Agropecu. 2003, 4, 55–71. [Google Scholar] [CrossRef]
- Traversa, D.; von Samson-Himmelstjerna, G. Anthelmintic resistance in sheep gastro-intestinal strongyles in Europe. Small Rumin. Res. 2016, 135, 75–80. [Google Scholar] [CrossRef]
- Atanásio-Nhacumbe, A.; Carybé, M.C.; Lambert, S.M.; Souza, B.P. Anthelmintic resistance in gastrointestinal nematodes of goats in Southern Mozambique. J. Vet. Med. Anim. Health 2017, 9, 313–319. [Google Scholar]
- Goolsby, M.K.; Leite-Browning, M.L.; Browning, R. Evaluation of parasite resistance to commonly used commercial anthelmintics in meat goats on humid subtropical pasture. Small Rumin. Res. 2017, 146, 37–40. [Google Scholar] [CrossRef]
- Herrera-Manzanilla, F.A.; Ojeda-Robertos, N.F.; González-Garduño, R.; Cámara-Sarmiento, R.; Torres-Acosta, J.F. Gastrointestinal nematode populations with multiple anthelmintic resistance in sheep farms from the hot humid tropics of Mexico. Vet. Parasitol. 2017, 9, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, B.A.; Mulvaney, C.J. Resistance to a triple-combination anthelmintic in Trichostrongylus spp. on a Commercial Sheep Farm in New Zealand. N. Z. Vet. J. 2017, 65, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.A.; Riet-Correa, B.; Estima-Silva, P.; Coelho, A.C.; Santos, B.L.; Costa, M.A.; Ruas, J.L.; Schild, A.L. Multiple anthelmintic resistance in Southern Brazil sheep flocks. Rev. Bras. Parasitol. Vet. 2017, 26, 427–432. [Google Scholar] [CrossRef]
- Garcia, C.; Sprenger, L.K.; Benavides, E.; Belträo, M. First Report of multiple anthelmintic resistance in nematodes of sheep in Colombia. An. Acad. Bras. Cienc. 2016, 88, 397–402. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Quantification of anthelmintic drug residues in milk and muscle tissues by liquid chromatography coupled to Orbitrap and liquid chromatography coupled to tandem mass spectrometry. Talanta 2011, 85, 991–1000. [Google Scholar] [CrossRef]
- Ortelli, D.; Cognard, E.; Jan, P.; Edder, P. Comprehensive fast multiresidue screening of 150 veterinary drugs in milk by ultra-performance liquid chromatography coupled to time of flight mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2363–2374. [Google Scholar] [CrossRef]
- Morley, N.J. Environmental risk and toxicology of human and veterinary waste pharmaceutical exposure to wild aquatic host-parasite relationships. Environ. Toxicol. Pharmacol. 2009, 27, 161–175. [Google Scholar] [CrossRef]
- Yoshimura, H.; Endoh, Y.S. Acute toxicity to freshwater organisms of antiparasitic drugs for veterinary use. Environ. Toxicol. 2005, 20, 60–66. [Google Scholar] [CrossRef]
- Kolar, L.; Kozuh, N.; Hogerwerf, L.; van Gestel, C.A. Toxicity of abamectin and doramectin to soil invertebrates. Environ. Pollut. 2008, 151, 182–189. [Google Scholar] [CrossRef]
- Costa, R.M.; Vaz, A.F.; Xavier, H.S.; Correia, M.T.; Carneiro-da-Cunha, M.G. Phytochemical screening of Phthirusa Pyrifolia leaf extracts: Free-radical scavenging activities and environmental toxicity. S. Afr. J. Bot. 2015, 99, 132–137. [Google Scholar] [CrossRef]
- Mkenda, P.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.; Mtei, K.; Belmain, S.R. Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 2015, 10, e0143530. [Google Scholar] [CrossRef] [PubMed]
- Chan-Pérez, J.I.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Hoste, H.; Castañeda-Ramírez, G.S.; Vilarem, G.; Mathieu, C. In Vitro susceptibility of ten Haemonchus contortus isolates from different geographical origins towards acetone: Water extracts of two tannin rich plants. Vet. Parasitol. 2016, 217, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kumarasingha, R.; Preston, S.; Yeo, T.C.; Lim, D.S.; Tu, C.L.; Palombo, E.A.; Shaw, J.M.; Gasser, R.B.; Boag, P.R. Anthelmintic activity of selected ethno-medicinal plant extracts on parasitic stages of Haemonchus contortus. Parasit. Vectors 2016, 9, 187. [Google Scholar] [CrossRef]
- Cabardo, D.E.; Portugaliza, H.P. Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int. J. Vet. Sci. Med. 2017, 5, 30–34. [Google Scholar] [CrossRef]
- Soldera-Silva, A.; Seyfried, M.; Campestrini, L.H.; Zawadzki-Baggio, S.F.; Minho, A.P.; Molento, M.B.; Maurer, J.B. Assessment of anthelmintic activity and bio-guided chemical analysis of Persea americana seed extracts. Vet. Parasitol. 2018, 251, 34–43. [Google Scholar] [CrossRef]
- Brunet, S.; Hoste, H. Monomers of condensed tannins affect the larval exsheathment of parasitic nematodes of ruminants. J. Agric. Food Chem. 2006, 54, 7481–7487. [Google Scholar] [CrossRef]
- Naumann, H.D.; Armstrong, S.A.; Lambert, B.D.; Muir, B.D.; Tedeschi, J.P.; Kothmann, M.M. Effect of molecular weight and concentration of legume condensed tannins on in vitro larval migration inhibition of Haemonchus contortus. Vet. Parasitol. 2014, 199, 93–98. [Google Scholar] [CrossRef]
- Quijada, J.; Fryganas, C.; Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I.; Hoste, H. Anthelmintic activities against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins. J. Agric. Food Chem. 2015, 63, 6346–6354. [Google Scholar] [CrossRef]
- Barrau, E.; Fabre, N.; Fouraste, I.; Hoste, H. Effect of bioactive compounds from sainfoin (Onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: Role of tannins and flavonol glycosides. Parasitology 2005, 131, 531–538. [Google Scholar] [CrossRef]
- Camurça-Vasconcelos, A.L.; Bevilaqua, C.M.; Morais, S.M.; Maciel, M.V.; Costa, C.T.; Macedo, I.T.; Olivera, L.M.; Braga, R.R.; Silva, R.A.; Vieira, L.S. Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet. Parasitol. 2007, 148, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Macedo, I.T.; Bevilaqua, C.M.; de Oliveira, L.; Camurça-Vasconcelos, A.L.; Vieira, L.S.; Oliveira, F.R.; Queiroz-Junior, E.M.; Portela, B.G.; Barros, R.S.; Chagas, A.C. Ovicidal and larvicidal activity in vitro of Eucalyptus globulus essential oils on Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2009, 18, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.M.; Morais, S.M.; Bevilaqua, C.M.; Luciano, J.H. Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vet. Parasitol. 2002, 109, 59–63. [Google Scholar] [CrossRef]
- Kiuchi, F.; Tsuda, Y.; Kondo, K.; Yoshimura, H.; Nishioka, I.; Nonaka, G. Studies on crude drugs effective on visceral Larva migrans. III. The bursting activity of tannins on dog roundworm larva. Chem. Pharm. Bull. 1988, 36, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Perrett, S.; Whitfield, P.J. Anthelmintic and pesticidal activity of Acorus gramineus (Araceae) is associated with phenylpropanoid asarones. Phytother. Res. 1995, 9, 405–409. [Google Scholar] [CrossRef]
- Morais, S.M.; Beviláqua, C.M.; Aprecida, L.; Moura de Assis, L. Chemical investigation of Spigelia anthelmia Linn. used in brazilian folk medicine as anthelmintic. Rev. Bras. Farmacogn. 2002, 12, 81–82. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhou, Z.; Jiang, D.X.; Han, J.; Wang, J.F.; Zhao, L.W.; Li, J. In Vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet. Parasitol. 2010, 171, 305–313. [Google Scholar] [CrossRef]
- Ríos-de Alvarez, L.; Jackson, F.; Greer, A.; Bartley, Y.; Bartley, D.J.; Grant, G.; Huntley, J.F. In Vitro screening of plant lectins and tropical plant extracts for anthelmintic properties. Vet. Parasitol. 2012, 186, 390–398. [Google Scholar] [CrossRef]
- Von Son-de Fernex, E.; Alonso-Diaz, M.; Valles-de la Mora, B.; Capetillo-Leal, C.M. In Vitro anthelmintic activity of five tropical legumes on the exsheathment and motility of Haemonchus contortus infective larvae. Exp. Parasitol. 2012, 131, 413–418. [Google Scholar] [CrossRef]
- Puerto-Abreu, M.; Arece-García, J.; López-Leyva, Y.; Roche, Y.; Molina, M.; Sanavria, A.; da Fonseca, A.H. In Vitro effect of Moringa oleifera and Gliricida sepium aqueus extracts in the development of non- parasitic stages of sheep gastrointestinal strongyles. Rev. Salud Anim. 2014, 36, 28–34. [Google Scholar]
- Wabo-Poné, J.; Kenne-Tameli, F.; Mpoame, M.; Pamo-Tedonkeng, E.; Bilong-Bilong, C.F. In Vitro activities of acetonic extracts from leaves of three forage legumes (Calliandra calotyrsus, Gliricidia sepium and Leucaena diversifolia) on Haemonchus contortus. Asian Pac. J. Trop. Med. 2011, 4, 125–128. [Google Scholar] [PubMed]
- Ademola, I.O.; Akanbi, A.I.; Idowu, S.O. Comparative nematocidal activity of chromatographic fractions of Leucaena leucocephala. Seed against gastrointestinal sheep nematodes. Pharm. Biol. 2005, 43, 599–604. [Google Scholar] [CrossRef]
- Alonso-Diaz, M.A.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Aguilar-Caballero, A.J.; Hoste, H. In Vitro larval migration and kinetics of exsheathment of Haemonchus contortus larvae exposed to four tropical tanniniferous plant extracts. Vet. Parasitol. 2008, 153, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Juárez, A.; Rojo-Rubio, R.; Arece-García, J.; Salem, A.Z.M.; Kholif, A.E.; Morales-Almaraz, E. In Vitro activity of Pithecellobium dulce and Lysiloma acapulcensis on exogenous development stages of sheep gastrointestinal strongyles. Ital. J. Anim. Sci. 2014, 13, 3104. [Google Scholar] [CrossRef][Green Version]
- León-Castro, Y.; Olivares-Pérez, J.; Rojas-Hernández, S.; Villa-Mancera, A.; Valencia-Almazán, M.T.; Hernández-Castro, E.; Córdova-Izquierdo, A.; Jiménez-Guillén, R. Effect of three fodder trees on Haemonchus contortus control and weight variations in kids. Ecosistemas Recur. Agropecu. 2015, 2, 193–201. [Google Scholar]
- Kabore, A.; Traore, A.; Nignan, M.; Gnanda, B.I.; Bamogo, V.; Tamboura, H.H.; Bélem, A.M. In Vitro anthelmintic activity of Leucaena leucocephala (Lam.) De Wit. (Mimosaceae) and Gliricidia sepium (Jacq.) Kunth ex Steud (Fabaceae) leave extracts on Haemonchus contortus ova and larvae. J. Chem. Pharm. Res. 2012, 4, 303–309. [Google Scholar]
- Soares, A.M.; de Araújo, S.A.; Lopes, S.G.; Costa-Junior, L.M. Anthelmintic activity of Leucaena leucocephala protein extracts on Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2015, 24, 396–401. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Gonzalez-Pech, P.G.; Parra-Tabla, V.P.; Mathieu, C. Is there a negative association between the content of condensed tannins, total phenols, and total tannins of tropical plant extracts and in vitro anthelmintic activity against Haemonchus contortus Eggs? Parasitol. Res. 2017, 116, 3341–3348. [Google Scholar] [CrossRef]
- Vargas-Magaña, J.J.; Torres-Acosta, J.F.; Aguilar-Caballero, A.J.; Sandoval-Castro, C.A.; Hoste, H.; Chan-Perez, J.A. Anthelmintic activity of acetone-water extracts against Haemonchus contortus eggs: Interactions between tannins and other plant secondary compounds. Vet. Parasitol. 2014, 206, 322–327. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; Rodriguez-Labastida, M.; Ortiz-Ocampo, G.I.; Gonzalez-Pech, P.G.; Ventura-Cordero, J.; Borges-Argaez, R.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Mathieu, C. An in vitro approach to evaluate the nutraceutical value of plant foliage against Haemonchus contortus. Parasitol. Res. 2018, 117, 3979–3991. [Google Scholar] [CrossRef]
- Pérez-Pérez, C.; Hernández-Villegas, M.M.; de la Cruz-Burelo, P.; Bolio-López, G.I.; Hernández-Bolio, G.I. In Vitro anthelmintic effect of methanolic leaf extract of Gliricidia sepium against gastrointestinale nematodes of sheep. Trop. Subtro. Agroecosyst. 2014, 17, 105–111. [Google Scholar]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An overview. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: London, UK, 2018; pp. 153–167. [Google Scholar]
- Torres, N.; Goicoechea, N.; Antolín, M.C. Antioxidant properties of leaves from different accessions of grapevine (Vitis vinifera L.) cv. Tempranillo after applying biotic and/or environmental modulator factors. Ind. Crops Prod. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; Mathieu, C.; Vilarem, G.; Hoste, H.; Mendoza-de-Gives, P.; González-Pech, P.G.; Torres-Acosta, J.F.; Sandoval-Castro, C.A. Age of Haemonchus contortus third stage infective larvae is a factor influencing the in vitro assessment of anthelmintic properties of tannin containing plant extracts. Vet. Parasitol. 2017, 243, 130–134. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Giovanelli, F.; Mattellini, M.; Fichi, G.; Flamini, G.; Perrucci, S. In Vitro anthelmintic activity of four plant-ferived compounds against sheep gastrointestinal nematodes. Vet. Sci. 2018, 5, 78. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Mondal, H.; Hossain, H.; Awang, K.; Saha, S.; Mamun-Ur-Rashid, S.; Islam, M.K.; Rahman, M.S.; Jahan, I.A.; Rahman, M.M.; Shilpi, J.A. Anthelmintic activity of ellagic acid, a major constituent of Alternanthera sessilis against Haemonchus contortus. Pak. Vet. J. 2015, 35, 58–62. [Google Scholar]
- Papetti, A.; Maietta, M.; Corana, F.; Marrubini, G.; Gazzani, G. Polyphenolic profile of green/red spotted italian Cichorium intybus salads by RP-HPLC-PDA-ESI-MSn. J. Food Compost. Anal. 2017, 63, 189–197. [Google Scholar] [CrossRef]
- Lowry, J.B.; Cook, N.; Wilson, R.D. Flavonol glycoside distribution in cultivars and hybrids of Leucaena leucocephala. J. Sci. Food Agric. 1984, 35, 401–407. [Google Scholar] [CrossRef]
- Manguro, L.O.; Ugi, I.; Lemen, P. Further flavonol glycosides of Embelia schimperi leaves. Bull. Chem. Soc. Ethiop. 2004, 18, 51–57. [Google Scholar]
- Regos, I. Chemical Characterisation of Low Molecular Weight Phenolic Compounds from the Forage Legume Sainfoin (Onobrychis viciifolia). Ph.D. Thesis, Technical University Munich, Munich, Germany, 2014. [Google Scholar]
- Jamous, R.M.; Ali-Shtayeh, M.S.; Abu-Zaitoun, S.Y.; Markovics, A.; Azaizeh, H. Effects of selected Palestinian plants on the in vitro exsheathment of the third stage larvae of gastrointestinal nematodes. BMC Vet. Res. 2017, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Baert, N. Oligomeric Ellagitannins of Epilobium Angustifolium: Quantification and Bioactivity Assessment; University of Turku: Turku, Finland, 2017. [Google Scholar]
- Zangueu, C.B.; Olounlade, A.P.; Ossokomack, M.; Djouatsa, Y.N.; Alowanou, G.G.; Azebaze, A.G.; Llorent-Martinez, E.; Fernández, M.L.; Dongmo, A.B.; Hounzangbe-Adote, M.S. In Vitro effects of aqueous extract from Maytenus senegalensis (Lam.) Exell stem bark on egg hatching, larval migration and adult worms of Haemonchus contortus. BMC Vet. Res. 2018, 14, 147. [Google Scholar] [CrossRef]
- Nigam, S.K.; Mitra, C.R. Pithecolobium dulce. V. Chemistry of the seed saponin and constituents of the leaves. Planta Med. 1970, 18, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Regos, I.; Urbanella, A.; Treutter, D. Identification and quantification of phenolic compounds from the forage legume sainfoin (Onobrychis viciifolia). J. Agric. Food Chem. 2009, 57, 5843–5852. [Google Scholar] [CrossRef] [PubMed]
- Morshed, N.; Moghal, M.M.; Amin, M.N.; Kibria, M.G.; Dewan, S.M. Investigation of in-vitro anthelmintic and cytotoxic activities of Artabotrys hexapetalus (family: Annonaceae) bark growing in Bangladesh. Trends Biotechnol. Res. 2012, 1, 27–30. [Google Scholar]
- M’rabet, Y.; Rokbeni, N.; Cluzet, S.; Boulila, A.; Richard, T.; Krisa, S.; Marzouki, L.; Casabianca, H.; Hosni, K. Profiling of phenolic compounds and antioxidant activity of Melia azedarach L. leaves and fruits at two stages of maturity. Ind. Crops Prod. 2017, 107, 232–243. [Google Scholar] [CrossRef]
- Li, T.; Yu, J. Studies on the chemical constituents of the leaves from Artabotrys hexapetalus. Yao Xue Xue Bao 1998, 33, 591–596. [Google Scholar]
- Cala, A.C.; Chagas, A.C.; Oliveira, M.C.; Matos, A.P.; Borges, L.M.; Sousa, L.A.; Souza, F.A.; Oliveira, G.P. In vitro anthelmintic effect of Melia azedarach L. and Trichilia claussenii C. against sheep gastrointestinal nematodes. Exp. Parasitol. 2012, 130, 98–102. [Google Scholar] [CrossRef]
- Akkari, H.; B’Chir, F.; Hajaji, S.; Rekik, M.; Sebai, E.; Hamza, H.; Darghouth, M.; Gharbi, M. Potential anthelmintic effect of Capparis spinosa (Capparidaceae) as related to its polyphenolic content and antioxidant activity. Vet. Med. 2016, 61, 308–316. [Google Scholar] [CrossRef]
- Castillo-Mitre, G.F.; Olmedo-Juarez, A.; Rojo-Rubio, R.; Gonzalez-Cortazar, M.; Mendoza-de-Gives, P.; Hernandez-Beteta, E.E.; Reyes-Guerrero, D.E.; López-Arellano, M.E.; Vásquez-Armijo, J.F.; Vargas-Ramirez, G.; et al. Caffeoyl and coumaroyl cerivatives from Acacia Cochliacantha exhibit ovicidal activity against Haemonchus contortus. J. Ethnopharmacol. 2017, 204, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jarret, R.; Russo, V.; Majetich, G.; Shimkus, J.; Bushway, R.; Perkins, B. Determination of capsinoids by HPLC-DAD in Capsicum species. J. Agric. Food Chem. 2009, 57, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Vinayaka, K.S.; Nandini, K.C.; Rakshitha, M.N.; Ramya, M.; Shruthi, J.; Shruthi, V.; Prashith, K.T.; Raghavendra, H.L. Proximate composition, antibacterial and anthelmintic activity of Capsicum frutescens (L.) var. Longa (Solanaceae) Leaves. Pharmacogn. J. 2010, 2, 486–491. [Google Scholar] [CrossRef]
- Kamal, A.T.M.M.; Chowdhury, K.A.A.; Chy, M.M.; Shill, L.K.; Chowdhury, S.; Chy, M.A.H.; Habib, M.Z. Evaluation of anthelmintic activity of seeds of Sesamum indicum L. and fruits of Capsicum frutescens L. J. Pharmacogn. Phytochem. 2015, 3, 256–259. [Google Scholar]
- Mengistu, G.; Hoste, H.; Karonen, M.; Salminen, J.P.; Hendriks, W.H.; Pellikaan, W.F. The in vitro anthelmintic properties of browse plant species against Haemonchus contortus is determined by the polyphenol content and composition. Vet. Parasitol. 2017, 237, 110–116. [Google Scholar] [CrossRef]
- Taylor, C.M.; Wang, Q.; Rosa, B.A.; Huang, S.C.; Powell, K.; Schedl, T.; Pearce, E.J.; Abubucker, S.; Mitreva, M. Discovery of anthelmintic drug targets and drugs using chokepoints in nematode metabolic pathways. PLoS Pathog. 2013, 9, e1003505. [Google Scholar] [CrossRef]
- Tyagi, R.; Bruce, A.R.; Makedonka, M. Omics-driven knowledge-based discovery of anthelmintic targets and drugs. In Silico Drug Design; Academic Press: Cambridge, MA, USA, 2019; pp. 329–358. [Google Scholar]
- Nguyen, D.M.; Seo, D.J.; Kim, K.Y.; Park, R.D.; Kim, D.H.; Han, Y.S.; Han, Y.S.; Kim, T.H.; Jung, W.J. Nematicidal activity of 3,4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59–60, 52–59. [Google Scholar] [CrossRef]
- Petkevičius, S.; Murrell, K.D.; Knudsen, K.B.; Jørgensen, H.; Roepstorff, A.; Laue, A.; Wachmann, H. Effects of short-chain fatty acids and lactic acids on survival of Oesophagostomum dentatum in pigs. Vet. parasitol. 2004, 122, 293–301. [Google Scholar] [CrossRef]
- Santos, N.S.; Santos, J.D.; Santos, F.O.; Serra, T.M.; Lima, H.G.; Botura, M.B.; Branco, A.; Batatinha, M.J. Ovicidal activity of succinic acid isolated from sisal waste (Agave sisalana) against gastrointestinal nematodes of goats. Cienc. Rural 2017, 47, e20170036. [Google Scholar] [CrossRef]
- Akkari, H.; Rtibi, K.; B’chir, F.; Rekik, M.; Darghouth, M.A.; Gharbi, M. In Vitro evidence that the pastoral Artemisia campestris species exerts an anthelmintic effect on Haemonchus contortus from sheep. Vet. Res. Commun. 2014, 38, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vincieri, F.F.; Prucher, D. Characterization of Matricaria recutita L. flower extracts by HPLC-MS and HPLC-DAD analysis. Chromatographia 2000, 51, 301–307. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Hajaji, S.; Alimi, D.; Jabri, M.A.; Abuseir, S.; Gharbi, M.; Akkari, H. Anthelmintic activity of Tunisian chamomile (Matricaria recutita L.) against Haemonchus contortus. J. Helminthol. 2018, 92, 168–177. [Google Scholar] [CrossRef]
- Yoon, Y.A.; Kim, H.; Lim, Y.; Shim, Y.H. Relationships between the larval growth inhibition of Caenorhabditis elegans by apigenin derivatives and their structures. Arch. Pharm. Res. 2006, 29, 582–586. [Google Scholar] [CrossRef]
- Kawasaki, I.; Jeong, M.H.; Oh, B.K.; Shim, Y.H. Apigenin inhibits larval growth of Caenorhabditis elegans through DAF-16 activation. FEBS Lett. 2010, 584, 3587–3591. [Google Scholar] [CrossRef]
- Gordon, H.M. Studies on anthelmintics for sheep: Dihydroxyanthraquinones and some other quinones. Aust. Vet. J. 1957, 33, 39–42. [Google Scholar] [CrossRef]
- Dhananjeyan, M.R.; Milev, Y.P.; Kron, M.A.; Nair, M.G. Synthesis and activity of substituted anthraquinones against a human filarial parasite, Brugia Malayi. J. Med. Chem. 2005, 48, 2822–2830. [Google Scholar] [CrossRef]
- Herrmann, A.; Svangard, E.; Claeson, P.; Gullbo, J.; Bohlin, L.; Goransson, U. Key role of glutamic acid for the cytotoxic activity of the cyclotide cycloviolacin O2. Cell. Mol. Life Sci. 2006, 63, 235–245. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Kotze, A.C.; Ireland, D.C.; Wang, C.K.; Craik, D.J. The anthelmintic activity of the cyclotides: Natural variants with enhanced activity. Chembiochem 2008, 9, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Benet-Buchholz, J.; Harder, A.; Etzel, W.; Schindler, M.; Thielking, G. Synthesis and anthelmintic activity of cyclohexadepsipeptides with (S,S,S,R,S,R)-configuration. Bioorg. Med. Chem. Lett. 2003, 13, 3285–3288. [Google Scholar] [CrossRef]
- Jeschke, P.; Benet-Buchholz, J.; Harder, A.; Etzel, W.; Schindler, M.; Gau, W.; Weiss, H.C. Synthesis and anthelmintic activity of substituted (R)-phenyllactic acid containing cyclohexadepsipeptides. Bioorg. Med. Chem. Lett. 2006, 16, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.C.; Niciura, S.C.; Molento, M.B. Practical Manual: Methodologies for the Diagnosis of Resistance and Detection of Active Substances in Ruminant Parasites; Embrapa Technology Information: Brasilia, Brazil, 2011; p. 153. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.; González, L.; Tablada, E.; Robledo, C. InfoStat Version 2011; Group InfoStat, FCA, National University of Cordoba: Cordoba, Argentina, 2011; Volume 8, pp. 195–199. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
Sample Availability: Samples of the compounds the Gliricidia sepium, Leucaena leucocephala and Pithecellobium dulce are available from the authors. |
| Classification | Gliricidia sepium | Leucaena leucocephala | Pithecellobium dulce |
|---|---|---|---|
| Glycosylated Flavonoids | Apigenin-di-C-dihexose-O-deoxyhexose * | Myricetin-3-O-hexoside * | Quercetin-3-glucoside * (Isoquercitrin) |
| Apigenin-di-C-dihexose-O-deoxyhexose isomer * | Myricetin-3-arabinoside | Luteolin-7-O-glucoside * (Cynaroside or Glucoluteolin) | |
| Apigenin-7-O-Glucoside * | Myrcetin rhamnose derivative | Kaempferol-3-O-rhamnoside (Afzelin) | |
| Rutin* | |||
| Quercetin-3-O-arabinoside | |||
| Quercetin-3-O-pentoside * | |||
| Quercetin 3-O-rhamnoside | |||
| Kaempferol-3-O-pentoside * | |||
| Kaempferol-3-O-rhamnoside * | |||
| Flavonoids | Luteolin * | ||
| Quercetin * | |||
| Apigenin * | |||
| Chrysoeriol * | |||
| Phenylpropanoids other than Flavonoids | p-coumaroyl hexose * | ||
| Caffeoyl hexoside * | |||
| Dihydro-p-coumaric acid isomer * | |||
| p-coumaric acid | |||
| Leu/dihydro-p-coumaric acid * | |||
| Phe /Dihydro-p-coumaric acid * | |||
| p-Coumaric acid derivative * | |||
| Methoxyphenols | Dihydrocapsiate * | Syringaldehyde syringate or derivative of quinic acid * | Dihydrocapsiate * |
| Syringaldehyde syringate or derivative of quinic acid * | |||
| Anthraquinonic Glycosides | Trihydroxyanthraquinone-O-methylgluconate-glucoside * | ||
| Trihydroxyanthraquinone-O-methylgluconate-deoxymethylgluconic * | |||
| Trihydroxyanthraquinone-O-methylgluconate * | |||
| Trihydroxyanthraquinone-O-methylgluconate isomer * | |||
| Amino acids | Glutamic acid * | ||
| N-Carbobenzyloxy-l-isoleucine * | |||
| Glycosylated phenolic acids | Phenyllactic acid-2-O-glucoside * | ||
| Fatty acids | Ázelaic acid * | Azelaic acid * |
| Plant Extract | Average and Standard Error of Larval Exsheathment Inhibition (%) | ||||
|---|---|---|---|---|---|
| 40 mg/mL | 20 mg/mL | 10 mg/mL | Control Tween | Control DMSO | |
| Gliricidia sepium | 91.28 ± 2.2 a | 45.14 ± 2.0 b | 8.89 ± 1.9 c | 2.08 ± 2.0 de | 5.01 ± 1.8 cde |
| Leucaena leucocephala | 41.01 ± 18.9 bc | 21.42 ± 5.6 c | 9.62 ± 1.7 c | 2.06 ± 1.0 de | 4.89 ± 1.1 cde |
| Pithecellobium dulce | 44.66 ± 20.5 bc | 23.35 ± 5.9 c | 7.44 ± 1.0 c | 2.04 ± 0.68 e | 4.82 ± 0.7 cde |
| Plant Extracts | Test | IC50 (mg/mL) | Lower Limit Confidence Level 95.0% | Upper Limit Confidence Level 95.0% | IC99 (mg/mL) | Lower Limit Confidence Level 95.0% | Upper Limit Confidence Level 95.0% |
|---|---|---|---|---|---|---|---|
| Gliricidia sepium | LEI | 22.44 | 20.50 | 24.74 | 65.44 | 59.18 | 73.47 |
| EHI | 1.97 | 1.76 | 2.20 | 5.46 | 4.89 | 6.23 | |
| Leucaena leucocephala | LEI | 43.35 | 37.99 | 50.98 | 101.05 | 86.63 | 122.52 |
| EHI | 4.31 | 3.90 | 4.79 | 11.45 | 10.29 | 12.98 | |
| Pithecellobium dulce | LEI | 41.77 | 36.39 | 49.48 | 103.03 | 87.83 | 125.88 |
| EHI | 3.91 | 3.53 | 4.35 | 10.36 | 9.29 | 11.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, N.; Areche, C.; Cubides-Cárdenas, J.; Escobar, N.; García-Beltrán, O.; Simirgiotis, M.J.; Céspedes, Á. In Vitro Anthelmintic Evaluation of Gliricidia sepium, Leucaena leucocephala, and Pithecellobium dulce: Fingerprint Analysis of Extracts by UHPLC-Orbitrap Mass Spectrometry. Molecules 2020, 25, 3002. https://doi.org/10.3390/molecules25133002
Romero N, Areche C, Cubides-Cárdenas J, Escobar N, García-Beltrán O, Simirgiotis MJ, Céspedes Á. In Vitro Anthelmintic Evaluation of Gliricidia sepium, Leucaena leucocephala, and Pithecellobium dulce: Fingerprint Analysis of Extracts by UHPLC-Orbitrap Mass Spectrometry. Molecules. 2020; 25(13):3002. https://doi.org/10.3390/molecules25133002
Chicago/Turabian StyleRomero, Néstor, Carlos Areche, Jaime Cubides-Cárdenas, Natalia Escobar, Olimpo García-Beltrán, Mario J. Simirgiotis, and Ángel Céspedes. 2020. "In Vitro Anthelmintic Evaluation of Gliricidia sepium, Leucaena leucocephala, and Pithecellobium dulce: Fingerprint Analysis of Extracts by UHPLC-Orbitrap Mass Spectrometry" Molecules 25, no. 13: 3002. https://doi.org/10.3390/molecules25133002
APA StyleRomero, N., Areche, C., Cubides-Cárdenas, J., Escobar, N., García-Beltrán, O., Simirgiotis, M. J., & Céspedes, Á. (2020). In Vitro Anthelmintic Evaluation of Gliricidia sepium, Leucaena leucocephala, and Pithecellobium dulce: Fingerprint Analysis of Extracts by UHPLC-Orbitrap Mass Spectrometry. Molecules, 25(13), 3002. https://doi.org/10.3390/molecules25133002








