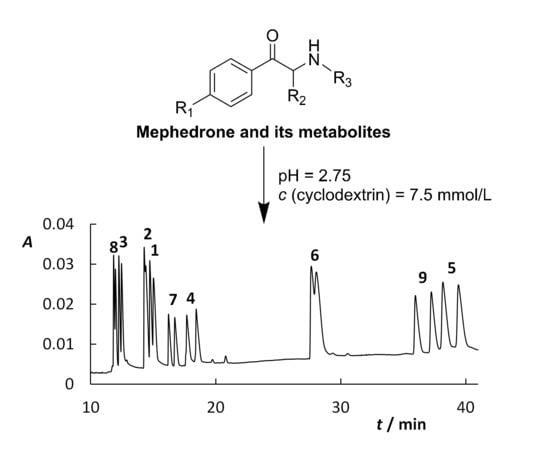
Enantioseparation and Determination of Mephedrone and Its Metabolites by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors
Abstract
1. Introduction
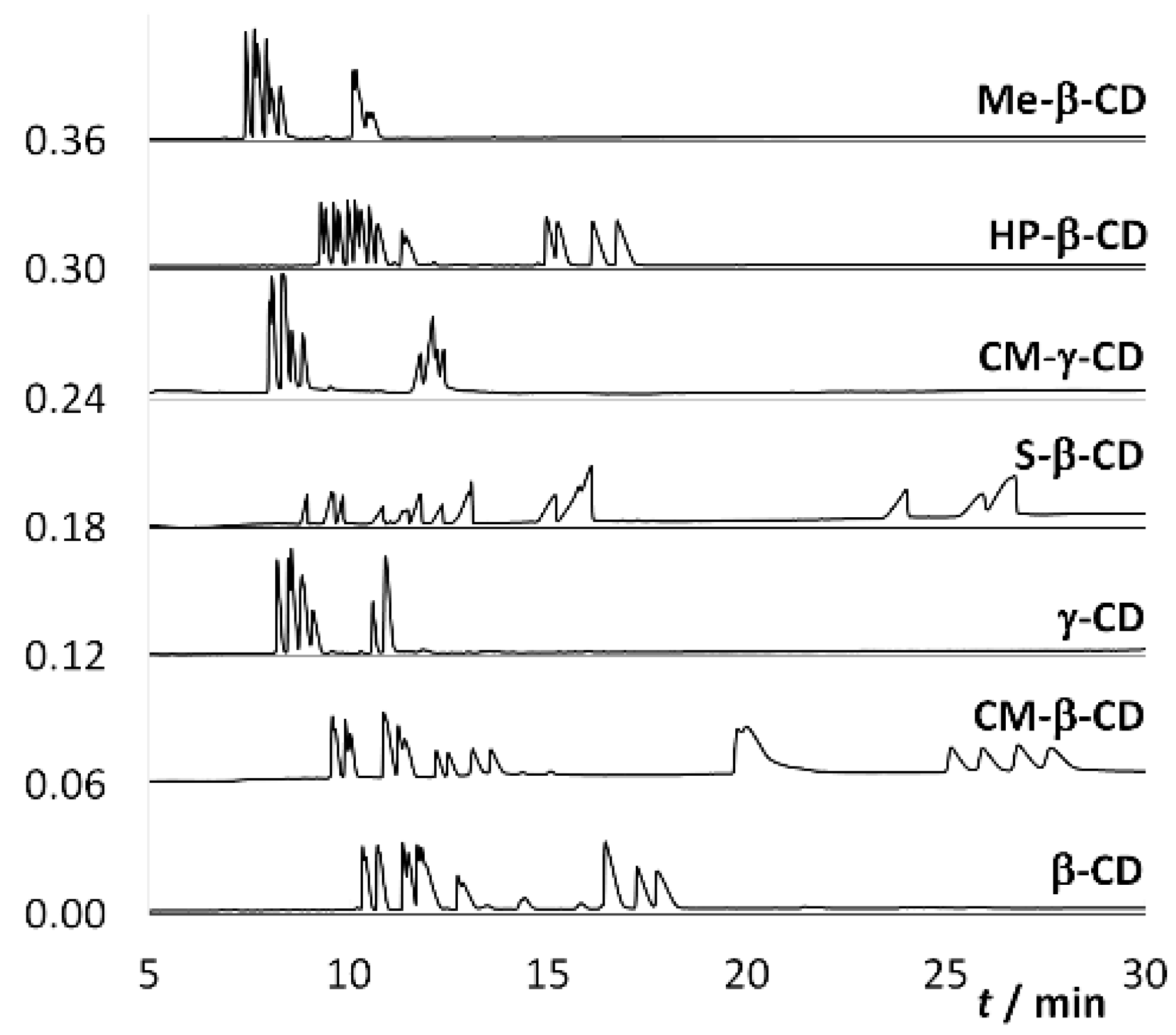
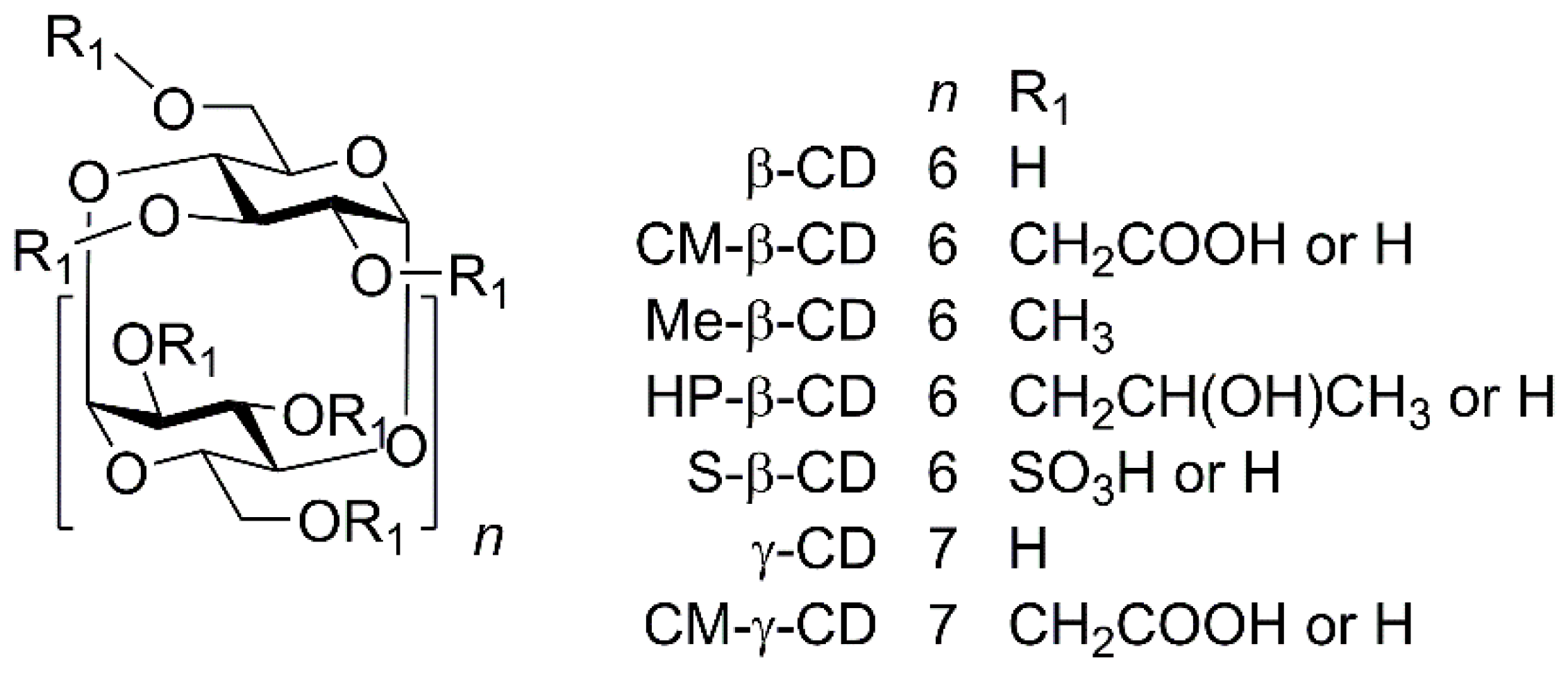
2. Results and Discussion
2.1. Cyclodextrin Optimization
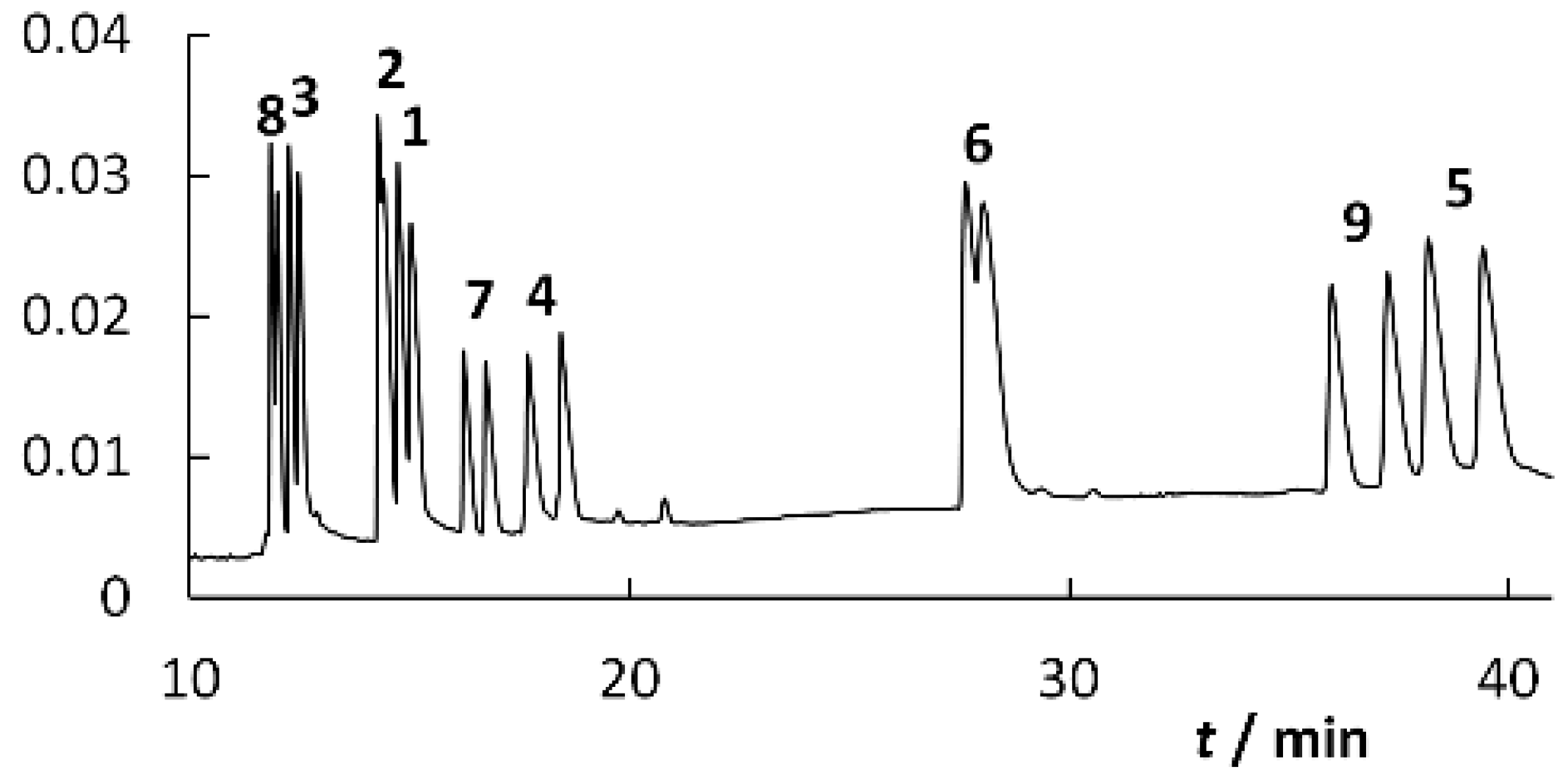
2.2. BGE Optimization
2.3. Calibration
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Equipment
3.3. CE Conditions
3.4. Sample Preparation
3.5. Simplex Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanchez:, S. Sur un homologue de l’ephedrine. Bull. Soc. Chim. Fr. 1929, 45, 284–286. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Available online: http://www.emcdda.europa.eu/publications/edr/trends-developments/2019_en (accessed on 5 May 2020).
- Busardo, F.P.; Di Trana, A.; Montanari, E.; Mauloni, S.; Tagliabracci, A.; Giorgetti, R. Is etizolam a safe medication? Effects on psychomotor perfomance at therapeutic dosages of a newly abused psychoactive substance. Forensic Sci. Int. 2019, 301, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.M.; Nelson, L.S. The toxicology of bath salts: A review of synthetic cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, S.; Savage, M.; Cavazos, C.; Bella, P. Thermal degradation of synthetic cathinones: Implications for forensic toxicology. J. Anal. Toxicol. 2016, 40, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldberg, J.; Gardos, G.; Cole, J.O. A controlled evaluation of pyrovalerone in chronically fatigued volunteers. Int. Pharmacopsychiatry 1973, 8, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Batisse, A.; Eiden, C.; Peyriere, H.; Djezzar, S. Use of new psychoactive substances to mimic prescription drugs: The trend in France. NeuroToxicology 2020, 79, 20–24. [Google Scholar] [CrossRef]
- Alremeithi, R.; Meetani, M.A.; Alaidaros, A.A.; Lanjawi, A.; Alsumaiti, K. Simultaneous Quantitative Determination of Synthetic Cathinone Enantiomers in Urine and Plasma Using GC-NCI-MS. J. Anal. Methods Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Majchrzak, M.; Celinski, R.; Kus, P.; Kowalska, T.; Sajewicz, M. The newest cathinone derivatives as designer drugs: An analytical and toxicological review. Forensic Toxicol. 2018, 1–18. [Google Scholar] [CrossRef]
- Usui, K.; Hayashizaki, Y.; Hashiyada, M.; Funayama, M. Rapid drug extraction from human whole blood using a modified QuEChERS extraction method. Leg. Med. 2012, 14, 286–296. [Google Scholar] [CrossRef]
- Toole, K.E.; Fu, S.; Shimmon, R.G.; Kraymen, N.; Taflaga, S.; Forensic, A.F. Color tests for the preliminary identification of methcathinone and analogues of methcathinone. Microgram J. 2012, 9, 27–32. [Google Scholar]
- Ellefsen, K.N.; Anizan, S.; Castaneto, M.S.; Desrosiers, N.A.; Martin, T.M.; Klette, K.L.; Huestis, M.A. Validation of the only commercially available immunoassay for synthetic cathinones in urine: Randox Drugs of Abuse V Biochip Array Technology. Drug Test. Anal. 2014, 6, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Paillet-Loilier, M.; Cesbron, A.; Bourgine, J.; Le, B.R.; Debruyne, D. Emerging drugs of abuse: Current perspectives on substituted cathinones. Subst. Abuse Rehabil. 2014, 5, 37–52. [Google Scholar] [PubMed]
- Daeid, N.N.; Savage, K.A.; Ramsay, D.; Holland, C.; Sutcliffe, O.B. Development of gas chromatography–mass spectrometry (GC–MS) and other rapid screening methods for the analysis of 16 ‘legal high’cathinone derivatives. Sci. Justice 2014, 54, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, A.; Johnston, M.R.; Brennan, M.; Davis, S.; Caldicott, D.G. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci. Int. 2010, 197, 59–66. [Google Scholar] [CrossRef]
- Fujii, H.; Hara, K.; Kageura, M.; Kashiwagi, M.; Matsusue, A.; Kubo, S. High throughput chiral analysis of urinary amphetamines by GC-MS using a short narrow-bore capillary column. Forensic Toxicol. 2009, 27, 75–80. [Google Scholar] [CrossRef]
- Meyer, M.R.; Wilhelm, J.; Peters, F.T.; Maurer, H.H. Beta-keto amphetamines: Studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2010, 397, 1225–1233. [Google Scholar] [CrossRef]
- Olesti, E.; Pujadas, M.; Papaseit, E.; Perez-Mana, C.; Pozo, O.J.; Farre, M.; de la Torre, R. GC–MS quantification method for mephedrone in plasma and urine: Application to human pharmacokinetics. J. Anal. Toxicol. 2017, 41, 100–106. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Olsen, K.H.; Johansen, S.S. Chiral separation and quantification of R/S-amphetamine, R/S-methamphetamine, R/S-MDA, R/S-MDMA, and R/S-MDEA in whole blood by GC-EI-MS. J. Chromatogr. B 2006, 842, 136–141. [Google Scholar] [CrossRef]
- Ribeiro, C.; Santos, C.; Gonçalves, V.; Ramos, A.; Afonso, C.; Tiritan, M.E. Chiral Drug Analysis in Forensic Chemistry: An Overview. Molecules 2018, 23, 262. [Google Scholar] [CrossRef]
- Mohr, S.; Weiß, J.A.; Spreitz, J.; Schmid, M.G. Chiral separation of new cathinone-and amphetamine-related designer drugs by gas chromatography–mass spectrometry using trifluoroacetyl-l-prolyl chloride as chiral derivatization reagent. J. Chromatogr. A 2012, 1269, 352–359. [Google Scholar] [CrossRef]
- Pedersen, A.J.; Dalsgaard, P.W.; Rode, A.J.; Rasmussen, B.S.; Müller, I.B.; Johansen, S.S.; Linnet, K. Screening for illicit and medicinal drugs in whole blood using fully automated SPE and ultra-high-performance liquid chromatography with TOF-MS with data-independent acquisition. J. Sep. Sci. 2013, 36, 2081–2089. [Google Scholar] [CrossRef]
- Paul, M.; Ippisch, J.; Herrmann, C.; Guber, S.; Schultis, W. Analysis of new designer drugs and common drugs of abuse in urine by a combined targeted and untargeted LC-HR-QTOFMS approach. Anal. Bioanal. Chem. 2014, 406, 4425–4441. [Google Scholar] [CrossRef]
- Concheiro, M.; Castaneto, M.; Kronstrand, R.; Huestis, M.A. Simultaneous determination of 40 novel psychoactive stimulants in urine by liquid chromatography–high resolution mass spectrometry and library matching. J. Chromatogr. A 2015, 1397, 32–42. [Google Scholar] [CrossRef]
- Mohr, S.; Taschwer, M.; Schmid, M.G. Chiral separation of cathinone derivatives used as recreational drugs by HPLC-UV using a CHIRALPAK® AS-H column as stationary phase. Chirality 2012, 24, 486–492. [Google Scholar] [CrossRef]
- Taschwer, M.; Seidl, Y.; Mohr, S.; Schmid, M.G. Chiral Separation of Cathinone and Amphetamine Derivatives by HPLC/UV Using Sulfated ß-Cyclodextrin as Chiral Mobile Phase Additive. Chirality 2014, 26, 411–418. [Google Scholar] [CrossRef]
- Pauk, V.; Zihlová, V.; Borovcova, L.; Havlicek, V.; Schug, K.; Lemr, K. Fast separation of selected cathinones and phenylethylamines by supercritical fluid chromatography. J. Chromatogr. A 2015, 1423, 169–176. [Google Scholar] [CrossRef]
- Geryk, R.; Kaliková, K.; Schmid, M.G.; Tesarová, E. Enantioselective separation of biologically active basic compounds in ultra-performance supercritical fluid chromatography. Anal. Chim. Acta 2016, 932, 98–105. [Google Scholar] [CrossRef]
- Carnes, S.; O’Brien, S.; Szewczak, A.; Tremeau-Cayel, L.; Rowe, W.F.; McCord, B.; Lurie, I.S. Comparison of ultra high performance supercritical fluid chromatography, ultra high performance liquid chromatography, and gas chromatography for the separation of synthetic cathinones. J. Sep. Sci. 2017, 40, 3545–3556. [Google Scholar] [CrossRef]
- Mohr, S.; Pilaj, S.; Schmid, M.G. Chiral separation of cathinone derivatives used as recreational drugs by cyclodextrin-modified capillary electrophoresis. Electrophoresis 2012, 33, 1624–1630. [Google Scholar] [CrossRef]
- Merola, G.; Fu, H.; Tagliaro, F.; Macchia, T.; McCord, B.R. Chiral separation of 12 cathinone analogs by cyclodextrin-assisted capillary electrophoresis with UV and mass spectrometry detection. Electrophoresis 2014, 35, 3231–3241. [Google Scholar] [CrossRef]
- Taschwer, M.; Weiß, J.A.; Kunert, O.; Schmid, M.G. Analysis and characterization of the novel psychoactive drug 4-chloromethcathinone (clephedrone). Forensic Sci. Int. 2014, 244, 56–59. [Google Scholar] [CrossRef]
- Nowak, P.M.; Olesek, K.; Wozniakiewicz, M.; Koscielniak, P. Simultaneous enantioseparation of methcathinone and two isomeric methylmethcathinones using capillary electrophoresis assisted by 2-hydroxyethyl-β-cyclodextrin. Electrophoresis 2018, 39, 2406–2409. [Google Scholar] [CrossRef] [PubMed]
- Baciu, T.; Borrull, F.; Calull, M.; Aguilar, C. Enantioselective determination of cathinone derivatives in human hair by capillary electrophoresis combined in-line with solid-phase extraction. Electrophoresis 2016, 37, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Martinez-Clemente, J.; Escubedo, E.; Pubill, D.; Camarasa, J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2012, 22, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Arnau, R.; Martinez-Clemente, J.; Pubill, D.; Escubedo, E.; Camarasa, J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: Butylone, mephedrone and methylone. Br. J. Pharmacol. 2012, 167, 407–420. [Google Scholar] [CrossRef]
- Chavant, F.; Boucher, A.; Le, B.R.; Debruyne, D.; Deheul, S. New synthetic drugs in addictovigilance. Therapie 2015, 70, 167–189. [Google Scholar] [CrossRef]
- Philogene-Khalid, H.L.; Hicks, C.; Reitz, A.B.; Liu-Chen, L.Y.; Rawls, S.M. Synthetic cathinones and stereochemistry: S enantiomer of mephedrone reduces anxiety- and depressant-like effects in cocaine- or MDPV-abstinent rats. Drug Alcohol Depend. 2017, 178, 119–125. [Google Scholar] [CrossRef]
- Schifano, F.; Albanese, A.; Fergus, S.; Stair, J.L.; Deluca, P.; Corazza, O.; Davey, Z.; Corkery, J.; Siemann, H.; Scherbaum, N.; et al. Mephedrone (4-methylmethcathinone; meow meow’): Chemical, pharmacological and clinical issues. Psychopharmacology (Heidelb. Ger.) 2011, 214, 593–602. [Google Scholar] [CrossRef]
- Ciechomska, M.; Wozniakiewicz, M.; Wietecha-Posluszny, R. Activity and biotransformation of three synthetic legal highs: Mephedrone, methylone and 3,4-methylenodioxypyrovalerone. Z Zagadnien Nauk Sadowych 2012, 89, 71–85. [Google Scholar]
- Karila, L.; Megarbane, B.; Cottencin, O.; Lejoyeux, M. Synthetic Cathinones: A New Public Health Problem. Curr. Neuropharmacol. 2015, 13, 12–20. [Google Scholar] [CrossRef]
- Papaseit, E.; Perez-Mana, C.; Mateus, J.A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farre, M. Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704. [Google Scholar] [CrossRef]
- Sichova, K.; Pinterova, N.; Horsley, R.R.; Lhotkova, E.; Stefkova, K.; Vejmola, C.; Uttl, L.; Kuchar, M.; Palenicek, T.; Pinterova, N.; et al. Mephedrone (4-Methylmethcathinone): Acute Behavioral Effects, Hyperthermic, and Pharmacokinetic Profile in Rats. Front. Psychiatry 2017, 8, 306. [Google Scholar] [CrossRef]
- Gregg, R.A.; Baumann, M.H.; Partilla, J.S.; Bonano, J.S.; Vouga, A.; Tallarida, C.S.; Velvadapu, V.; Smith, G.R.; Peet, M.M.; Reitz, A.B. Stereochemistry of mephedrone neuropharmacology: Enantiomer-specific behavioural and neurochemical effects in rats. Br. J. Pharmacol. 2015, 172, 883–894. [Google Scholar] [CrossRef]
- Linhart, I.; Himl, M.; Zidkova, M.; Balikova, M.; Lhotkova, E.; Palenicek, T. Metabolic profile of mephedrone: Identification of nor-mephedrone conjugates with dicarboxylic acids as a new type of xenobiotic phase II metabolites. Toxicol. Lett. 2016, 240, 114–121. [Google Scholar] [CrossRef]
- Pozo, O.J.; Ibanez, M.; Sancho, J.V.; Lahoz-Beneytez, J.; Farre, M.; Papaseit, E.; de la Torre, R.; Hernandez, F. Mass spectrometric evaluation of mephedrone in vivo human metabolism: Identification of phase I and phase II metabolites, including a novel succinyl conjugate. Drug Metab. Dispos. 2015, 43, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Bermejo, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M.L. Chiral capillary electrophoresis. TrAC Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Rezanka, P.; Navratilova, K.; Rezanka, M.; Kral, V.; Sykora, D. Application of cyclodextrins in chiral capillary electrophoresis. Electrophoresis 2014, 35, 2701–2721. [Google Scholar] [CrossRef]
- Catai, J.R.; Carrilho, E. Simplex optimization of electrokinetic injection of DNA in capillary electrophoresis using dilute polymer solution. Electrophoresis 2003, 24, 648–654. [Google Scholar] [CrossRef]
- Rezanka, P.; Navratilova, K.; Rysava, H.; Masek, J.; Rokosova, L.; Blahova, M.; Rezanka, M.; Jindrich, J.; Sykora, D.; Kral, V. Influence of substituent position and cavity size of all regioisomers of monocarboxymethyl-α-, β-, and γ-cyclodextrins on their apparent stability constants of their complexes with both enantiomers of Tröger’s base. J. Sep. Sci. 2016, 39, 980–985. [Google Scholar] [CrossRef]
- Řezanková, K.; Kohoutová, R.; Kuchař, M.; Král, V.; Řezanka, P. Enantioseparation of novel psychoactive chiral amines and their mixture by capillary electrophoresis using cyclodextrins as chiral selectors. Chem. Pap. 2018, 72, 2737–2743. [Google Scholar] [CrossRef]
- Jurasek, B.; Himl, M.; Jurok, R.; Hajkova, K.; Vobinuskova, A.; Rezanka, P.; Kuchar, M. Synthesis of methoxetamine, its metabolites and deuterium labelled analog as analytical standards and their HPLC and chiral capillary electrophoresis separation. RSC Adv. 2017, 7, 56691–56696. [Google Scholar] [CrossRef]
- Szeman, J.; Ganzler, K.; Salgo, A.; Szejtli, J. Effect of the degree of substitution of cyclodextrin derivatives on chiral separations by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 1996, 728, 423–431. [Google Scholar] [CrossRef]
- Sabbah, S.; Scriba, G.K.E. Separation of dipeptide and tripeptide enantiomers in capillary electrophoresis using carboxymethyl-β-cyclodextrin and succinyl-β-cyclodextrin: Influence of the amino acid sequence, nature of the cyclodextrin and pH. Electrophoresis 2001, 22, 1385–1393. [Google Scholar] [CrossRef]
- Nowak, P.M.; Olesek, K.; Wozniakiewicz, M.; Mitoraj, M.; Sagan, F.; Koscielniak, P. Cyclodextrin-induced acidity modification of substituted cathinones studied by capillary electrophoresis supported by density functional theory calculations. J. Chromatogr. A 2018, 1580, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Zloh, M. An analysis of the ‘legal high’mephedrone. Bioorg. Med. Chem. Lett. 2010, 20, 4135–4139. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Holmen, A.; Nagard, M.; Lindberg, W. Rapid screening of pKa values of pharmaceuticals by pressure-assisted capillary electrophoresis combined with short-end injection. J. Chromatogr. A 2002, 979, 369–377. [Google Scholar] [CrossRef]
- Glicksberg, L.; Winecker, R.; Miller, C.; Kerrigan, S. Postmortem distribution and redistribution of synthetic cathinones. Forensic Toxicol. 2018, 36, 291–303. [Google Scholar] [CrossRef]
- Spendley, W.; Hext, G.R.; Himsworth, F.R. Sequential application of simplex designs in optimisation and evolutionary operation. Technometrics 1962, 4, 441–461. [Google Scholar] [CrossRef]
- Rezanka, P.; Rysava, H.; Havlik, M.; Jakubek, M.; Sykora, D.; Kral, V. Enantioseparation of Troeger’s Base Derivatives by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors. Chirality 2013, 25, 379–383. [Google Scholar] [CrossRef] [PubMed]
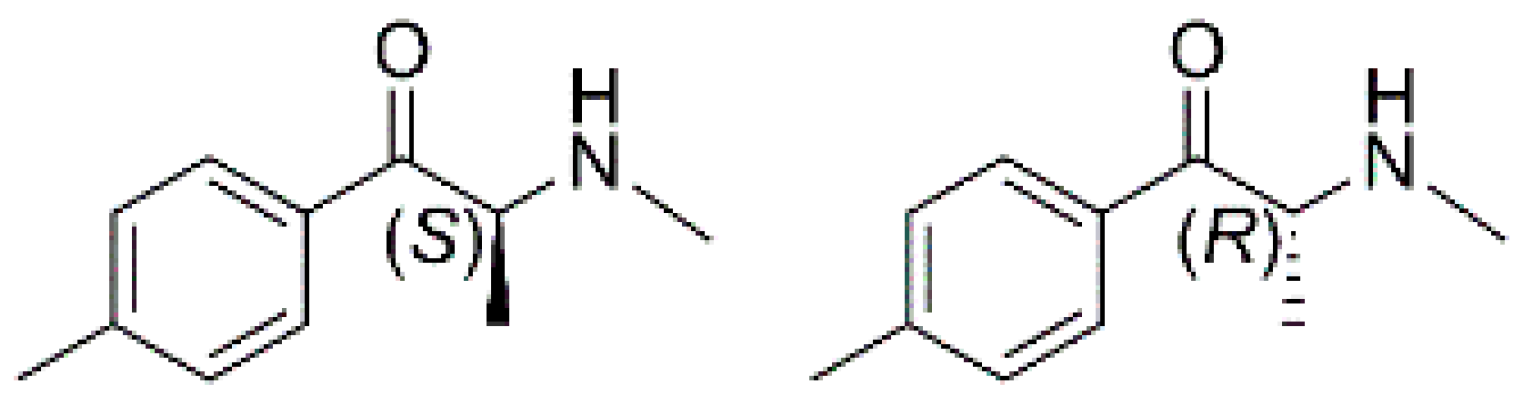
Sample Availability: Samples of the compounds 1–9 (Figure 2) are available from the authors. |



| Analyte | a | L(a) | b | L(b) | R2 | ||
|---|---|---|---|---|---|---|---|
| mmol·L−1 | mmol·L−1 | ||||||
| 1 | 0 | 32.60 | ± | 0.27 | 0.991 | ||
| 2 | 0 | 27.76 | ± | 0.16 | 0.995 | ||
| 3 | −5.47 | ± | 1.31 | 23.54 | ± | 1.14 | 0.807 |
| 4 | −5.07 | ± | 0.65 | 19.68 | ± | 0.29 | 0.967 |
| 5 | 0 | 26.14 | ± | 0.21 | 0.989 | ||
| 6 | −3.55 | ± | 1.07 | 36.01 | ± | 0.83 | 0.959 |
| 7 | 0 | 21.04 | ± | 0.20 | 0.991 |
| Analyte | LOD mmol·L−1 | Linear Concentration Range | ||
|---|---|---|---|---|
| LOQ | mmol·L−1 | |||
| 1 | 0.35 | 1.17 | − | 5.00 |
| 2 | 0.28 | 0.92 | − | 5.00 |
| 3 | 0.61 | 2.02 | − | 2.20 |
| 4 | 0.53 | 1.76 | − | 5.00 |
| 5 | 0.46 | 1.53 | − | 5.00 |
| 6 | 0.31 | 1.04 | − | 2.20 |
| 7 | 0.21 | 0.71 | − | 2.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Řezanka, P.; Macková, D.; Jurok, R.; Himl, M.; Kuchař, M. Enantioseparation and Determination of Mephedrone and Its Metabolites by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors. Molecules 2020, 25, 2879. https://doi.org/10.3390/molecules25122879
Řezanka P, Macková D, Jurok R, Himl M, Kuchař M. Enantioseparation and Determination of Mephedrone and Its Metabolites by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors. Molecules. 2020; 25(12):2879. https://doi.org/10.3390/molecules25122879
Chicago/Turabian StyleŘezanka, Pavel, Denisa Macková, Radek Jurok, Michal Himl, and Martin Kuchař. 2020. "Enantioseparation and Determination of Mephedrone and Its Metabolites by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors" Molecules 25, no. 12: 2879. https://doi.org/10.3390/molecules25122879
APA StyleŘezanka, P., Macková, D., Jurok, R., Himl, M., & Kuchař, M. (2020). Enantioseparation and Determination of Mephedrone and Its Metabolites by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors. Molecules, 25(12), 2879. https://doi.org/10.3390/molecules25122879