Concentrated Bioshell Calcium Oxide (BiSCaO) Water Kills Pathogenic Microbes: Characterization and Activity
Abstract
1. Introduction
2. Results
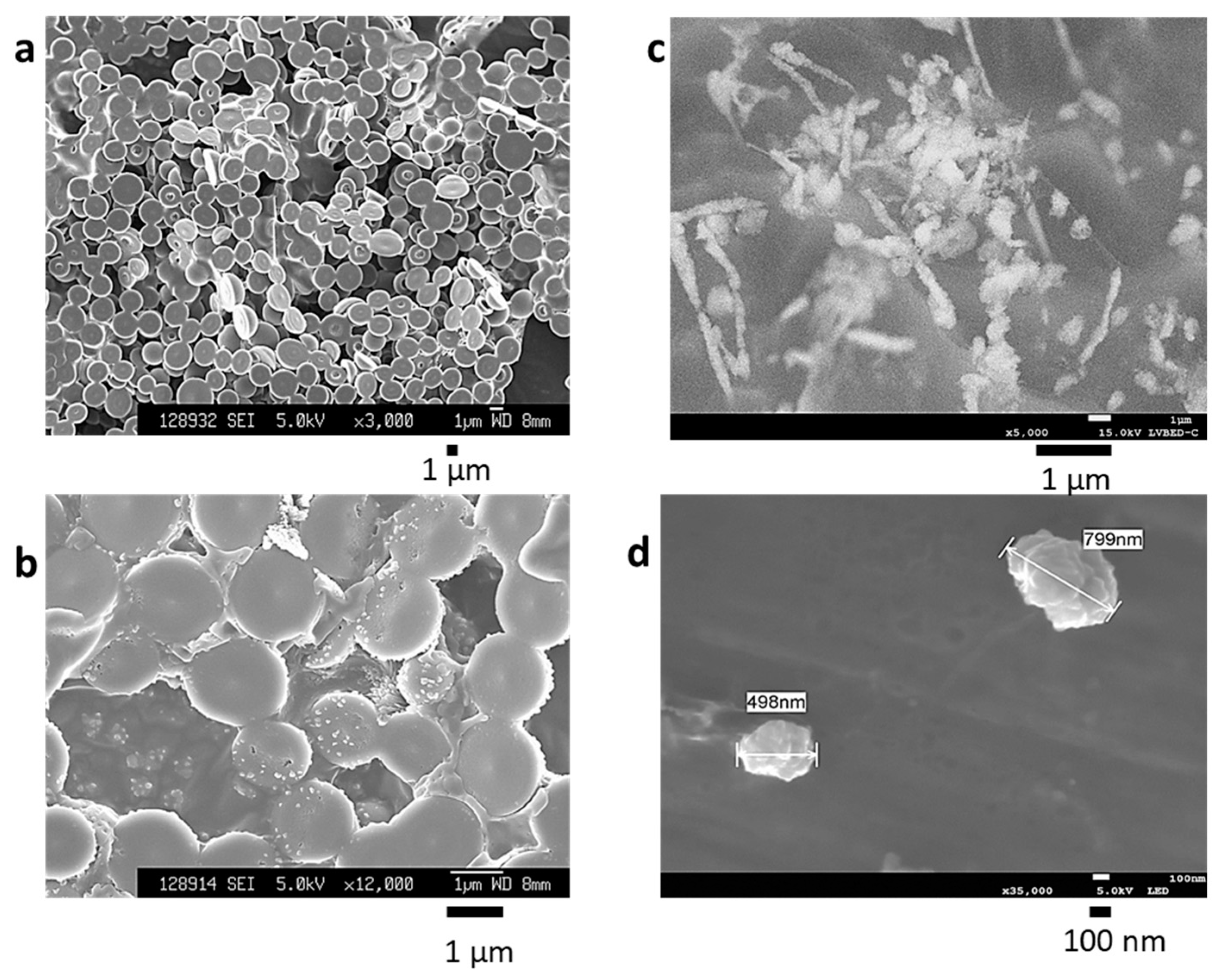
2.1. Characterization of BiSCaO Water
2.2. Assay for the Disinfection Activity of BiSCaO Water against Pathogenic Bacterias and Viruses In Vitro
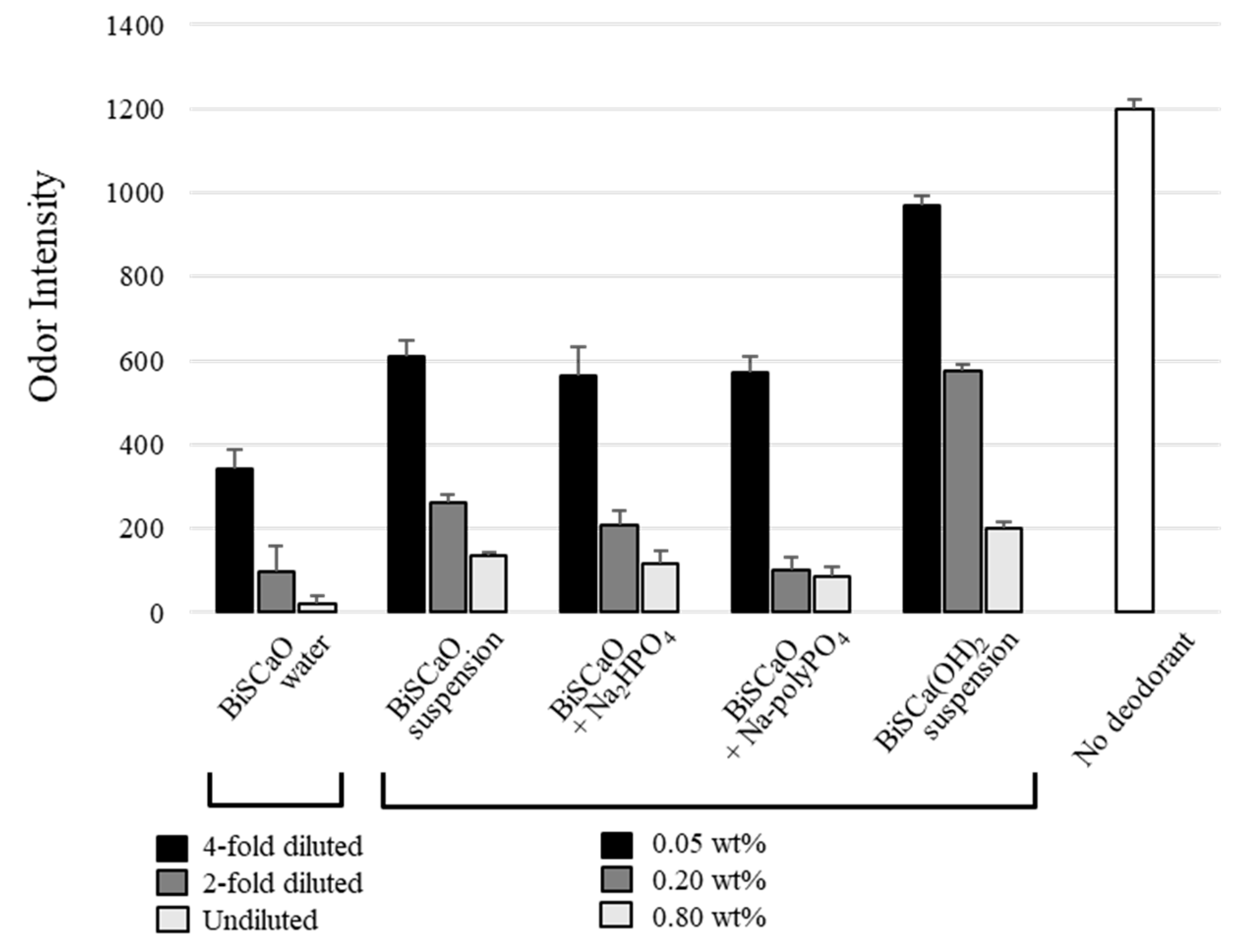
2.3. Deodorization Activity of BiSCaO Water, Dispersion, and Colloidal Dispersion
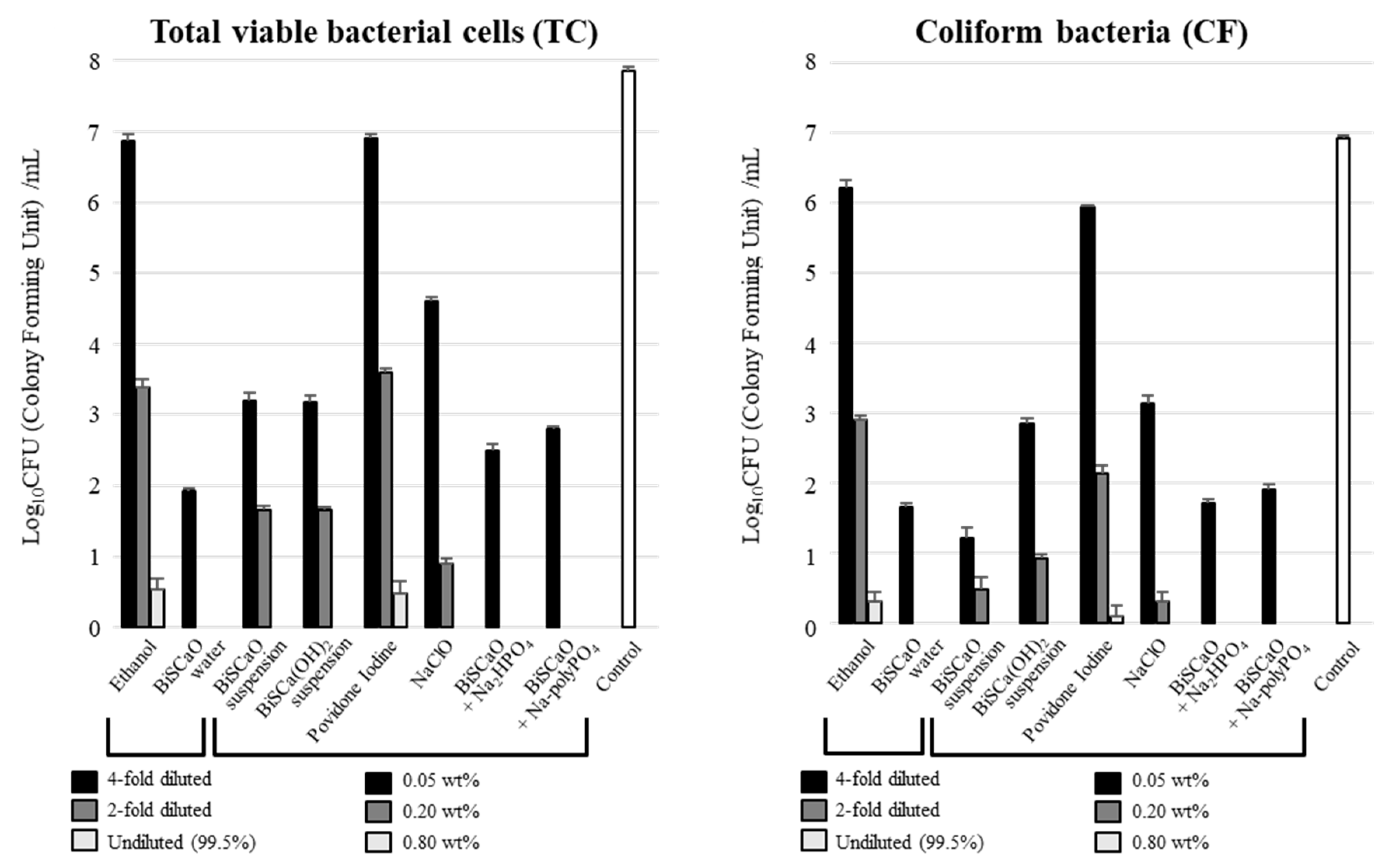
2.4. Microbicidal Efficacy of BiSCaO Water, Suspension, Dispersion, and Colloidal Dispersion
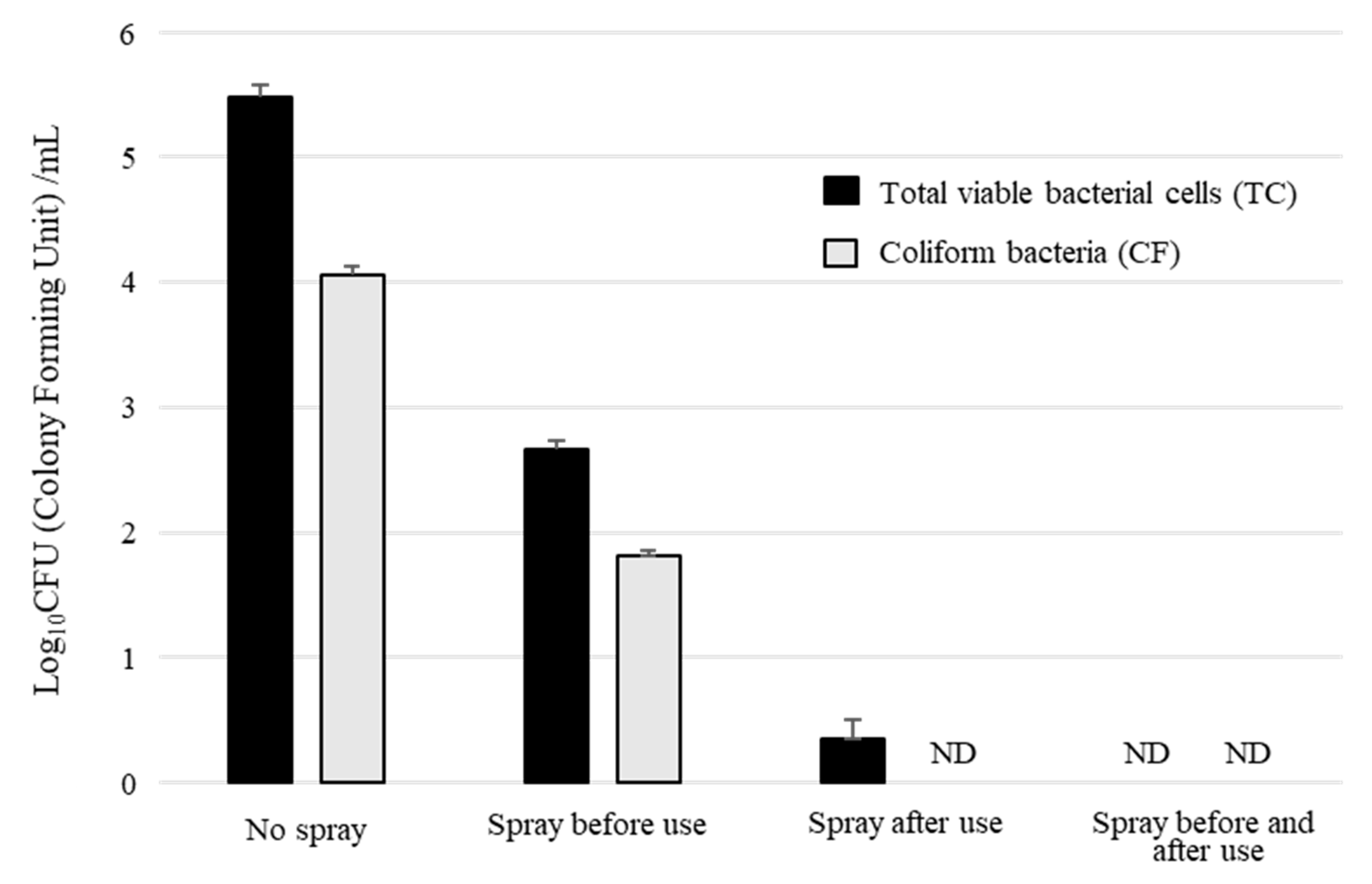
2.5. Sterilization by BiSCaO Water Sprayed on a Mask Surface
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of BiSCaO Water
4.2. Assay of the Disinfection Activity of BiSCaO Water against Pathogenic Microbes In Vitro
4.3. Deodorization Activity of BiSCaO Water
4.4. Microbicidal Efficacy of BiSCaO Water
4.5. Sterilization by BiSCaO Water Sprayed on Mask Surfaces
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wiercinski, F.J. Calcium, An overview—1989. Biol. Bull. 1989, 176, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Chou, K.S.; Huang, Y.K. A novel method to make regenerable core-shell calcium-based sorbants. J. Environ. Manag. 2006, 79, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J. Antimicrobial Characteristics of Heated Scallop Shell Powder and Its Application. Biocontrol. Sci. 2011, 16, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Fujimoto, R.; Sawai, J.; Kikuchi, M.; Yahata, S.; Satoh, S. Antibacterial characteristics of heated scallop-shell nano-particles. Biocontrol. Sci. 2014, 19, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Thammakarn, C.; Satoh, K.; Suguro, A.; Hakim, H.; Ruenphet, S.; Takehara, K. Inactivation of Avian Influenza Virus, Newcastle Disease Virus and Goose Parvovirus Using Solution of Nano-Sized Scallop Shell Powder. J. Vet. Med. Sci. 2014, 76, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Miyoshi, H.; Kojima, H. Sporicidal kinetics of Baccillus subtilis spores by heated scallop shell powder. J. Food Prot. 2003, 66, 1482–1485. [Google Scholar] [CrossRef]
- Xing, R.; Qin, Y.; Guan, X.; Liu, S.; Yu, H.; Li, P. Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Kubo, M.; Ohshima, Y.; Irie, F.; Kikuchi, M.; Sawai, J. Disinfection Treatment of Heated Scallop-Shell Powder on Biofilm of Escherichia coli ATCC 25922 Surrogated for E. coli O157:H7. J. Biomater. Nanobiotechnol. 2013, 4, 10–19. [Google Scholar] [CrossRef]
- Sawai, J.; Nagasawa, K.; Kikuchi, M. Ability of heated acallop-shell powder to disinfect staphylococcus aureus biofilm. Food Sci. Technol. Res. 2013, 19, 561–568. [Google Scholar] [CrossRef]
- Shimamura, N.; Irie, F.; Yamakawa, T.; Kikuchi, M.; Sawai, J. Heated Scallop-Shell Powder Treatment for Deactivation and Removal of Listeria sp. Biofilm Formed at a Low Temperature. Biocontrol. Sci. 2015, 20, 153–157. [Google Scholar] [CrossRef]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Kuwabara, M.; Takayama, T.; Hiruma, S.; Murakami, K.; Fujita, M.; Yokoe, H. Comparison of various disinfectants on bactericidal activity under organic matter contaminated water. Biocontrol. Sci. 2019, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Sato, Y.; Ishihara, M.; Nakamura, S.; Takayama, T.; Murakami, K.; Fujita, M.; Yokoe, H. Skin cleansing technique with disinfectant using improved high-velocity steam-air micro mist jet spray. Biocontrol. Sci. 2020, 25, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J. Chronic wounds and bacteria. Clinical relevance, detection and therapy. Hautarzt 2014, 65, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Selim, P.; Bashford, C.; Grossman, C. Evidence-based practice: Tap water cleansing of leg ulcers in the community. J. Clin. Nurse 2001, 10, 372–379. [Google Scholar] [CrossRef]
- Moore, Z.; Cowman, S. A systematic review of wound cleansing for pressure ulcers. J. Clin. Nurse 2008, 17, 1963–1972. [Google Scholar] [CrossRef]
- Ishihara, M.; Murakami, K.; Fukuda, K.; Nakamura, S.; Kuwabara, M.; Hattori, H.; Fujita, M.; Kiyosawa, T.; Yokoe, H. Stability of weak acidic hypochlorous acid solution with microbicidal activity. Biocontrol. Sci. 2017, 22, 223–227. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.G.; Kang, J.W.; Cho, H.J.; Kim, H.S.; Byeon, H.K.; Yoon, J.H. Effects of a low concentration hypochlorous acid nasal irrigation solution on bacteria, fungi, and virus. Laryngoscope 2008, 118, 1862–1867. [Google Scholar] [CrossRef]
- Kuwabara, M.; Ishihara, M.; Fukuda, K.; Nakamura, S.; Murakami, K.; Sato, Y.; Yokoe, H.; Kiyosawa, T. Disinfection by Hypochlorous Acid for Pseudomonas aeruginosa-Infected Wounds in Diabetic db/db Mice. Wound Med. 2018, 23, 1–5. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Ishihara, M.; Kinoda, J.; Hattori, H.; Nakamura, S.; Ono, T.; Miyahira, Y.; Matsui, T. Development of antimicrobial biomaterials produced from chitin-nanofiber sheet/silver nanoparticle composites. J. Nanobiotechnol. 2014, 12, 49. [Google Scholar] [CrossRef]
- Ishihara, M.; Nguyen, V.Q.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of silver nanoparticles onto different surface structures of chitin/chitosan and correlations with antimicrobial activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Sato, Y.; Takayama, T.; Nakamura, S.; Fukuda, K.; Murakami, K.; Yokoe, H.; Kiyosawa, T. Healing of Pseudomonas aeruginosa-infected wounds in diabetic db/db mice by weakly acidic hypochlorous acid cleansing and silver nanoparticle/chitin nanofiber sheet covering. Wound Med. 2020, 28, 100183. [Google Scholar] [CrossRef]
- Takayama, T.; Ishihara, M.; Sato, Y.; Nakamura, S.; Fukuda, K.; Murakami, K.; Yokoe, H. Bioshell calcium oxide (BiSCaO) for cleansing and healing of pseudomonas aeruginosa-infected wounds in hairless rats. Bio-Med. Mater. Eng. 2020, 31, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ishihara, M.; Murakami, K.; Nakamura, S.; Sato, Y.; Kuwabara, M.; Fujita, M.; Kiyosawa, T.; Yokoe, H. Cleaning technique using high-velocity steam-air micromist jet spray. J. Med. Eng. Technol. 2017, 41, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Hiruma, S.; Murakami, K.; Fujita, M.; Yokoe, H. Preparation and application of bioshell calcium oxide (BiSCaO) nanoparticles-dispersions with bactericidal activity. Molecules 2019, 24, 3415. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ohata, H.; Inoue, A.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Murakami, K.; Hiruma, S.; Yokoe, H. Application of colloidal dispersions of bioshell calcium oxide (BiSCaO) for disinfection. Polymers 2019, 11, 1991. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Mori, Y.; Tagawa, T.; Fujita, M.; Kuno, T.; Suzuki, S.; Matsui, T.; Ishihara, M. Simple and environmentally friendly preparation and size control of silver nanoparticles using an inhomogeneous system with silver-containing glass powder. J. Nanopart. Res. 2011, 13, 2799–2806. [Google Scholar] [CrossRef]
- Hewitt, C.J.; Bellara, S.R.; Andreani, A.; Nebe-Von-Caron, G.; McFarlane, C.M. An evaluation of the antibacterial action of ceramic powder slurries using multi-parameter flow cytometry. Biotechnol. Lett. 2001, 23, 667–675. [Google Scholar] [CrossRef]
- Geller, C.; Varbanov, M.; Duval, R.E. Human coronavirus: Insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 2012, 4, 3044–3068. [Google Scholar] [CrossRef]
- Wilson, J.P.; Mills, J.G.; Prather, I.D.; Dimitrijerich, S.D. A toxicity index of skin and wound cleaners used on in vitro fibroblasts and keratinocytes. Adv. Skin. Wound Care 2005, 18, 373–378. [Google Scholar] [CrossRef] [PubMed]
- McCauley, R.L.; Linares, H.A.; Herndon, D.N.; Robson, M.C.; Heggers, J.P. In vitro toxicity of topical antimicrobial agents to human fibroblasts. J. Surg. Res. 1989, 3, 269–274. [Google Scholar] [CrossRef]
- Kinoda, J.; Ishihara, M.; Hattori, H.; Nakamura, S.; Fukuda, K.; Yokoe, H. Cytotoxicity of silver nanoparticle and chitin–nanofiber sheet composites caused by oxidative stress. Nanomaterials 2016, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, M.T.; Hart, A.; Henckol, J.; Sadoghi, P.; Sell, R.; Moutn, C. COVID-19 coronavirus: Recommended personal protective equipment for the orthopaedic and trauma surgeon. Knee Surg. Sports Traumatol. Anthroscopy 2020, 28, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Shen, C.; Xia, N.; Song, W.; Fan, M.; Cowling, B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, 434–436. [Google Scholar] [CrossRef]
Sample Availability: The materials using in this study was commercially available bioshell calcium oxide (BiSCaO; Plus Lab Co. Ltd., Kanagawa, Japan). |

| Bacterial Strain | 0 min | 1 min | 5 min | 15 min |
|---|---|---|---|---|
| E. coli | (CFU) | (CFU) | (CFU) | (CFU) |
| ― | 3.6 × 105 | <10 * | <10 * | |
| E. coli (control) | 6.7 × 105 | ― | ― | 6.1 × 105 |
| E. coli (O157:H7) | ― | 6.4 × 105 | 4.6 × 102 | <10 * |
| E. coli (O157:H7) (control) | 6.9 × 105 | ― | ― | 6.6 × 105 |
| P. aeruginosa | ― | 1.4 × 104 | <10 * | <10 * |
| P. aeruginosa (control) | 2.7 × 105 | ― | ― | 4.0 × 105 |
| Salmonella | ― | 2.1 × 104 | <10 * | <10 * |
| Salmonella (control) | 7.1 × 105 | ― | ― | 7.7 × 105 |
| S. aureus | ― | 1.1 × 105 | 1.1 × 104 | <10 * |
| S. aureus (control) | 3.4 × 105 | ― | ― | 4.7 × 105 |
| Virus Strain | 0 min | 1 min | 5 min | 15 min |
|---|---|---|---|---|
| Feline calicivirus | <1.5 * | <1.5 * | <1.5 * | |
| (TCID50/mL) | (TCID50/mL) | (TCID50/mL) | (TCID50/mL) | |
| Feline calicivirus (control) | 6.7 | ― | ― | 7.0 |
| Influenza A (H1N1) | 1.7 | <1.5 * | <1.5 * | |
| Influenza A (H1N1) (control) | 6.0 | ― | ― | 6.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S.; Ishihara, M.; Sato, Y.; Takayama, T.; Hiruma, S.; Ando, N.; Fukuda, K.; Murakami, K.; Yokoe, H. Concentrated Bioshell Calcium Oxide (BiSCaO) Water Kills Pathogenic Microbes: Characterization and Activity. Molecules 2020, 25, 3001. https://doi.org/10.3390/molecules25133001
Nakamura S, Ishihara M, Sato Y, Takayama T, Hiruma S, Ando N, Fukuda K, Murakami K, Yokoe H. Concentrated Bioshell Calcium Oxide (BiSCaO) Water Kills Pathogenic Microbes: Characterization and Activity. Molecules. 2020; 25(13):3001. https://doi.org/10.3390/molecules25133001
Chicago/Turabian StyleNakamura, Shingo, Masayuki Ishihara, Yoko Sato, Tomohiro Takayama, Sumiyo Hiruma, Naoko Ando, Koichi Fukuda, Kaoru Murakami, and Hidetaka Yokoe. 2020. "Concentrated Bioshell Calcium Oxide (BiSCaO) Water Kills Pathogenic Microbes: Characterization and Activity" Molecules 25, no. 13: 3001. https://doi.org/10.3390/molecules25133001
APA StyleNakamura, S., Ishihara, M., Sato, Y., Takayama, T., Hiruma, S., Ando, N., Fukuda, K., Murakami, K., & Yokoe, H. (2020). Concentrated Bioshell Calcium Oxide (BiSCaO) Water Kills Pathogenic Microbes: Characterization and Activity. Molecules, 25(13), 3001. https://doi.org/10.3390/molecules25133001






