Treatment with a New Barbituric Acid Derivative Exerts Antiproliferative and Antimigratory Effects against Sorafenib Resistance in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
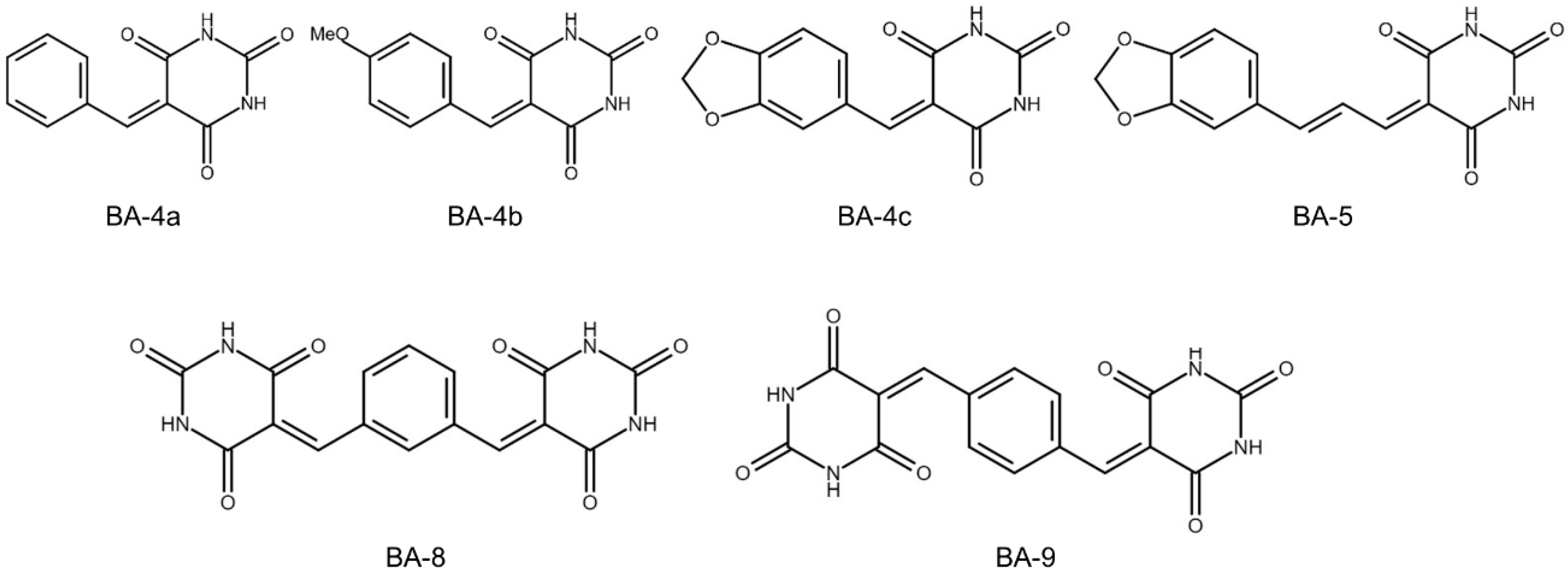
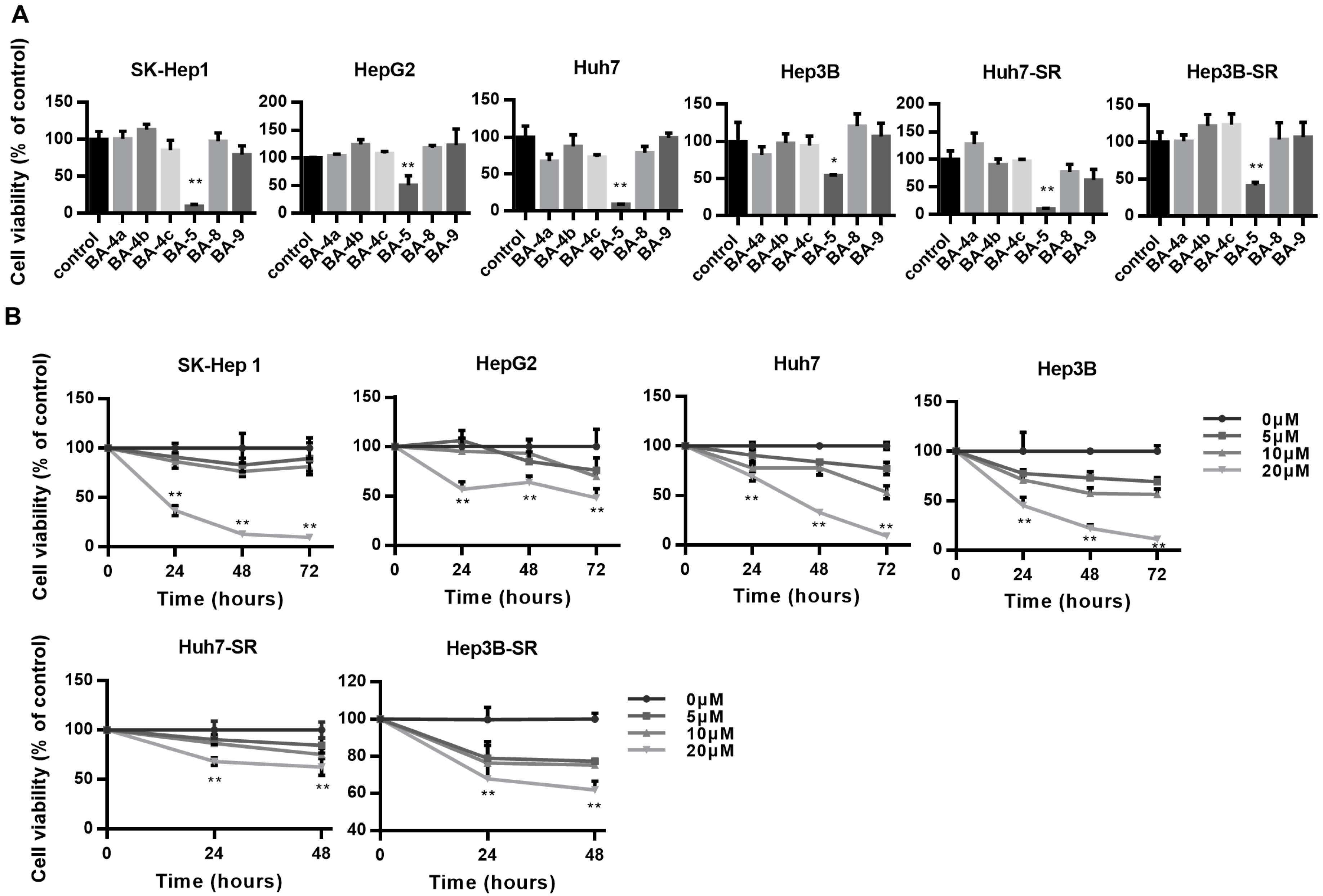
2.1. BA-5 Possessed the Best Ability to Decrease Cell Viability in Both Parental and Sorafenib-Resistant HCC Cells
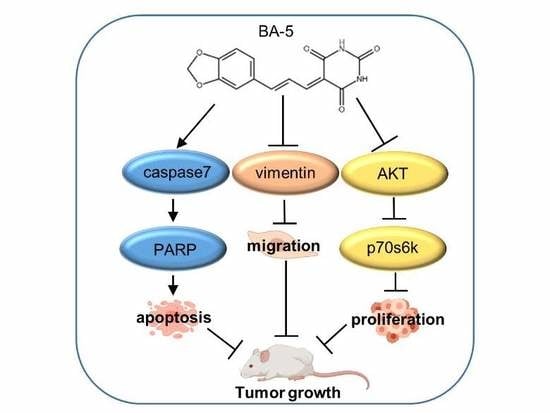
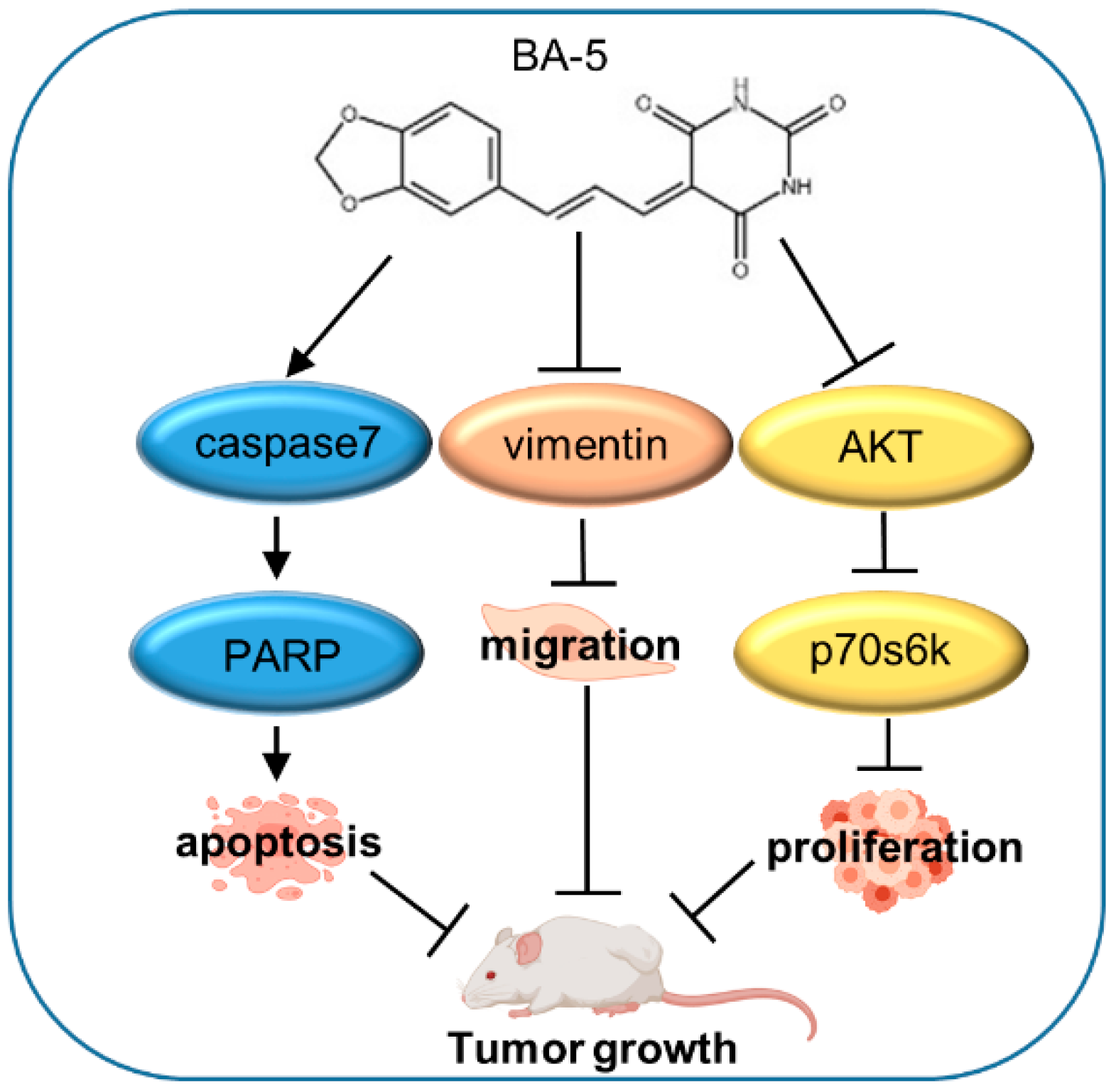
2.2. BA-5 Treatment Inhibited HCC and HCC-SR Cell Proliferation by Blocking AKT Signaling Pathways
2.3. Treatment with BA-5 Activated the Apoptosis Signaling Pathway
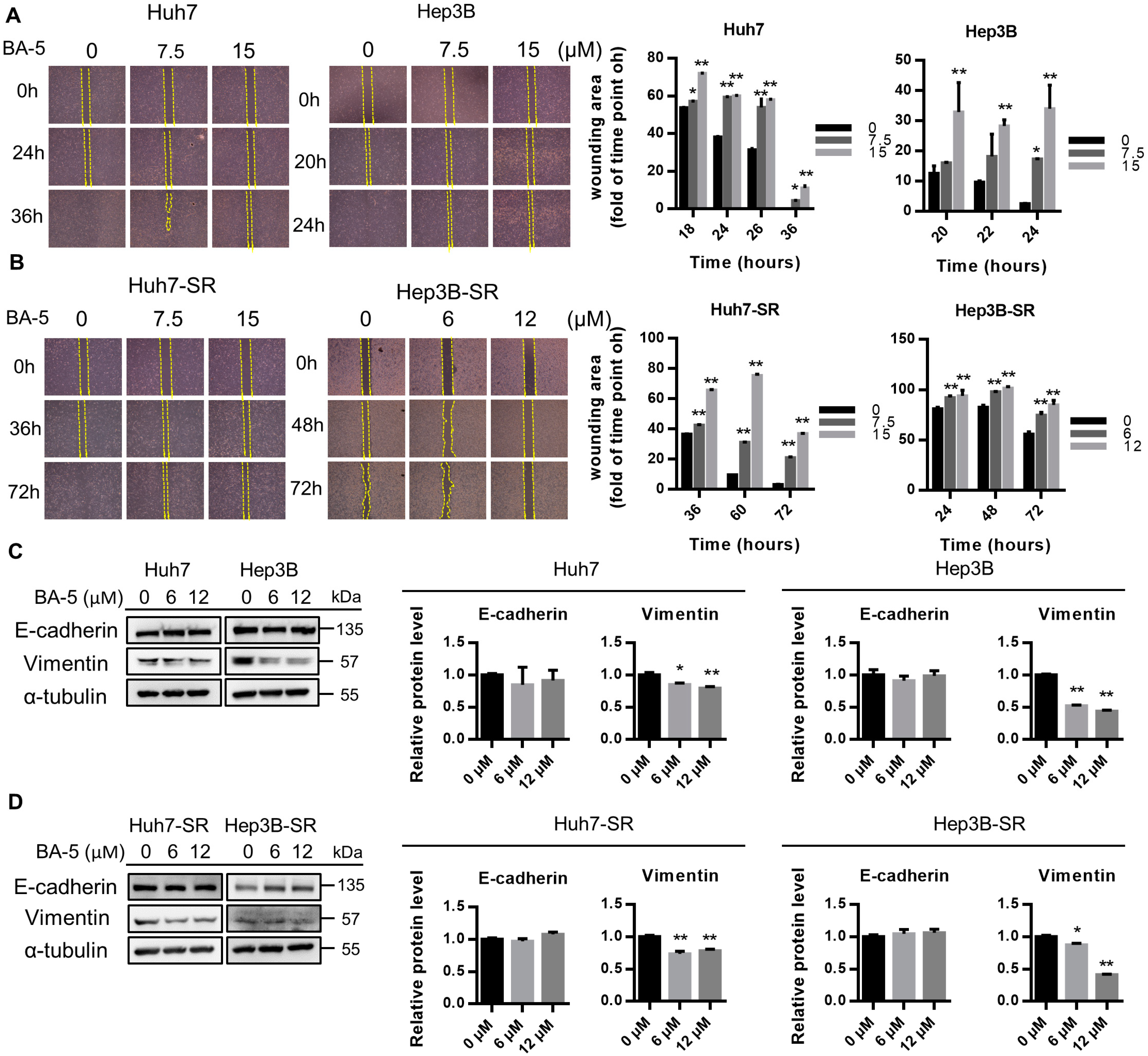
2.4. BA-5 Treatment Inhibited the Migration Ability of HCC and HCC-SR Cells
2.5. Genes and Biological Functions Affected by BA-5 Treatment in Both HCC and HCC-SR Cells
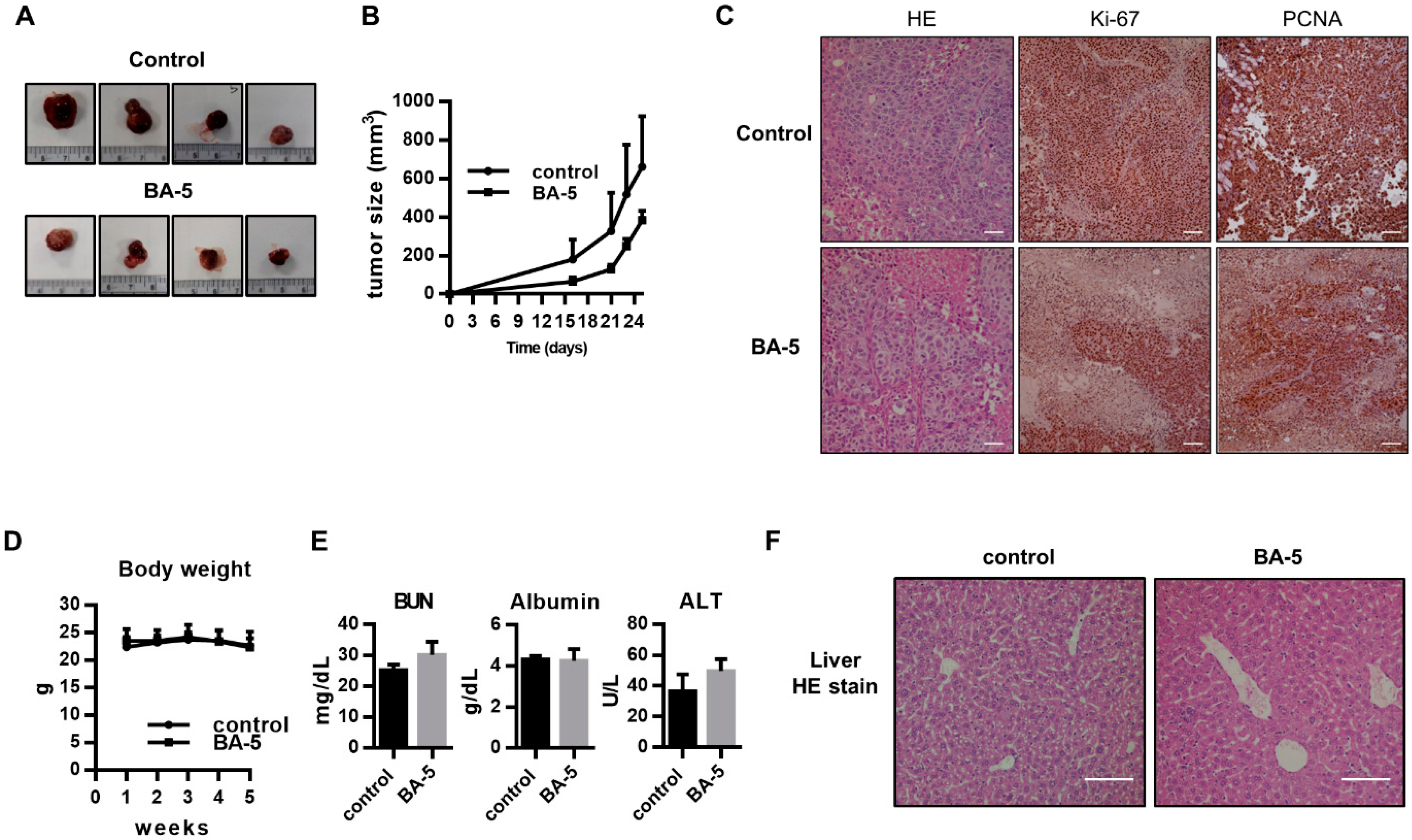
2.6. BA-5 Suppressed Tumor Growth in an HCC Xenograft Mouse Model
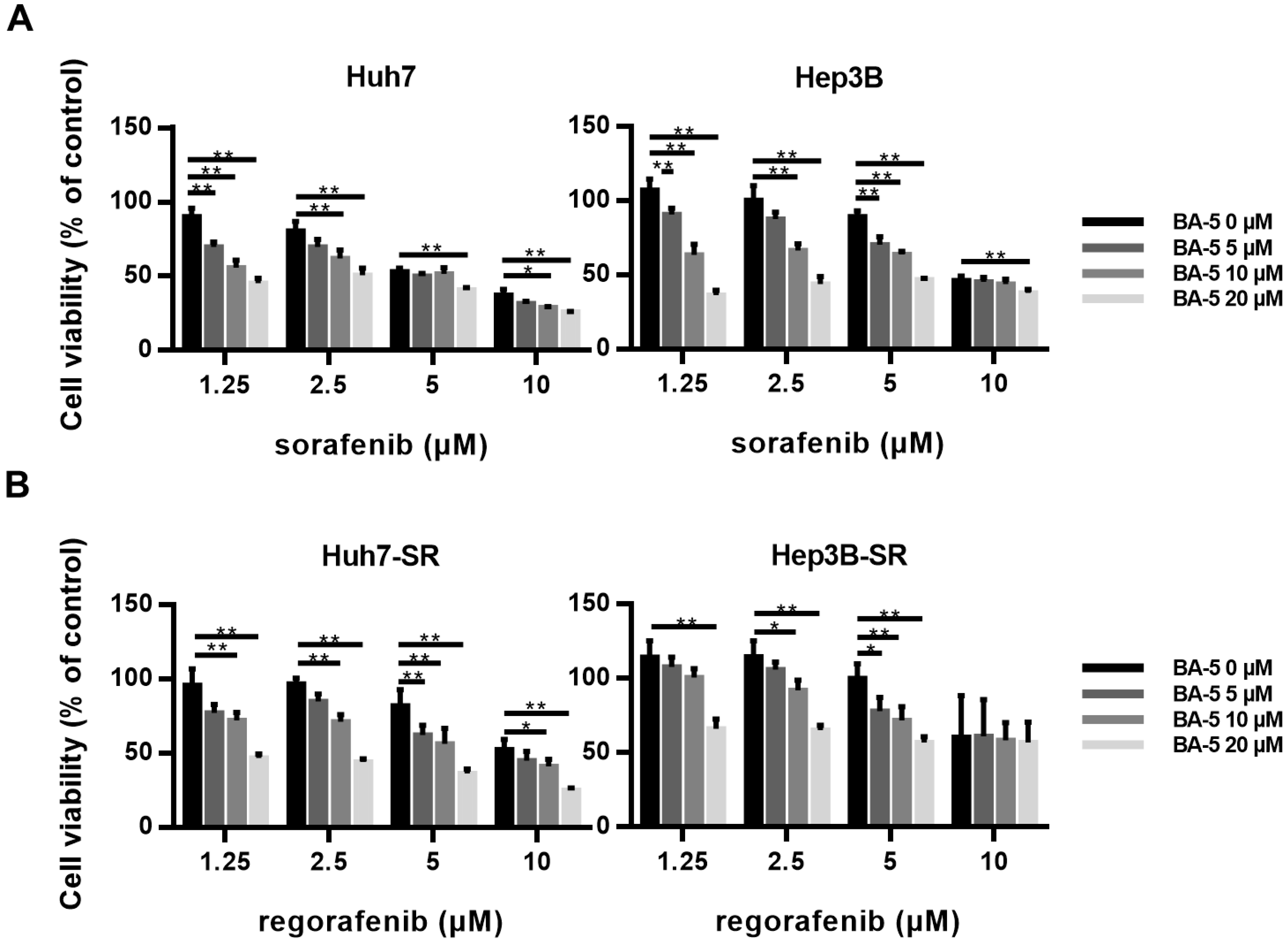
2.7. The Combination of BA-5 with a Low Dose of Regorafenib Synergistically Inhibited HCC-SR Cell Viability
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drugs Treatment and the Coefficient of Drug Interaction
4.3. MTT Cell Viability Assay
4.4. Wound Healing Migration Assay
4.5. Western Blot Analysis
4.6. Gene Expression Profiling
4.7. Subcutaneous Xenograft Model
4.8. Immunohistochemistry (IHC) Staining and Blood Biochemical Parameters
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A.J. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, G.L.; Dempster, J.; Meler, J.D.; Orr, D.W.; Walberg, M.W.; Brown, B.; Berger, B.D.; O’Connor, J.K.; Goldstein, R.M. Hepatocellular carcinoma: Management of an increasingly common problem. Proc. (Bayl. Univ. Med. Cent.) 2008, 21, 266–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- London, W.T.; McGlynn, K.A. Liver cancer. In Cancer Epidemiology and Prevention, 3rd ed.; Schottenfeld, D., Fraumeni, J., Jr., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 763–786. [Google Scholar]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Khan, Z.; Alloghbi, A.; Said Ahmed, T.S.; Ashraf, M.; Hammouda, D.M. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina 2019, 55, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannelli, G.; Koudelkova, P.; Dituri, F.; Mikulits, W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol. 2016, 65, 798–808. [Google Scholar] [CrossRef] [Green Version]
- Kulik, L.; El-Serag, H.B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef]
- Copur, M.S. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 2498–2499. [Google Scholar]
- Montironi, C.; Montal, R.; Llovet, J.M. New Drugs Effective in the Systemic Treatment of Hepatocellular Carcinoma. Clin. Liver Dis. 2019, 14, 56–61. [Google Scholar] [CrossRef]
- Mendez-Blanco, C.; Fondevila, F.; Garcia-Palomo, A.; Gonzalez-Gallego, J.; Mauriz, J.L. Sorafenib resistance in hepatocarcinoma: Role of hypoxia-inducible factors. Exp. Mol. Med. 2018, 50. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, Z.H.; Qu, X.J. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin. Pharmacol. Toxicol. 2015, 116, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, R.; Zhao, J.; Liu, J.; Ying, H.; Yan, H.; Zhou, S.; Liang, Y.; Huang, D.; Liang, X. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015, 367, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-F.; Chen, H.-L.; Tai, W.-T.; Feng, W.-C.; Hsu, C.-H.; Chen, P.-J.; Cheng, A.-L. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2011, 337, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Zhai, B.; Sun, W.; Hu, F.; Cheng, H.; Xu, J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeyer, A. Mittheilungen aus dem organischen Laboratorium des Gewerbeinstitutes in Berlin: Untersuchungen über die Harnsäuregruppe. Justus Liebigs Annalen der Chemie. 1864, 130, 129–175. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.; Von Mering, J. Über eine neue Klasse von Schlafmitteln. Therapie Gegenwart. 1903, 44, 97–100. [Google Scholar]
- Knabe, J.; Büch, H.; Reinhardt, J. Derivatives of barbituric acid, 32. Central nervous activity of racemic and optically active barbituric acids with basic substituents. Arch. Pharm. 1982, 315, 832–839. [Google Scholar] [CrossRef]
- Archana Rani, P.; Bajaj, K.; Srivastava, V.K.; Chandra, R.; Kumar, A. Synthesis of newer indolyl/phenothiazinyl substituted 2-oxo/thiobarbituric acid derivatives as potent anticonvulsant agents. Arzneimittelforschung 2003, 53, 301–306. [Google Scholar] [CrossRef]
- Sokmen, B.B.; Ugras, S.; Sarikaya, H.Y.; Ugras, H.I.; Yanardag, R. Antibacterial, Antiurease, and Antioxidant Activities of Some Arylidene Barbiturates. Appl. Biochem. Biotech. 2013, 171, 2030–2039. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Cao, R.; Yi, W.; Chen, Z.; Wen, H.; Ma, L.; Song, H. Inhibitory effects of 5-benzylidene barbiturate derivatives on mushroom tyrosinase and their antibacterial activities. Eur. J. Med. Chem. 2009, 44, 4235–4243. [Google Scholar] [CrossRef]
- Faidallah, H.M.; Khan, K.A. Synthesis and biological evaluation of new barbituric and thiobarbituric acid fluoro analogs of benzenesulfonamides as antidiabetic and antibacterial agents. J. Fluorine Chem. 2012, 142, 96–104. [Google Scholar] [CrossRef]
- Naguib, F.N.; Levesque, D.L.; Wang, E.-C.; Panzica, R.P.; El Kouni, M.H. 5-Benzylbarbituric acid derivatives, potent and specific inhibitors of uridine phosphorylase. Biochem. Pharmacol. 1993, 46, 1273–1283. [Google Scholar] [CrossRef]
- Dhorajiya, B.D.; Ibrahim, A.S.; Badria, F.A.; Dholakiya, B.Z. Design and synthesis of novel nucleobase-based barbiturate derivatives as potential anticancer agents. Med. Chem. Res. 2014, 23, 839–847. [Google Scholar] [CrossRef]
- Dhorajiya, B.D.; Dholakiya, B.Z.; Mohareb, R.M. Hybrid probes of aromatic amine and barbituric acid: Highly promising leads for anti-bacterial, anti-fungal and anti-cancer activities. Med. Chem. Res. 2014, 23, 3941–3952. [Google Scholar] [CrossRef]
- Ramisetti, S.R.; Pandey, M.K.; Lee, S.Y.; Karelia, D.; Narayan, S.; Amin, S.; Sharma, A.K. Design and synthesis of novel thiobarbituric acid derivatives targeting both wild-type and BRAF-mutated melanoma cells. Eur. J. Med. Chem. 2018, 143, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, S.; Zheng, H.; Chen, J.; Lin, L.; Ye, X.; Chen, Z.; Xu, Q.; Chen, T.; Yang, J.; et al. Synthesis and biological activity of novel barbituric and thiobarbituric acid derivatives against non-alcoholic fatty liver disease. Eur. J. Med. Chem. 2011, 46, 2003–2010. [Google Scholar] [CrossRef]
- Zheng, H.; Li, S.; Ma, L.; Cheng, L.; Deng, C.; Chen, Z.; Xie, C.; Xiang, M.; Jiang, W.; Chen, L. A novel agonist of PPAR-γ based on barbituric acid alleviates the development of non-alcoholic fatty liver disease by regulating adipocytokine expression and preventing insulin resistance. Eur. J. Pharmacol. 2011, 659, 244–251. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Suk, F.-M.; Liu, C.-L.; Chen, T.-L.; Twu, Y.-C.; Hsu, M.-H.; Liao, Y.-J. Antifibrotic Effects of a Barbituric Acid Derivative on Liver Fibrosis by Blocking the NF-κB Signaling Pathway in Hepatic Stellate Cells. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Jha, R.; Prins, P.A.; Wang, H.; Chacha, M.; Hartley, M.L.; He, A.R. Regorafenib in advanced hepatocellular carcinoma (HCC): Considerations for treatment. Cancer Chemother. Pharmacol. 2017, 80, 945–954. [Google Scholar] [CrossRef]
- Berasain, C. Hepatocellular carcinoma and sorafenib: Too many resistance mechanisms? Gut 2013, 62, 1674–1675. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S.; Letouze, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Munoz, F.; Ucha-Udabe, R.; Alamo, C. The history of barbiturates a century after their clinical introduction. Neuropsychiatr. Dis. Treat. 2005, 1, 329–343. [Google Scholar] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Liu, G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Lui, V.W.; Yeo, W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011, 7, 1149–1167. [Google Scholar] [CrossRef]
- Nakanishi, K.; Sakamoto, M.; Yamasaki, S.; Todo, S.; Hirohashi, S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer 2005, 103, 307–312. [Google Scholar] [CrossRef]
- Minguez, B.; Tovar, V.; Chiang, D.; Villanueva, A.; Llovet, J.M. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr. Opin. Gastroenterol. 2009, 25, 186–194. [Google Scholar] [CrossRef]
- Zhai, B.; Hu, F.; Jiang, X.; Xu, J.; Zhao, D.; Liu, B.; Pan, S.; Dong, X.; Tan, G.; Wei, Z.; et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol. Cancer. Ther. 2014, 13, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, Q.; Liu, J.; Cao, H. Inhibition of the PI3K/Akt signaling pathway reverses sorafenib-derived chemo-resistance in hepatocellular carcinoma. Oncol. Lett. 2018, 15, 9377–9384. [Google Scholar] [CrossRef] [PubMed]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; Gonzalez-Baron, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Cole, D.C.; Brooijmans, N.; Bursavich, M.G.; Curran, K.J.; Ellingboe, J.W.; Gibbons, J.J.; Hollander, I.; Hu, Y.; Kaplan, J.; et al. Discovery of potent and selective inhibitors of the mammalian target of rapamycin (mTOR) kinase. J. Med. Chem. 2009, 52, 7081–7089. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.M.; Nowak, P.; Brooijmans, N.; Bursavich, M.G.; Dehnhardt, C.; Delos Santos, E.; Feldberg, L.R.; Hollander, I.; Kim, S.; Lombardi, S.; et al. Novel purine and pyrazolo[3,4-d]pyrimidine inhibitors of PI3 kinase-alpha: Hit to lead studies. Bioorg. Med. Chem. Lett. 2010, 20, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovas. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Wen, X.; Lin, Z.Q.; Liu, B.; Wei, Y.Q. Caspase-mediated programmed cell death pathways as potential therapeutic targets in cancer. Cell Prolif. 2012, 45, 217–224. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.G.; Epping, M.; Kruyt, F.A.; Giaccone, G. Apoptosis: Target of cancer therapy. Clin. Cancer Res. 2002, 8, 2024–2034. [Google Scholar]
- Tiwari, N.; Gheldof, A.; Tatari, M.; Christofori, G. EMT as the ultimate survival mechanism of cancer cells. Semin. Cancer Biol. 2012, 22, 194–207. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Rev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [Green Version]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Cai, X.; Fan, D. Roles of epithelial-mesenchymal transition in cancer drug resistance. Curr. Cancer Drug Targets 2013, 13, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, J.; Zhang, Y.; Cai, H.; Chen, X.; Sun, D. Regorafenib reverses HGF-induced sorafenib resistance by inhibiting epithelial-mesenchymal transition in hepatocellular carcinoma. FEBS 2019, 9, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refolo, M.G.; Lippolis, C.; Carella, N.; Cavallini, A.; Messa, C.; D’Alessandro, R.J.I. Chlorogenic acid improves the regorafenib effects in human hepatocellular carcinoma cells. Int. J. Mol. Sci. 2018, 19, 1518. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.J.; Fang, C.C.; Yen, C.H.; Hsu, S.M.; Wang, C.K.; Huang, S.F.; Liang, Y.C.; Lin, Y.Y.; Chu, Y.T.; Arthur Chen, Y.M. Niemann-Pick type C2 protein regulates liver cancer progression via modulating ERK1/2 pathway: Clinicopathological correlations and therapeutical implications. Int. J. Cancer 2015, 137, 1341–1351. [Google Scholar] [CrossRef]
- Suk, F.M.; Liu, C.L.; Hsu, M.H.; Chuang, Y.T.; Wang, J.P.; Liao, Y.J. Treatment with a new benzimidazole derivative bearing a pyrrolidine side chain overcomes sorafenib resistance in hepatocellular carcinoma. Sci. Rep. 2019, 9, 17259. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Cao, S.-S.; Zhen, Y.-S. Potentiation of antimetabolite antitumor activity in vivo by dipyridamole and amphotericin B. Cancer Chemother. Pharmacol. 1989, 24, 181–186. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the corresponding author (M.H.H.). |



| Description | Count | % | p Value |
|---|---|---|---|
| Upregulation | |||
| HIF-1 signaling pathway | 3 | 7.1 | 0.016 |
| Downregulation | |||
| Pathways in cancer | 15 | 7.2 | 0.00037 |
| TNF signaling pathway | 7 | 3.3 | 0.0022 |
| Cytokine-cytokine receptor interaction | 10 | 4.8 | 0.0034 |
| Apoptosis | 5 | 2.4 | 0.0076 |
| Rap1 signaling pathway | 8 | 3.8 | 0.017 |
| PI3K-Akt signaling pathway | 10 | 4.8 | 0.03 |
| Signaling pathways regulating pluripotency of stem cells | 6 | 2.9 | 0.032 |
| Central carbon metabolism in cancer | 4 | 1.9 | 0.047 |
| Regulation of actin cytoskeleton | 7 | 3.3 | 0.049 |
| p53 signaling pathway | 4 | 1.9 | 0.052 |
| Complement and coagulation cascades | 4 | 1.9 | 0.056 |
| Leukocyte transendothelial migration | 5 | 2.4 | 0.057 |
| Melanoma | 4 | 1.9 | 0.06 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 2 | 1.0 | 0.062 |
| Sphingolipid signaling pathway | 5 | 2.4 | 0.065 |
| Chemokine signaling pathway | 6 | 2.9 | 0.086 |
| FoxO signaling pathway | 5 | 2.4 | 0.089 |
| BA-5 (20 µM) | |||||
|---|---|---|---|---|---|
| Sorafenib (µM) | Huh7 | Hep3B | Regorafenib (µM) | Hhh7-SR | Hep3B-SR |
| 1.25 | 0.65 | 0.67 | 1.25 | 0.79 | 0.73 |
| 2.5 | 0.81 | 0.70 | 2.5 | 0.75 | 0.74 |
| 5 | 0.92 | 0.84 | 5 | 0.74 | 0.82 |
| 10 | 0.89 | 1.15 | 10 | 0.81 | 1.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.-J.; Hsu, S.-M.; Chien, C.-Y.; Wang, Y.-H.; Hsu, M.-H.; Suk, F.-M. Treatment with a New Barbituric Acid Derivative Exerts Antiproliferative and Antimigratory Effects against Sorafenib Resistance in Hepatocellular Carcinoma. Molecules 2020, 25, 2856. https://doi.org/10.3390/molecules25122856
Liao Y-J, Hsu S-M, Chien C-Y, Wang Y-H, Hsu M-H, Suk F-M. Treatment with a New Barbituric Acid Derivative Exerts Antiproliferative and Antimigratory Effects against Sorafenib Resistance in Hepatocellular Carcinoma. Molecules. 2020; 25(12):2856. https://doi.org/10.3390/molecules25122856
Chicago/Turabian StyleLiao, Yi-Jen, Shih-Ming Hsu, Chia-Ying Chien, Yuan-Hsi Wang, Ming-Hua Hsu, and Fat-Moon Suk. 2020. "Treatment with a New Barbituric Acid Derivative Exerts Antiproliferative and Antimigratory Effects against Sorafenib Resistance in Hepatocellular Carcinoma" Molecules 25, no. 12: 2856. https://doi.org/10.3390/molecules25122856
APA StyleLiao, Y.-J., Hsu, S.-M., Chien, C.-Y., Wang, Y.-H., Hsu, M.-H., & Suk, F.-M. (2020). Treatment with a New Barbituric Acid Derivative Exerts Antiproliferative and Antimigratory Effects against Sorafenib Resistance in Hepatocellular Carcinoma. Molecules, 25(12), 2856. https://doi.org/10.3390/molecules25122856