Anti-Proliferative, Analgesic and Anti-Inflammatory Properties of Syzygium mundagam Bark Methanol Extract
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity Analyses
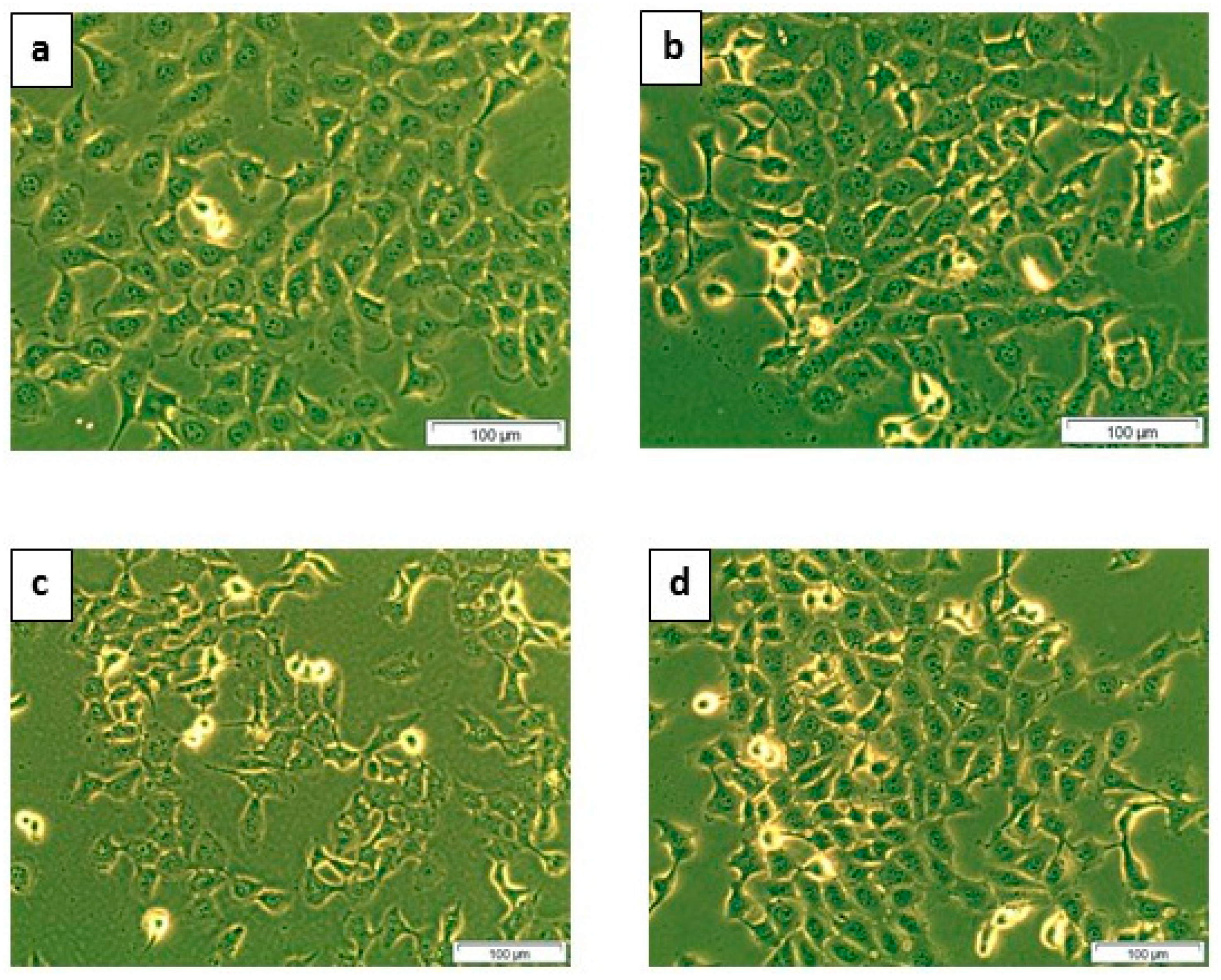
2.1.1. Cellular Morphology
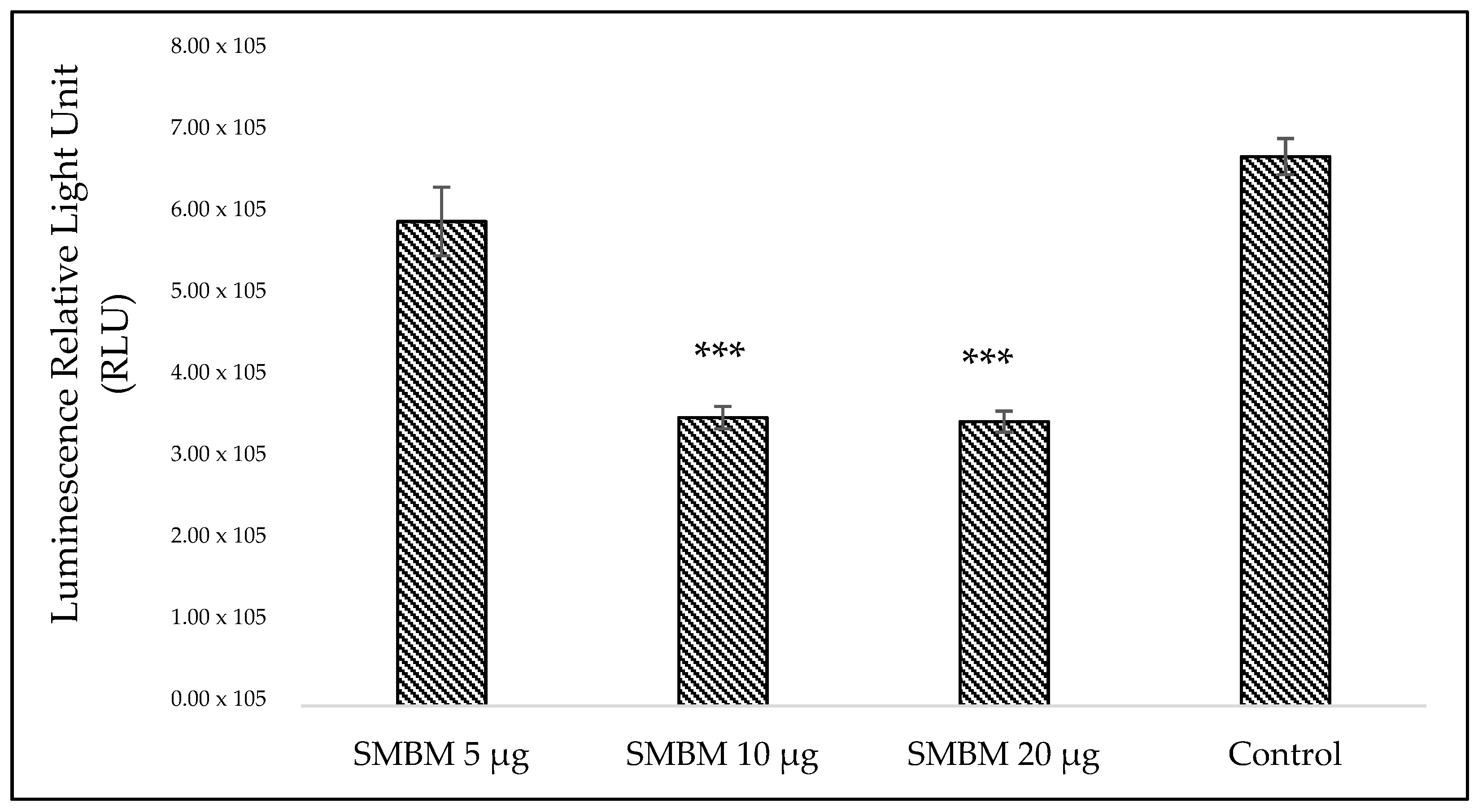
2.1.2. Adenosine Triphosphate (ATP) Cell Metabolism Assay
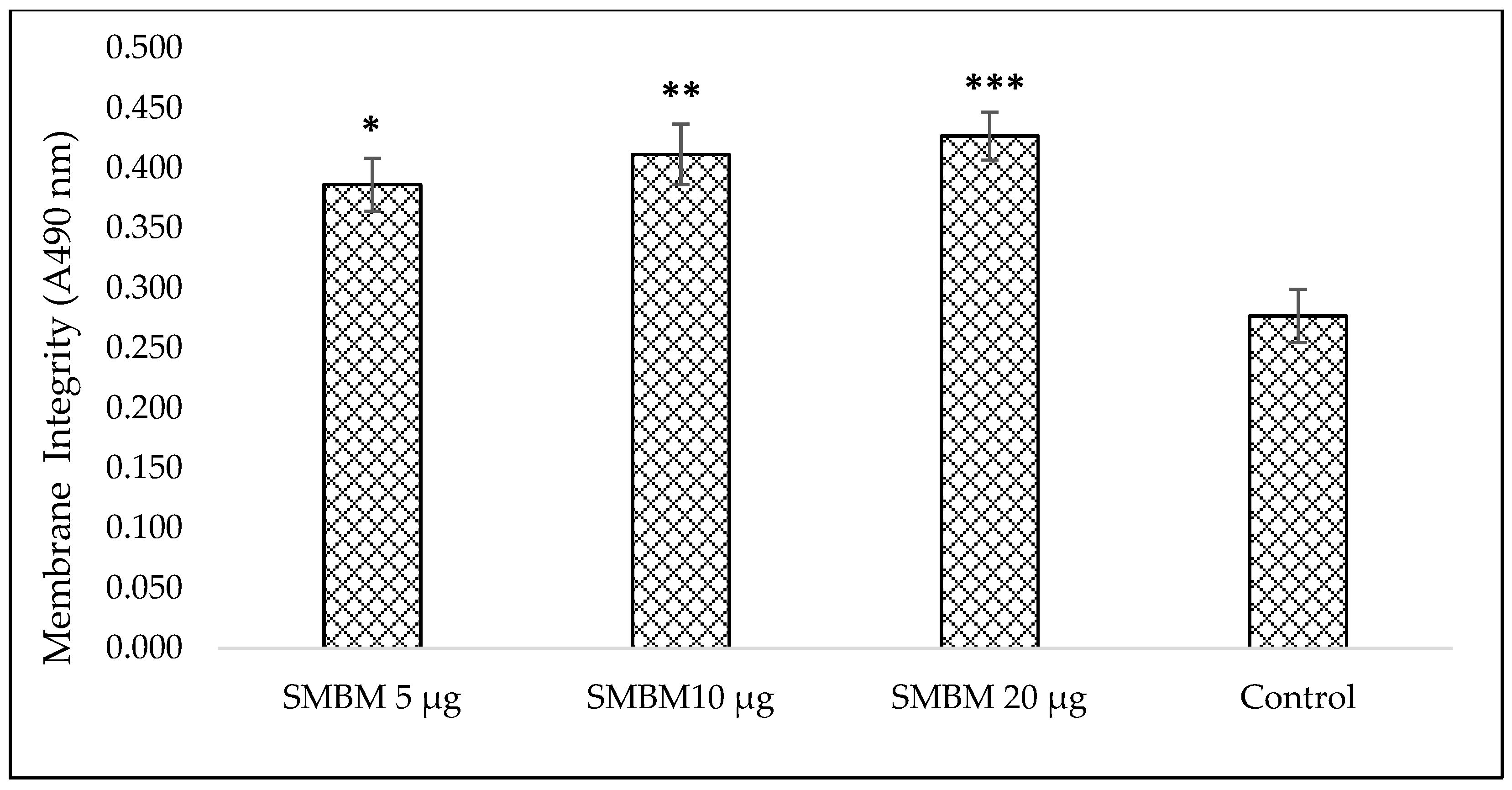
2.1.3. Lactate Dehydrogenase (LDH) Assay for Cytotoxicity
2.1.4. Hoechst Stain
2.2. Acute Toxicity Study
2.3. Analgesic Test
2.3.1. Acetic Acid Induced Writhing Test
2.3.2. Formalin Induced Paw Licking in Mice
2.3.3. Hot Plate Method
2.4. Anti-Inflammatory Activity
2.4.1. Carrageenan Induced Paw Oedema
2.4.2. Egg Albumin Induced Paw Oedema
2.4.3. The Cotton Pellet Implanted Granuloma
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Materials
4.2. Successive Solvent Extraction
4.3. Cytotoxicity Analyses
4.3.1. Cell Culture and Treatment
4.3.2. Cellular Morphology-Inverted Microscopy
4.3.3. Cellular Proliferation-Adenosine Triphosphate (ATP) Luminescent Assay
4.3.4. Cytotoxicity-Lactate Dehydrogenase (LDH) Assay
4.3.5. Hoechst Stain
4.4. Animals and Ethical Approval
4.5. Acute Toxicity
4.6. Analgesic Test
4.6.1. Acetic Acid Induced Writhing Test
4.6.2. Formalin-Induced Paw Licking in Mice
4.6.3. Hot Plate Method
4.7. Anti-Inflammatory Activity
4.7.1. Carrageenan Induced Paw Oedema
4.7.2. Egg Albumin Induced Paw Oedema
4.7.3. The Cotton Pellet Implanted Granuloma
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- List, M.A.; Stracks, J.; Colangelo, L.; Butler, P.; Ganzenko, N.; Lundy, D.; Sullivan, P.; Haraf, D.; Kies, M.; Goodwin, W.; et al. How Do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J. Clin. Oncol. 2000, 18, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Schreinemachers, D.M.; Everson, R.B. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 1994, 5, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Suraneni, M.V.; Reddy, G.V.; Reddanna, P. Horizons in Cancer Research; Watanabe, H.S., Ed.; Nova Science Publishers: New York, NY, USA, 2011; Volume 47, pp. 1–18. [Google Scholar]
- Wallace, J.L.; Vong, L. NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr. Opin. Investig. Dr. 2008, 9, 1151–1156. [Google Scholar]
- Corley, D.A.; Kerlikowske, K.; Verma, R.; Buffler, P. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta analysis. Gastroenterol 2003, 124, 47–56. [Google Scholar] [CrossRef]
- Simmons, D. The use of animal models in studying genetic disease: Transgenesis and induced mutation. Nat. Edu. 2008, 1, 70. [Google Scholar]
- Ibrahim, B.; Sowemimo, A.; van Rooyen, A.; Vande Venter, M. Anti-inflammatory, analgesic and antioxidant activities of Cyathula prostrata (Linn.) Blume (Amaranthaceae). J. Ethnopharmacol. 2012, 141, 282–289. [Google Scholar] [CrossRef]
- Muruganadan, S.; Srinivasan, K.; Chandra, S.; Tandan, S.K.; Lal, J.; Raviprakash, V. Anti-inflammatory activity of Syzygium cumini bark. Fitoterapia 2001, 72, 365–375. [Google Scholar]
- Oudhia, P. Positive and Negative Impacts on Health when Chyawanprash is given with Syzygium calophyllifolium Walp. Based Herbal Formulations, Pankaj Oudhia’s Expert Comments on World Patents on Herbal Medicines, Audio Bank on Biodiversity and Traditional Healing. 2012. Available online: http://www.pankajoudhia.com (accessed on 22 June 2016).
- Narayanan, M.K.R.; Anilkumar, N.; Balakrishnan, V.; Sivadasan, M.; Ahmed Alfarhan, H.; Alatar, A.A. Wild edible plants used by the Kattunaikka, Paniya and Kuruma tribes of Wayanad District, Kerala, India. J. Med. Plants Res. 2011, 5, 3520–3529. [Google Scholar]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Guana, Y.; Li, X.; Zou, H.; Yan, X.; Zhao, C.; Wang, J.; Chend, L.; Zhanga, Y.A. Multiparameter Characterization Confirms Apoptosis as the Primary Cause of Reduced Self-renewal Capacity in Cultured Human Fetal Neural Stem Cells. Cell Physiol. Biochem. 2016, 38, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Hisama, M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001, 49, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Ogunwande, I.; Olawore, N.; Ekundayo, O.; Walker, T.M.; Schmidt, J.M.; Setzer, W.N. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147–152. [Google Scholar] [CrossRef]
- Yoo, C.B.; Han, K.T.; Cho, K.S.; Ha, J.; Park, H.J.; Nam, J.H.; Kil, U.H.; Lee, K.T. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anticariogenic and cytotoxic activity of clove essential oil (Eugenia caryophyllata) against a large number of oral pathogens. Ann. Microb. 2010, 60, 599–604. [Google Scholar] [CrossRef]
- Kumar, P.; Febriyanti, R.; Sofyan, F.; Luftimas, D.E.; Abdulah, R. Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmac. Res. 2014, 6, 350–354. [Google Scholar] [CrossRef]
- Bai, L.Y.; Lin, W.Y.; Chiu, C.F.; Weng, J.R. Anti-tumor activities of triterpenes from Syzygium kusukusense. Nat. Prod. Commun. 2014, 9, 1557–1558. [Google Scholar] [CrossRef]
- Li, L.; Adams, L.S.; Chen, S.; Killian, C.; Ahmed, A.; Seeram, N.P. Eugenia jambolana Lam. berry extract inhibit growth and induces apoptosis of human breast cancer but not non tumor-genic breast cells. J. Agric. Food Chem. 2009, 57, 826–831. [Google Scholar] [CrossRef]
- Ren, Y.; Anaya-Eugenio, G.D.; Czarnecki, A.A.; Ninh, T.N.; Yuan, C.; Chai, H.B.; Soejarto, D.D.; Burdette, J.E.; Carcache de Blanco, E.J.; Kinghorn, A.D. Cytotoxic and NF-κB and mitochondrial transmembrane potential inhibitory pentacyclic triterpenoids from Syzygium corticosum and their semi-synthetic derivatives. Bioorg. Med. Chem. 2018, 26, 4452–4460. [Google Scholar] [CrossRef]
- Chandran, R.; George, B.P.; Abrahamse, H.; Thangaraj, P. Therapeutic effects of Syzygium mundagam bark methanol extracts on Type-2 diabetic complications in rats. Biomed. Pharmacother. 2017, 95, 167–174. [Google Scholar] [CrossRef]
- Chandran, R.; Parimelazhagan, T.; George, B.P. Antihyperglycemic activity of the bark methanolic extract of Syzygium mundagam in diabetic rats. Alex. J. Med. 2017, 53, 317–324. [Google Scholar] [CrossRef][Green Version]
- Goyal, M.; Ghosh, M.; Nagori, B.P.; Sasmal, D. Analgesic and anti-inflammatory studies of cyclopeptide alkaloid fraction of leaves of Ziziyphus nummularia. Saudi. J. Biol. Sci. 2013, 20, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Hiruma-Lima, C.; Gracioso, J.; Bighetti, E.; Germonsén Robineou, L.; Souza Brito, A.R. The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J. Ethnopharmacol. 2000, 71, 267–274. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Ghani, Z.D.F.A.; Nor, R.N.S.R.M.; Gopalan, H.K.; Sulaiman, M.R.; Jais, A.M.; Somchit, M.N.; Kader, A.A.; Ripin, J. Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J. Nat. Med. 2008, 62, 179–187. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation, Pharmacological Assay, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 702–704. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Tsai, H.-Y.; Wu, T.-S. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995, 61, 2–8. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Fereidoni, M.; Semnanian, S.; Kamalinejad, M.; Saremi, S. Antinociceptive and anti-inflammatory effects of Sambucus ebulus rhizome extract in rats. J. Ethnopharm. 1998, 61, 229–235. [Google Scholar] [CrossRef]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef]
- Rali, S.; Oyedeji, O.O.; Aremu, O.O.; Oyedeji, A.O.; Nkeh-Chungag, B.N. Semisynthesis of Derivatives of Oleanolic Acid from Syzygium aromaticum and Their Antinociceptive and Anti-Inflammatory Properties. Mediat. Inflamm 2016, 2016, 8401843. [Google Scholar] [CrossRef]
- Mollika, S.; Islam, N.; Parvin, N.; Kabir, A.; Sayem, M.W.; Nesa, L.; Saha, R. Evaluation of analgesic, anti-inflammatory and CNS activities of the methanolic extract of Syzygium samarangense leave. Glob. J. Pharm. 2014, 8, 39–46. [Google Scholar]
- Taher, Y.A.; Samud, A.M.; El-Taher, F.E.; Ben-Hussin, G.; Elmezogi, J.S.; Al-Meddawi, B.F.; Salem, H.A. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J. Med. 2015, 10, 28685. [Google Scholar] [CrossRef] [PubMed]
- Okpo, S.; Fatokun, F.; Adeyemi, O. Analgesic and anti-inflammatory activity of Crinum glaucum aqueous extract. J. Ethnopharmacol. 2001, 78, 207–211. [Google Scholar] [CrossRef]
- Chandran, R.; Abrahamse, H.; Thangaraj, P. Cytotoxic, analgesic and anti-inflammatory properties of Syzygium calophyllifolium bark. Biomed. Pharm. 2018, 103, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, M.C.; Anand, S.; Balaganur, V.; Kumari, R.R.; More, A.S.; Kumar, D.; Bhadoria, B.K.; Tandan, S.K. Analgesic activity of Eugenia jambolana leave constituent: A dikaempferol rhamnopyranoside from ethyl acetate soluble fraction. Pharm. Biol. 2014, 52, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Quintans, J.S.; Brito, R.G.; Aquino, P.G.; França, P.H.; Siqueira-Lima, P.S.; Santana, A.E.G.; Ribiero, E.A.N.; Salvador, M.J.; Aroujo-Junior, J.X.; Quintans-Junior, L.J. Antinociceptive activity of Syzygium cumini leaves ethanol extract on orofacial nociception protocols in rodents. Pharm. Biol. 2014, 52, 762–766. [Google Scholar] [CrossRef]
- Ecobichon, D.J. The Basis of Toxicology Testing, 2nd ed.; CRC Press: New York, NY, USA, 1997; pp. 43–86. [Google Scholar]
- Shang, J.H.; Cai, X.H.; Feng, T.; Zhao, Y.L.; Wang, J.K.; Zhang, L.Y.; Yan, M.; Luo, X.D. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J. Ethnopharmacol. 2010, 129, 174–181. [Google Scholar] [CrossRef]
- Zhu, Z.Z.; Ma, K.J.; Ran, X.; Zhang, H.; Zheng, C.J.; Han, T.; Zhang, Q.Y.; Qin, L.P. Analgesic, anti-inflammatory and antipyretic activities of the petroleum ether fraction from the ethanol extract of Desodium podocarpum. J. Ethnopharmacol. 2011, 133, 1126–1131. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimback, D. Synthetic analgesics: II. Dithyienylbutenylamines and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 544–547. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenan-induced oedema in hind paw of the rats as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Okokon, J.E.; Nwafor, P.A. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak. J. Pharm. Sci. 2010, 23, 385–392. [Google Scholar] [PubMed]
- Winter, C.A.; Porter, C.C. Effect of alterations in the side chain upon anti-inflammatory and liver glycogen activities of hydrocortisone esters. J. Am. Pharm. Assoc. 1957, 46, 515–519. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Syzygium mundagam bark methanol extract are available compounds are available from the authors. |


| Groups | Doses (mg/kg b. wt.) | No. of Writhes (in 30 s) | % Inhibition |
|---|---|---|---|
| SMBM | 100 | 28 ± 3.49 | 7.44 |
| SMBM | 200 | 15.5 ± 2.75 | 48.76 |
| Control | 30.25 ± 9.73 | ||
| Aspirin | 150 | 14.5 ± 1.19 | 52.07 |
| Groups | Dose (mg/kg b. wt.) | Latency Period (in sec) | % Inhibition | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | |||
| Control | - | 3.50 ± 0.30 | 6.00 ± 0.40 | 5.30 ± 0.60 | 8.80 ± 2.1 | 4.80 ± 1.3 | 6.50 ± 0.6 | - |
| SMBM | 100 | 2.25 ± 0.25 | 4.75 ± 0.85 | 4.00 ± 0.41 | 6.00 ± 0.71 | 4.75 ± 0.85 | 8.50 ± 0.29 | 52 |
| SMBM | 200 | 3.50 ± 0.29 | 4.25 ± 0.63 | 6.75 ± 0.48 * | 4.25 ± 0.48 | 6.25 ± 1.55 * | 10.50 ± 0.65 *** | 57.14 |
| Pentazocine | 5 | 3.50 ± 0.29 | 3.75 ± 0.25 | 4.25 ± 0.25 | 4.50 ± 0.96 | 5.00 ± 0.41 | 8.50 ± 0.87 | 40 |
| Paw Thickness (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Dose (mg/kg b. wt.) | 0th h | 1st h | 2nd h | 3rd h | 4th h | % Inhibition |
| Control | - | 3.97 ± 0.15 | 6.27 ± 0.27 | 6.12 ± 0.85 | 5.93 ± 0.35 | 6.09 ± 0.84 | - |
| SMBM | 100 | 4.28 ± 0.20 | 6.39 ± 0.18 | 7.02 ± 0.45 | 6.42 ± 0.40 | 6.32 ± 0.76 | 95.19 |
| SMBM | 200 | 4.27 ± 0.15 | 6.74 ± 0.16 | 7.11 ± 0.77 | 6.68 ± 0.77 | 6.04 ± 0.84 * | 96.88 |
| Indomethacin | 10 | 4.29 ± 0.41 | 6.05 ± 0.15 | 7.12 ± 0.31 | 6.35 ± 0.32 | 6.10 ± 0.45 * | 96.71 |
| Groups | Dose (mg/kg b. wt.) | Paw Thickness (mm) | |||||
|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | % Inhibition | ||
| Control | 4.44 ± 0.06 | 5.63 ± 0.22 | 5.86 ± 0.26 | 5.97 ± 0.45 | 5.67 ± 0.16 | ||
| SMBM | 200 | 4.61 ± 0.14 | 6.00 ± 0.61 | 5.93 ± 0.54 | 4.67 ± 0.43 * | 5.05 ± 0.48 | 77.97 |
| SMBM | 100 | 4.62 ± 0.25 | 5.07 ± 0.14 | 5.36 ± 0.34 | 4.96 ± 0.15 | 4.75 ± 0.42 | 68.31 |
| Indomethacin | 10 | 5.03 ± 0.30 | 5.54 ± 0.28 | 4.88 ± 0.29 | 5.55 ± 0.43 | 4.38 ± 0.40 * | 61.33 |
| Groups | Dose (mg/kg b. wt.) | Initial Pellet wt (mg) | Mean wt on 8th Day (mg) | Mean Increase in Pellet wt (mg) | % Inhibition |
|---|---|---|---|---|---|
| Control | - | 50 | 129.00 | 79 ± 3.61 | - |
| SMBM | 100 | 50 | 121.00 | 71 ± 5.20 | 10.13 |
| SMBM | 200 | 50 | 67.77 | 17.67 ± 2.34 *** | 77.64 |
| Indomethacin | 10 | 20 | 83.33 | 33.33 ± 2.73 *** | 57.81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandran, R.; George, B.P.; Abrahamse, H. Anti-Proliferative, Analgesic and Anti-Inflammatory Properties of Syzygium mundagam Bark Methanol Extract. Molecules 2020, 25, 2900. https://doi.org/10.3390/molecules25122900
Chandran R, George BP, Abrahamse H. Anti-Proliferative, Analgesic and Anti-Inflammatory Properties of Syzygium mundagam Bark Methanol Extract. Molecules. 2020; 25(12):2900. https://doi.org/10.3390/molecules25122900
Chicago/Turabian StyleChandran, Rahul, Blassan P. George, and Heidi Abrahamse. 2020. "Anti-Proliferative, Analgesic and Anti-Inflammatory Properties of Syzygium mundagam Bark Methanol Extract" Molecules 25, no. 12: 2900. https://doi.org/10.3390/molecules25122900
APA StyleChandran, R., George, B. P., & Abrahamse, H. (2020). Anti-Proliferative, Analgesic and Anti-Inflammatory Properties of Syzygium mundagam Bark Methanol Extract. Molecules, 25(12), 2900. https://doi.org/10.3390/molecules25122900








