An Eco-Friendly Hydrophobic Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction for the Determination of Neonicotinoid Insecticide Residues in Water, Soil and Egg Yolk Samples
Abstract
1. Introduction
2. Results and Discussion
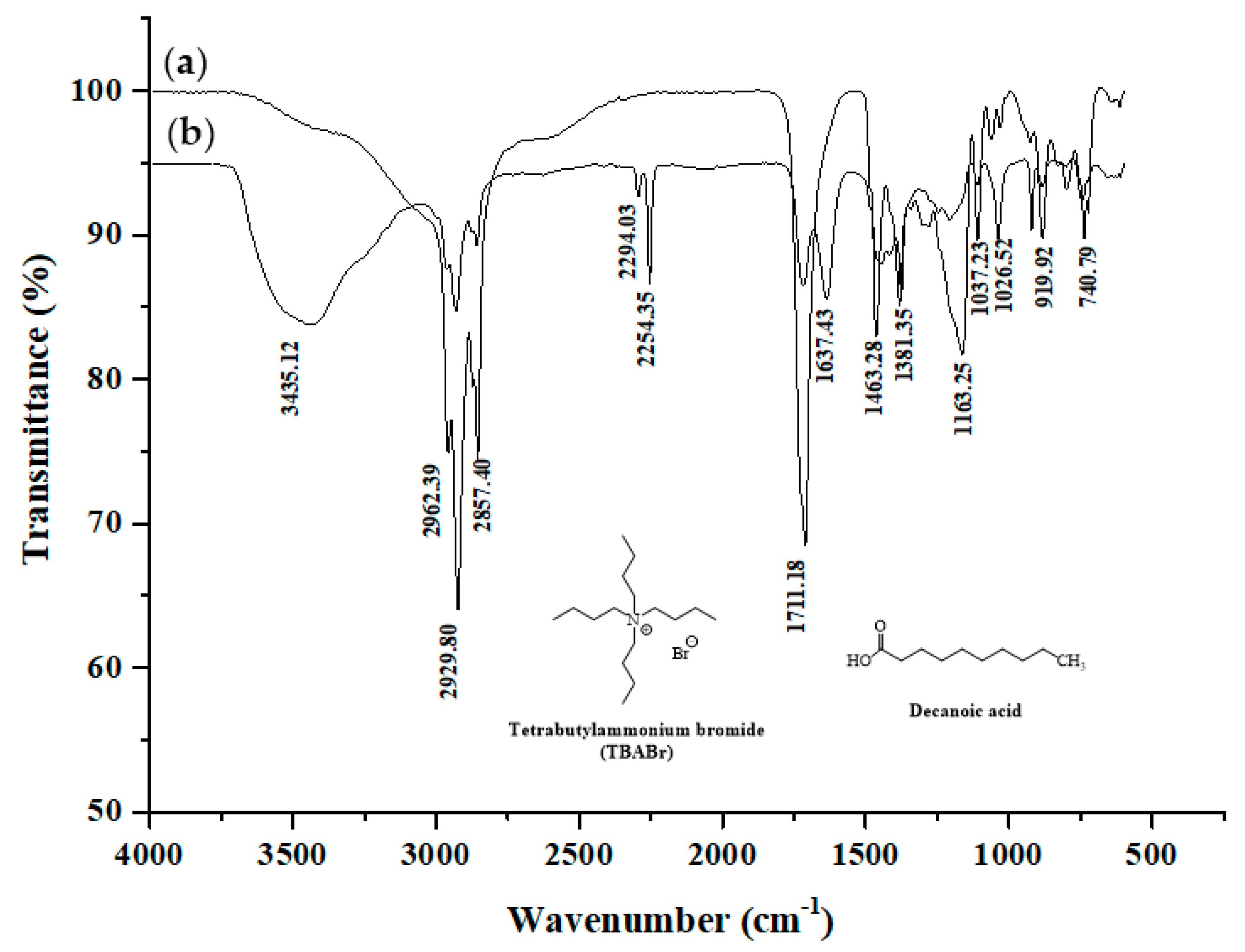
2.1. Characterization of Hydrophobic DES (TBABr with Decanoic Acid)
2.2. Optimization of Hydrophobic Deep Eutectic Solvent Based on Dispersive Liquid–Liquid Microextraction Procedure
2.2.1. Selection of Hydrophobic DES and Its Volume
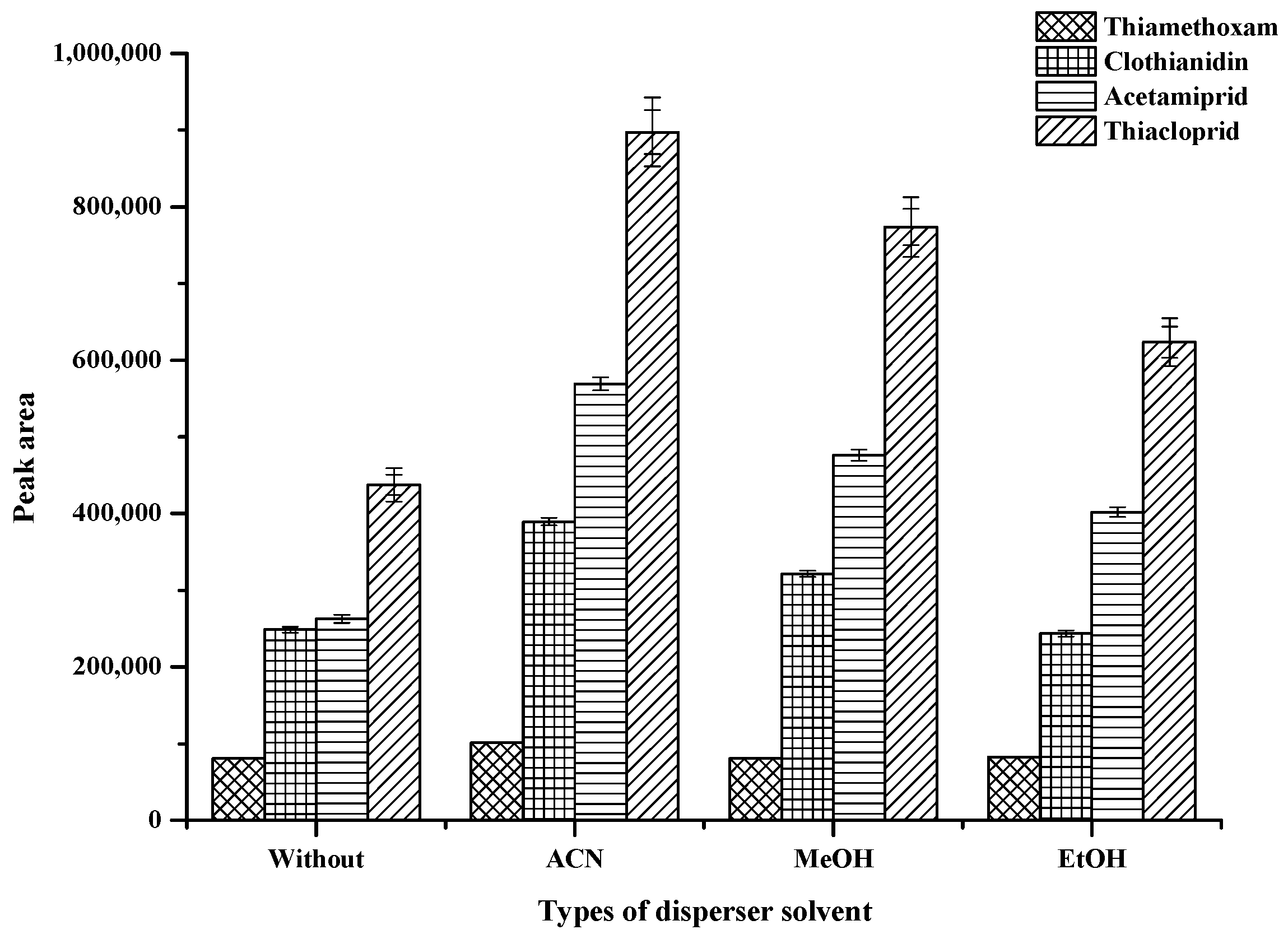
2.2.2. Effect of Type and Volume of Disperser Solvents
2.2.3. Effect of Type and Concentration of Surfactant
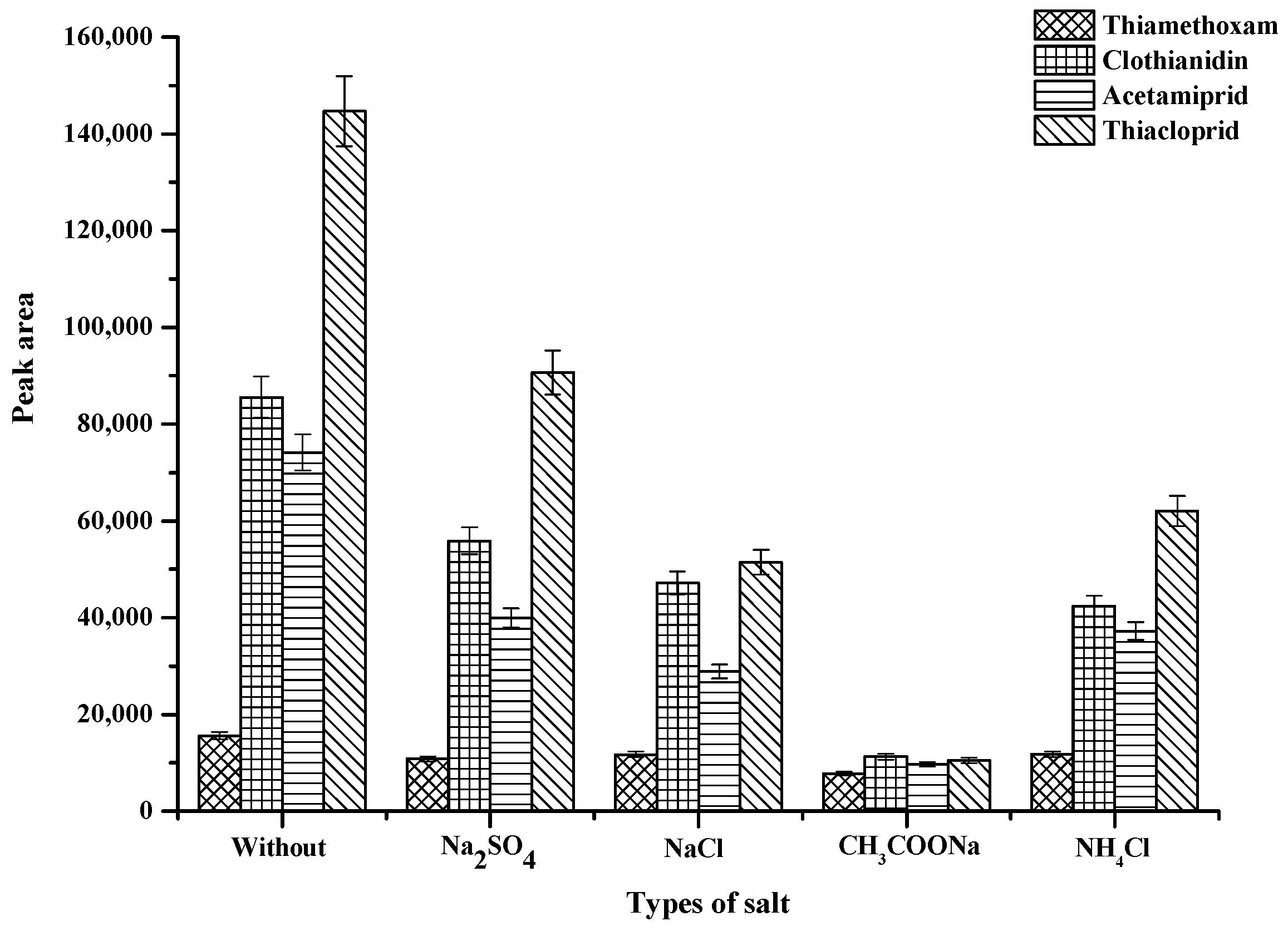
2.2.4. Effect of Salts Addition
2.2.5. Effect of Vortex and Centrifugation Time
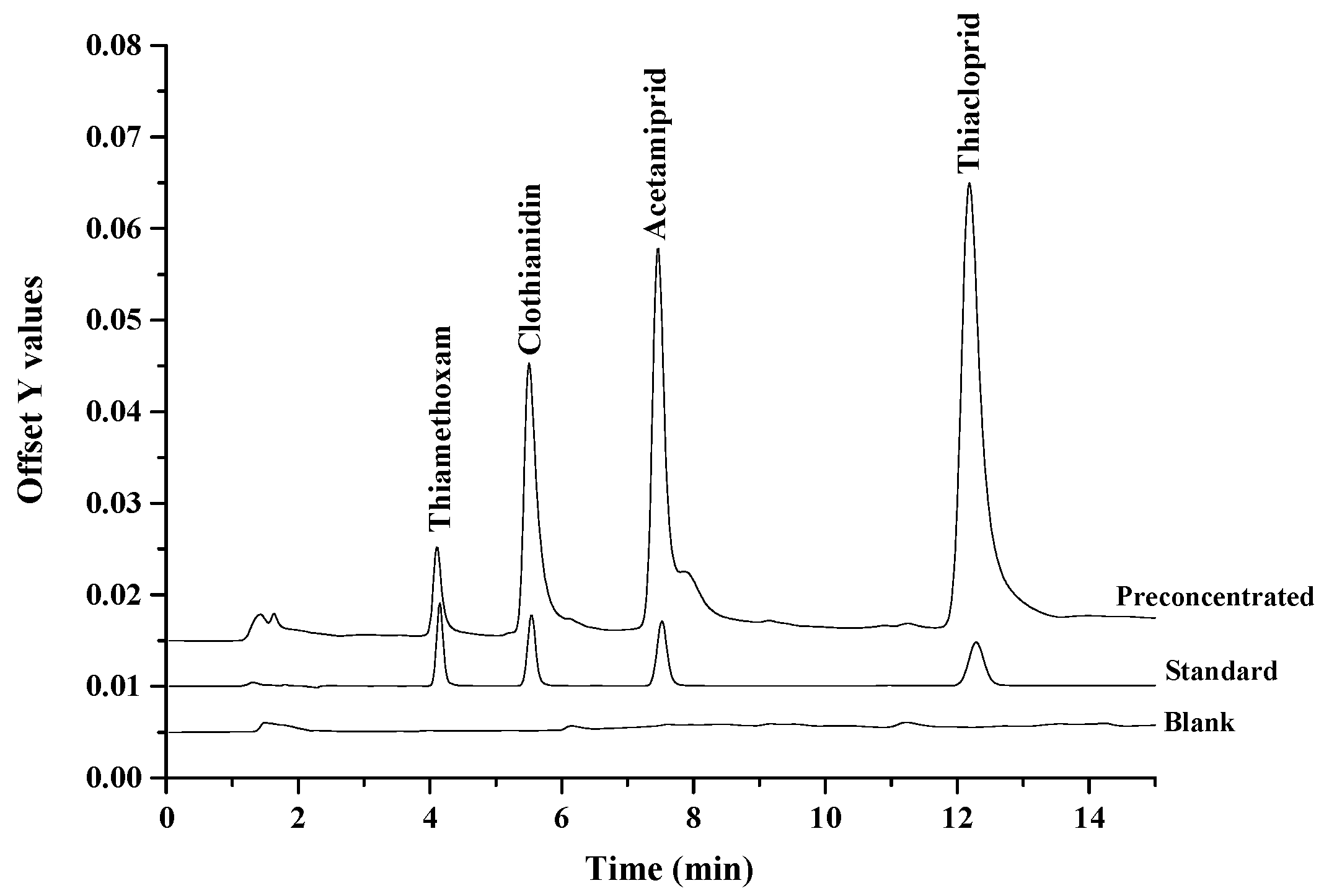
2.3. Analytical Performance of the Proposed Method
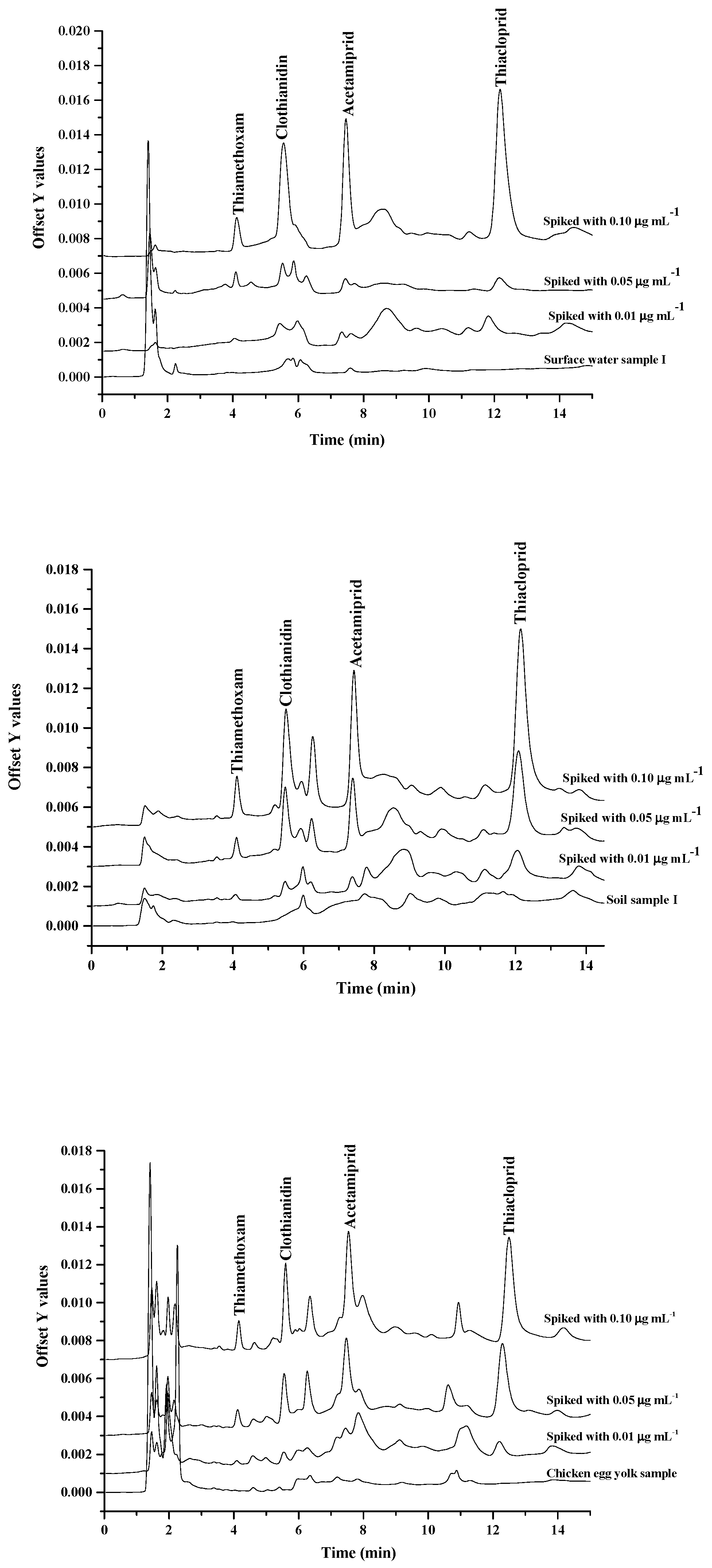
2.4. Analysis of Real Samples
2.5. Comparison of the Proposed Hydrophobic Deep Eutectic Solvent Based on Dispersive Liquid–Liquid Microextraction With Other Sample Preparation Methods
3. Experimental
3.1. Chemicals and Reagents
3.2. Chromatographic Conditions
3.3. Synthetic of Hydrophobic Deep Eutectic Solvent
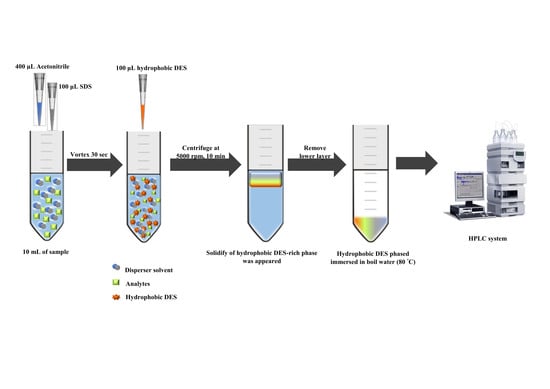
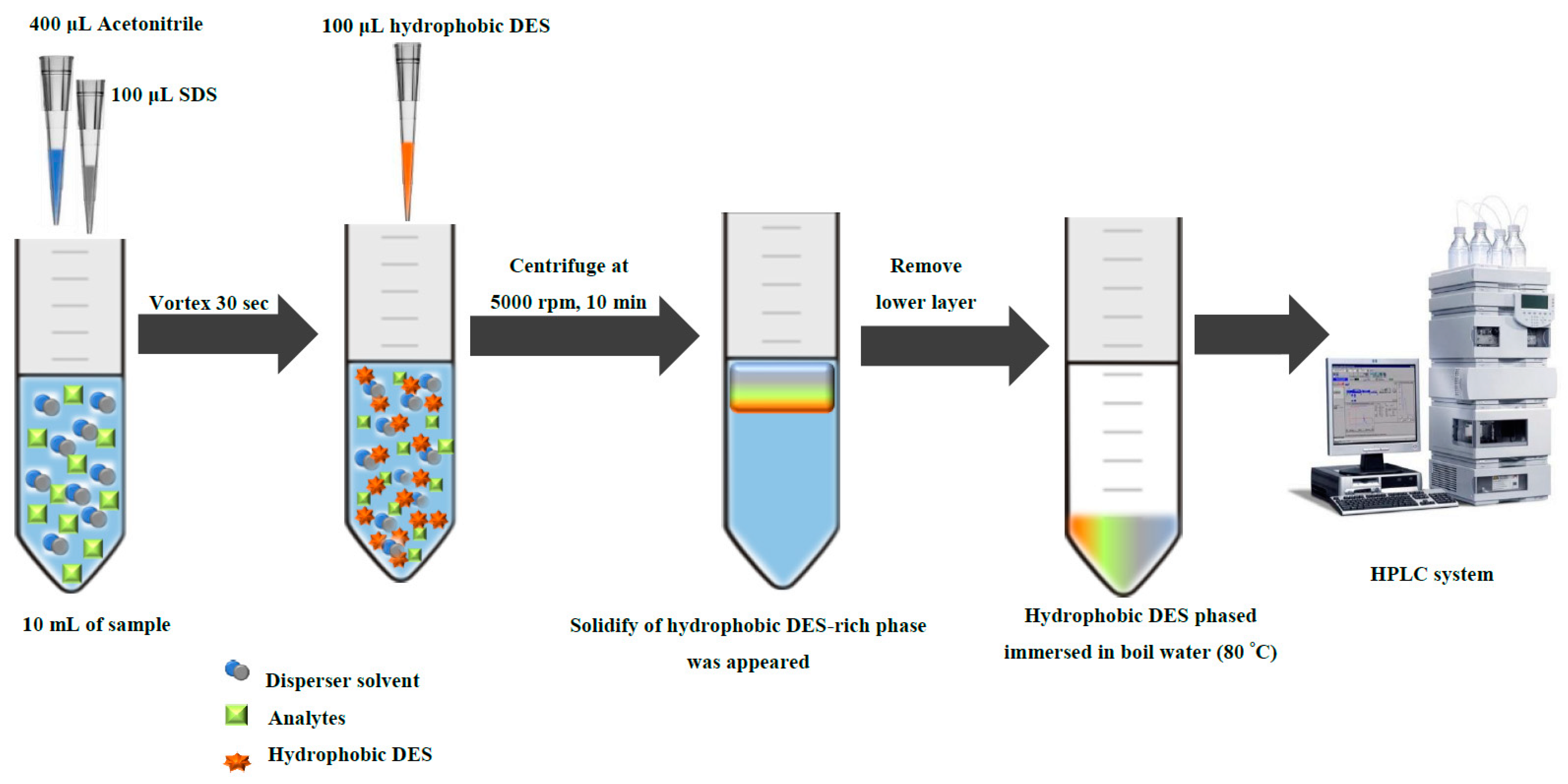
3.4. Hydrophobic Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction
3.5. Sample Preparation
3.5.1. Surface Water
3.5.2. Soil
3.5.3. Egg Yolk
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Gani, R.; Jiménez-González, C.; Constable, D.J.C. Method for selection of solvents for promotion of organic reactions. Comput. Chem. Eng. 2005, 29, 1661–1676. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.E.; Jang, H.W.; Lim, T.G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F.; Poole, S.K. Extraction of organic compounds with room temperature ionic liquids. J. Chromatogr. A 2010, 1217, 2268–2286. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Li, Y.; Lin, Y.; Zhang, H.; Zhou, Y. A novel poly(deep eutectic solvent)-based magnetic silica composite for solid-phase extraction of trypsin. Anal. Chim. Acta 2016, 946, 64–72. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Makoś, P.; Przyjazny, A.; Boczkaj, G. Hydrophobic deep eutectic solvents as “green” extraction media for polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2018, 1570, 28–37. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Crespo, E.A.; Pontes, P.V.A.; Silva, L.P.; Bülow, M.; Maximo, G.J.; Batista, E.A.C.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids. ACS Sustain. Chem. Eng. 2018, 6, 8836–8846. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Wang, X.; Deng, Z. Hexafluoroisopropanol-based hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of pyrethroids in tea beverages and fruit juices. Food Chem. 2018, 274, 891–899. [Google Scholar]
- Zhu, S.; Zhou, J.; Jia, H.; Zhang, H. Liquid–liquid microextraction of synthetic pigments in beverages using a hydrophobic deep eutectic solvent. Food Chem. 2018, 243, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tereshatov, E.E.; Boltoeva, M.Y.; Folden, C.M. First evidence of metal transfer into hydrophobic deep eutectic and low-transition-temperature mixtures: Indium extraction from hydrochloric and oxalic acids. Green Chem. 2016, 18, 4616–4622. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Yin, X.; Wang, C.; Wang, Z. Dispersive liquid-liquid microextraction combined with sweeping micellar electrokinetic chromatography for the determination of some neonicotinoid insecticides in cucumber samples. Food Chem. 2012, 133, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, D.J.G.P.; Zubeir, L.F.; Van Den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Dwamena, A.K. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations. 2019, 6, 9. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Wang, P.; Yang, X.; Wang, J.; Cui, J.; Dong, A.J.; Zhao, H.T.; Zhang, L.W.; Wang, Z.Y.; Xu, R.B.; Li, W.J.; et al. Multi-residue method for determination of seven neonicotinoid insecticides in grains using dispersive solid-phase extraction and dispersive liquid-liquid micro-extraction by high performance liquid chromatography. Food Chem. 2012, 134, 1691–1698. [Google Scholar] [CrossRef]
- Rezaee, M.; Yamini, Y.; Faraji, M. Evolution of dispersive liquid-liquid microextraction method. J. Chromatogr. A 2010, 1217, 2342–2357. [Google Scholar] [CrossRef]
- Milatovic, D. Chapter 23-Insecticides. In Biomarkers in Toxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Decourtye, A.; Devillers, J. Ecotoxicity of neonicotinoid insecticides to bees BT-Insect Nicotinic Acetylcholine Receptors. Insect Nicotinic Acetylcholine Recept. 2010, 683, 85–95. [Google Scholar]
- Biever, R.C.; Hoberg, J.R.; Jacobson, B.; Dionne, E.; Sulaiman, M.; McCahon, P. Icon® Rice Seed Treatment Toxicity To Crayfish (Procambarus clarkii) in Experimental Rice Paddies. Environ. Toxicol. Chem. 2003, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Total Environ. 2016, 566, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Bamorowat, M.; Mogaddam, M.R.A. Ringer tablet-based ionic liquid phase microextraction: Application in extraction and preconcentration of neonicotinoid insecticides from fruit juice and vegetable samples. Talanta 2016, 160, 211–216. [Google Scholar] [CrossRef]
- Singh, S.B.; Foster, G.D.; Khan, S.U. Microwave-Assisted Extraction for the Simultaneous Determination of Thiamethoxam, Imidacloprid, and Carbendazim Residues in Fresh and Cooked Vegetable Samples. J. Agric. Food Chem. 2004, 52, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Seccia, S.; Fidente, P.; Montesano, D.; Morrica, P. Determination of neonicotinoid insecticides residues in bovine milk samples by solid-phase extraction clean-up and liquid chromatography with diode-array detection. J. Chromatogr. A 2008, 1214, 115–120. [Google Scholar] [CrossRef]
- García, M.D.G.; Galera, M.M.; Valverde, R.S.; Galanti, A.; Girotti, S. Column switching liquid chromatography and post-column photochemically fluorescence detection to determine imidacloprid and 6-chloronicotinic acid in honeybees. J. Chromatogr. A 2007, 1147, 17–23. [Google Scholar] [CrossRef]
- Jovanov, P.; Guzsvány, V.; Franko, M.; Lazić, S.; Sakač, M.; Šarić, B.; Banjaca, V. Multi-residue method for determination of selected neonicotinoid insecticides in honey using optimized dispersive liquid-liquid microextraction combined with liquid chromatography-tandem mass spectrometry. Talanta 2013, 111, 125–133. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.; Lee, J.; Lee, J.; Kim, B.J.; Kim, J.H. Simultaneous analysis of 310 pesticide multiresidues using UHPLC-MS/MS in brown rice, orange, and spinach. Chemosphere 2018, 207, 519–526. [Google Scholar] [CrossRef]
- Xiong, C.; Ruan, J.; Cai, Y.; Tang, Y. Extraction and determination of some psychotropic drugs in urine samples using dispersive liquid-liquid microextraction followed by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2009, 49, 572–578. [Google Scholar] [CrossRef]
- Alshana, U.; Ertaş, N.; Göʇer, N.G. Determination of parabens in human milk and other food samples by capillary electrophoresis after dispersive liquid-liquid microextraction with back-extraction. Food Chem. 2015, 181, 1–8. [Google Scholar] [CrossRef]
- Lu, R.; Gan, W.; Wu, B.H.; Zhang, Z.; Guo, Y.; Wang, H.F. C−H Stretching Vibrations of Methyl, Methylene and Methine Groups at the Vapor/Alcohol (n = 1−8) Interfaces. J. Phys. Chem. B 2005, 109, 14118–14129. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicka, M.; Starczewska, B.; Syperek, I. Micellar extractions with anionic surfactant SDS and the mixture of SDS/OSAS for determination lovastatin in river samples. J. Mol. Liq. 2013, 187, 320–325. [Google Scholar] [CrossRef]
- Wang, S.; Yi, L.; Ye, L.; Cao, J.; Du, L.; Peng, L.; Xu, J.; Zhang, Q. Microwave-assisted micellar extraction of organic and inorganic iodines using zwitterionic surfactants. J. Chromatogr. A 2017, 1509, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, X.; Wang, X.; Shen, J.; Ding, S. Determination of neonicotinoid insecticides residues in bovine tissues by pressurized solvent extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, K.P.; Bernal, J.L.; Nozal, M.J.; Martín, M.T.; Bernal, J. Determination of seven neonicotinoid insecticides in beeswax by liquid chromatography coupled to electrospray-mass spectrometry using a fused-core column. J. Chromatogr. A 2013, 1285, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Dankyi, E.; Gordon, C.; Carboo, D.; Fomsgaard, I.S. Quantification of neonicotinoid insecticide residues in soils from cocoa plantations using a QuEChERS extraction procedure and LC-MS/MS. Sci. Total Environ. 2014, 499, 276–283. [Google Scholar] [CrossRef]
- Sánchez-Hernández, L.; Hernández-Domínguez, D.; Bernal, J.; Neusüß, C.; Martín, M.T.; Bernal, J.L. Capillary electrophoresis-mass spectrometry as a new approach to analyze neonicotinoid insecticides. J. Chromatogr. A 2014, 1359, 317–324. [Google Scholar] [CrossRef]
- Pavle, Q.; Lazic, S.; Franko, M.; Sakac, M. Development of HPLC-DAD method for determination of neonicotinoids in honey. J. Food Compos. Anal. 2015, 40, 106–113. [Google Scholar]
- Breja, A.M.; Morar, F. Multiresidue analysis of 70 pesticides from soil by gas chromatography-time-of-flight mass spectrometry GC TOF-MS. In The 6th Edition of the Interdisciplinarity in Engineering International Conference; “Petru Maior” University of Tîrgu Mureş: Târgu Mureș, Romania, 2012; pp. 167–172. [Google Scholar]
- Arnnok, P.; Patdhanagul, N.; Burakham, R. Dispersive solid-phase extraction using polyaniline-modified zeolite NaY as a new sorbent for multiresidue analysis of pesticides in food and environmental samples. Talanta 2017, 164, 651–661. [Google Scholar] [CrossRef]
- Hu, X.Z.; Wang, J.X.; Feng, Y.Q. Determination of benzimidazole residues in edible animal food by polymer monolith microextraction combined with liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2010, 58, 112–119. [Google Scholar] [CrossRef]
- Vichapong, J.; Santaladchaiyakit, Y.; Burakham, R.; Srijaranai, S. Determination of Benzimidazole Anthelminthics in Eggs by Advanced Microextraction with High-Performance Liquid Chromatography. Anal. Lett. 2015, 48, 617–631. [Google Scholar] [CrossRef]
- Domínguez-álvarez, J.; Mateos-Vivas, M.; García-Gómez, D.; Rodríguez-Gonzalo, E.; Carabias-Martínez, R. Capillary electrophoresis coupled to mass spectrometry for the determination of anthelmintic benzimidazoles in eggs using a QuEChERS with preconcentration as sample treatment. J. Chromatogr. A 2013, 1278, 166–174. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not from the authors. |

| Analyte | Linear Range (µg·mL−1) | Linear Equation | r2 | LOD (µg mL−1) | LOQ (µg mL−1) | RSD (%) | EF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| tR (n = 5 × 3) | Peak Area (n = 5 × 3) | |||||||||
| Intra Day | Inter Day | Intra Day | Inter Day | |||||||
| Thiamethoxam | 0.003–1 | y = 203,253x + 366.71 | 0.9996 | 0.001 | 0.003 | 0.10 | 0.20 | 1.37 | 4.13 | 10 |
| Clothianidin | 0.003–1 | y = (1 × 106)x + 691.91 | 0.9991 | 0.001 | 0.003 | 0.19 | 0.29 | 1.67 | 3.42 | 10 |
| Acetamiprid | 0.009–1 | y = 875,385x − 2215.8 | 0.9989 | 0.003 | 0.009 | 0.18 | 0.21 | 1.07 | 3.88 | 30 |
| Thiacloprid | 0.009–1 | y = (2 × 106)x − 5337 | 0.9994 | 0.003 | 0.009 | 0.32 | 0.46 | 1.63 | 4.57 | 30 |
| Method *. | Linear Range | Extraction Solvents | Solvent Usage | Extraction Time | LOD | % Recovery | Ref. |
|---|---|---|---|---|---|---|---|
| DLLME-LC-MS/MS | 1.5–100.0 μg·kg−1 | acetonitrile/dichloromethane | 2.5 mL | 20 min | 0.5–1.0 μg·kg−1 | 74.3–113.9 | [28] |
| PSE-LC-MS/MS | - | MeOH | 5 mL | 20 min | 0.8–1.5 μg·kg−1 | 83.2–101.9 | [35] |
| LLE-LC-ESI-MS | 2.0–1000 μg·kg−1 | n-hexane/isopropanol (8:2%v/v) | 15 mL | 18 min | 0.6–2.3 μg·kg−1 | 85–105 | [36] |
| QuEChERS-LC-MS/MS | 1.56–400 μg·L−1 | 1% acetic acid in acetonitrile | 10 mL | 50 min | 1.0–3.0 μg·kg−1 | 72–104.8 | [37] |
| Capillary-ESI-MS | 4.0–1000 μg·L−1 | n-hexane/isopropanol (8:2%v/v) | 15 mL | 25 min | 2.6–4.7 μg·L−1 | 60–71 | [38] |
| DLLME-HPLC-DAD | 1.5–100 μg·L−1 | acetonitrile/dichloromethane | 2.5 mL | 17 min | 1.5–2.5 μg·L−1 | 73.1–118.3 | [39] |
| Hydrophobic DES-DLLME-HPLC-UV | 3–1000 μg·L−1 | hydrophobic DES | 600 μL | 10.3 min | 1–3 μg·L−1 | 60–114 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachangoon, R.; Vichapong, J.; Santaladchaiyakit, Y.; Burakham, R.; Srijaranai, S. An Eco-Friendly Hydrophobic Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction for the Determination of Neonicotinoid Insecticide Residues in Water, Soil and Egg Yolk Samples. Molecules 2020, 25, 2785. https://doi.org/10.3390/molecules25122785
Kachangoon R, Vichapong J, Santaladchaiyakit Y, Burakham R, Srijaranai S. An Eco-Friendly Hydrophobic Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction for the Determination of Neonicotinoid Insecticide Residues in Water, Soil and Egg Yolk Samples. Molecules. 2020; 25(12):2785. https://doi.org/10.3390/molecules25122785
Chicago/Turabian StyleKachangoon, Rawikan, Jitlada Vichapong, Yanawath Santaladchaiyakit, Rodjana Burakham, and Supalax Srijaranai. 2020. "An Eco-Friendly Hydrophobic Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction for the Determination of Neonicotinoid Insecticide Residues in Water, Soil and Egg Yolk Samples" Molecules 25, no. 12: 2785. https://doi.org/10.3390/molecules25122785
APA StyleKachangoon, R., Vichapong, J., Santaladchaiyakit, Y., Burakham, R., & Srijaranai, S. (2020). An Eco-Friendly Hydrophobic Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction for the Determination of Neonicotinoid Insecticide Residues in Water, Soil and Egg Yolk Samples. Molecules, 25(12), 2785. https://doi.org/10.3390/molecules25122785