Immobilization of Naringinase from Aspergillus Niger on a Magnetic Polysaccharide Carrier
Abstract
1. Introduction
2. Results and Discussion
2.1. Immobilization of Naringinase by Adsorption on Magnetic Carrier
2.2. Desorption
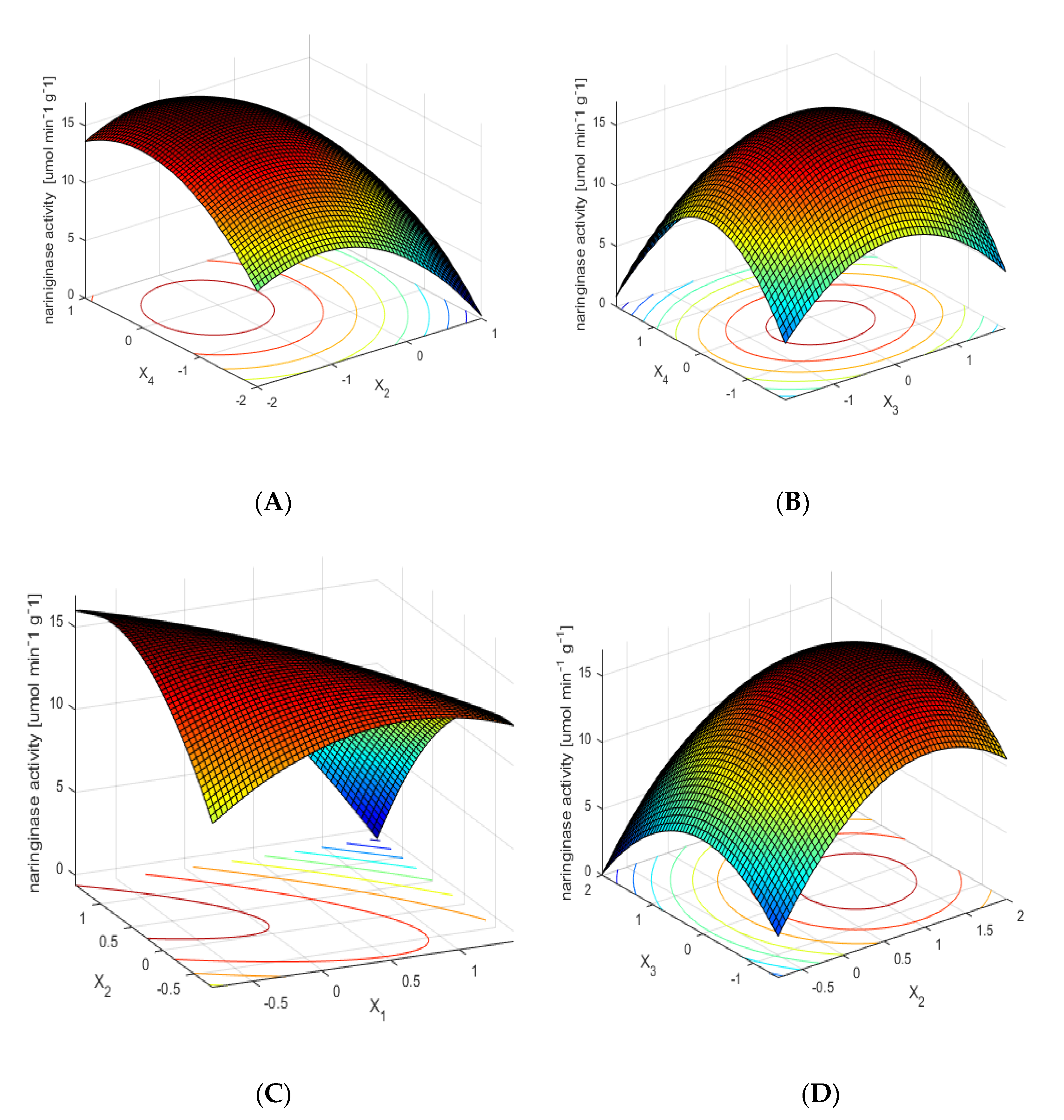
2.3. Optimization of the Process of Naringinase Immobilization on a Polysaccharide Magnetic Carrier
- f—activity of immobilized naringinase (µmol min−1 g−1 of the carrier);
- x1—pH (nondimensional values);
- x2—immobilization time (nondimensional values);
- x3—concentration of naringinase preparation (nondimensional values);
- x4—temperature (nondimensional values).
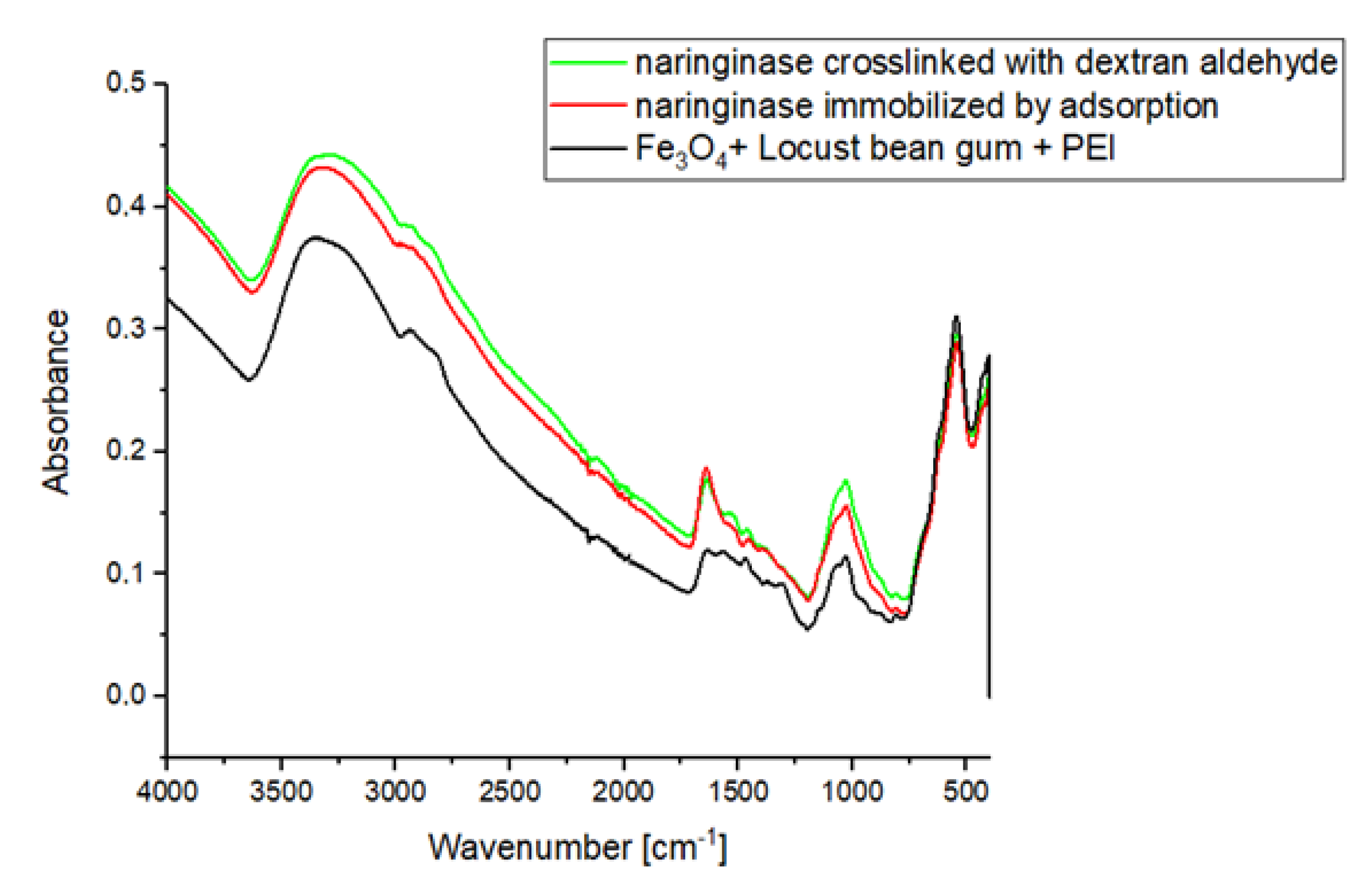
2.4. Crosslinking of Bound Naringinase with Dextran Aldehyde
2.5. Characteristics of Naringinase from A. Niger KMS
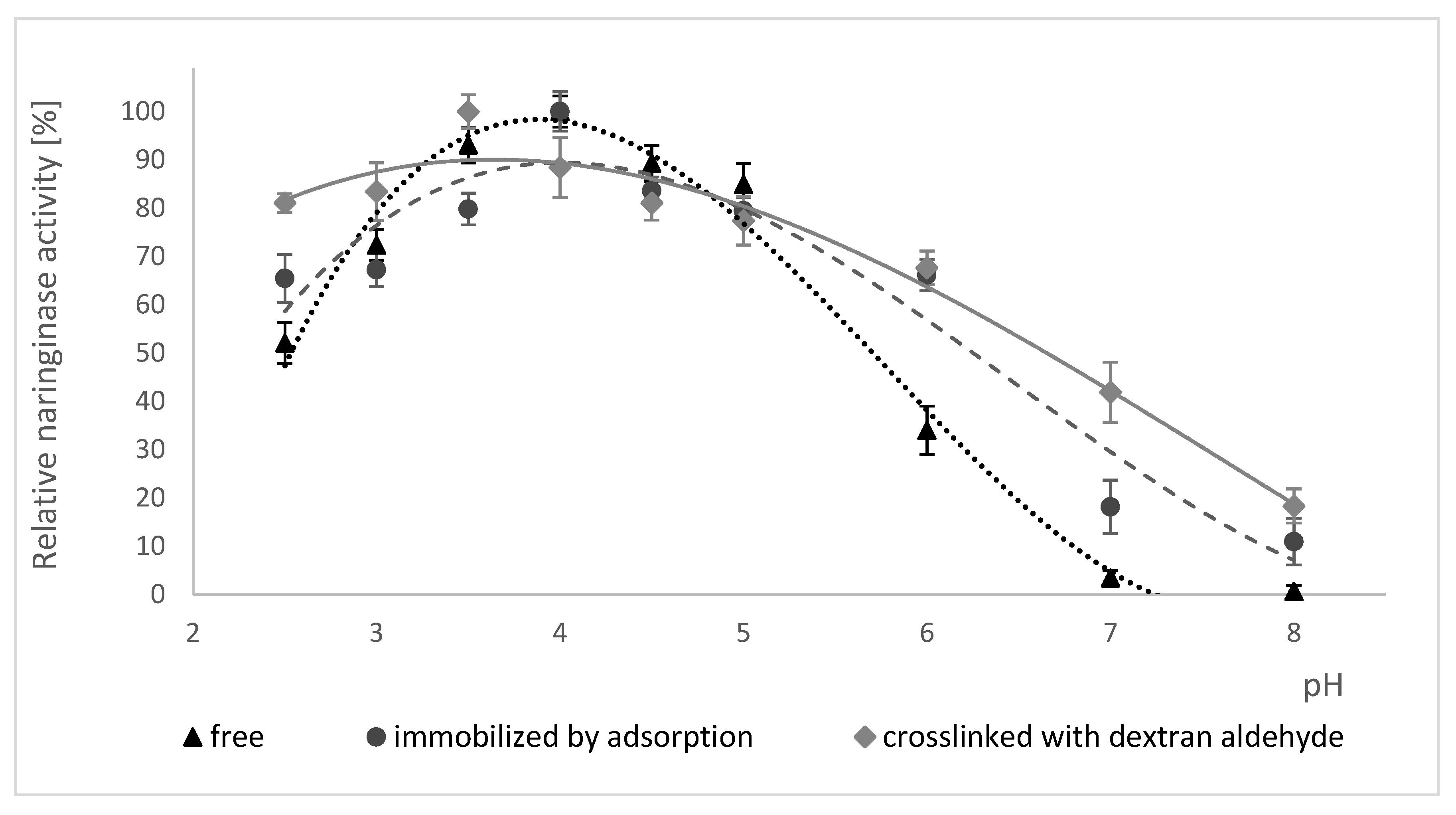
2.5.1. Effect of pH on Naringinase Activity
2.5.2. Effect of Incubation in Buffers with Different pH on the Activity of Free, Adsorption-Immobilized and Crosslinked Naringinase
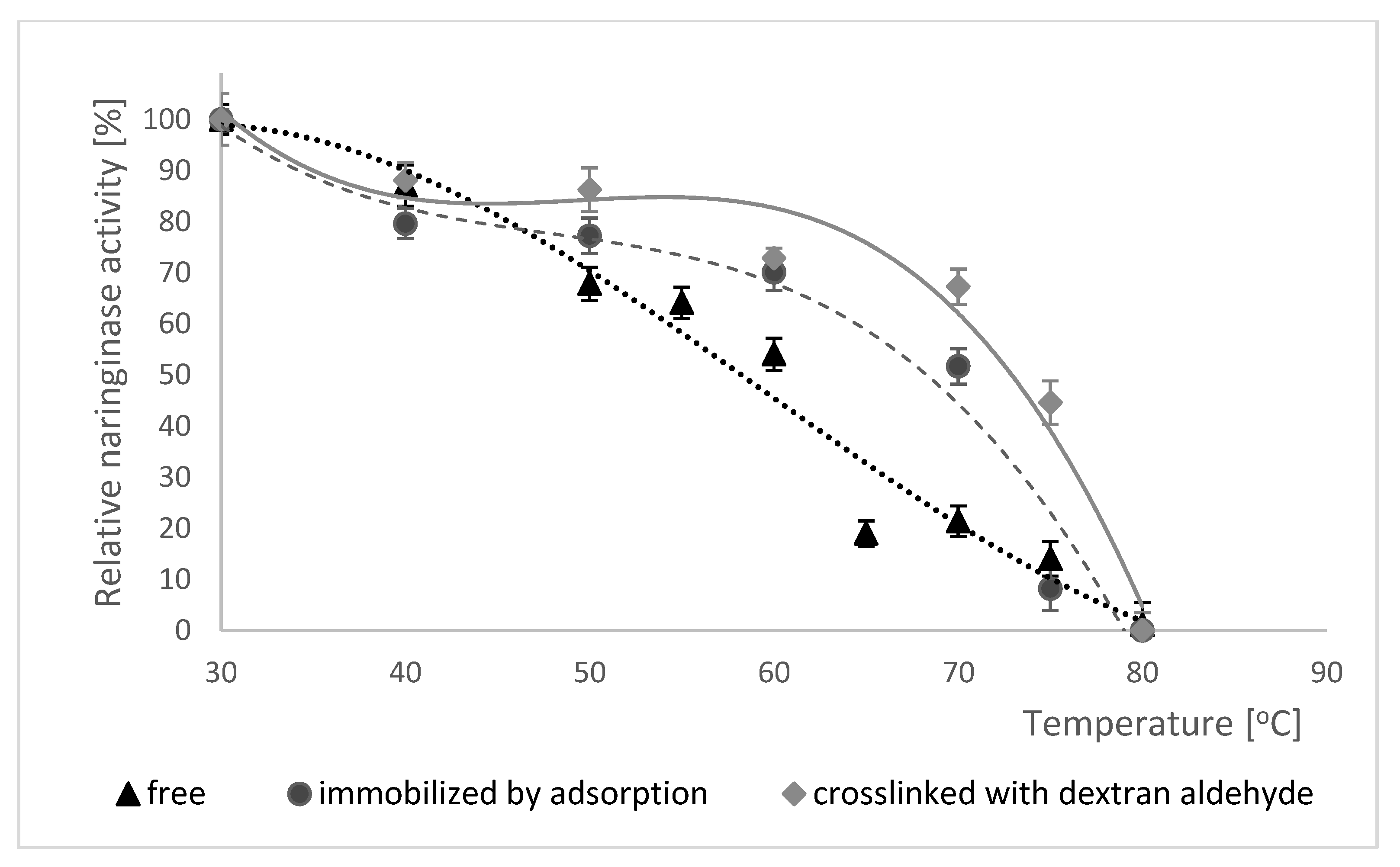
2.5.3. Effect of Temperature on Naringinase Activity
2.5.4. Thermal Stability of Naringinase
2.6. Determination of Activation and Deactivation Energy Values and the Naringinase Half-Life
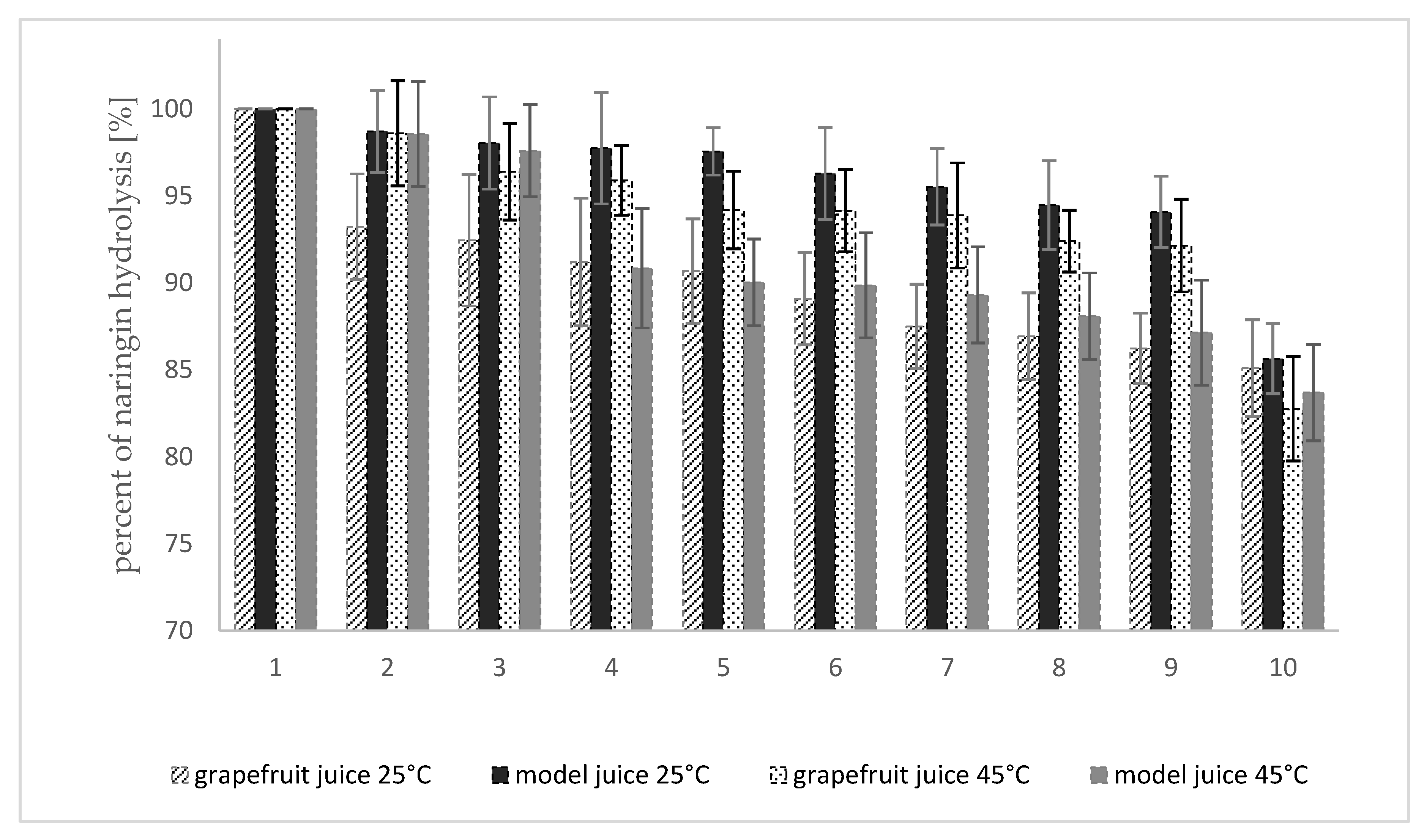
2.7. Operational Stability of Naringinase Crosslinked with Dextran Aldehyde
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.2.1. Determination of Free Naringinase Activity
3.2.2. Determination of Activity of Immobilized Naringinase and Naringinase Crosslinked with Dextran Aldehyde
3.2.3. Determination of Naringin Content in Juice
3.2.4. Determination of Protein Concentration
3.3. Immobilization of Naringinase
3.3.1. Receiving the Magnetic Carriers
3.3.2. Carrier Activation
3.3.3. Measurement of Carrier Particle Size
3.3.4. Infrared Spectroscopy
3.3.5. Immobilization of Naringinase on the Polysaccharide Magnetic Carrier
3.3.6. Calculation of Efficiency of the Naringinase Immobilization Process
- Xa—the difference between the total activity of the enzyme used for immobilization and the activity of the immobilized enzyme;
- Xa0—the total activity of the enzyme used for immobilization.
- Xb—the difference between the total amount of protein used for immobilization and the amount of protein present in the filtrate after washing the immobilized enzyme;
- Xb0—the amount of protein used for immobilization.
3.3.7. Desorption of the Enzyme from the Surface of the Carrier and its Reimmobilization
3.3.8. Optimization of the Naringinase Immobilization Process
3.3.9. Crosslinking Bound Naringinase with Dextran Aldehyde
3.4. Characteristics of a Free, Immobilized and Crosslinked Biocatalyst
3.4.1. Effect of pH on Naringinase Activity
3.4.2. Effect of Incubation in Buffers with Different pH on Naringinase Activity
3.4.3. Effect of Temperature on Naringinase Activity
3.4.4. Thermal Stability of Naringinase
3.5. Determination of Activation and Deactivation Energy and Naringinase Half-Life
- ν—rate of enzymatic reaction (μmol·min−1·g−1);
- k0—constant (min−1);
- E—enzyme concentration (μmol·g−1);
- Ea—activation energy (J·mol−1);
- R—gas constant (J·mol−1·K−1);
- T—temperature (K).
- ν—rate of enzymatic reaction (μmol·h−1·g−1);
- k—kinetic constant (h−1);
- E0—initial enzyme concentration (μmol·g−1);
- Kd—deactivation constant (h−1);
- t—time (h).
- Kd—deactivation constant (h−1);
- Kd0—constant (h−1);
- Ed—activation energy of the thermal deactivation process (J·mol−1);
- R—gas constant (J·mol−1·K−1);
- T—temperature (K).
- τ—half-life (h);
- Kd—deactivation constant (h−1).
3.6. Operational Stability of Crosslinked Naringinase
3.7. Statistical Compilation of Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribeiro, M.H. Naringinases: Occurrence, characteristics, and applications. Appl. Microbiol. Biotechnol. 2011, 90, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.; Wray, V.; Just, U.; Hiller, K. Enzymatic hydrolysis of the cytotoxic triterpenoid glycoside virgaureasaponin 1. Phytochemistry 1998, 49, 153–156. [Google Scholar] [CrossRef]
- Pogorzelski, E.; Wilkowska, A. Flavour enhancement through the enzymatic hydrolysis of glycosidic aroma precursors in juices and wine beverages: A review. Flavour Fragr. J. 2007, 22, 206–213. [Google Scholar] [CrossRef]
- Da Silva, C.M.G.; Contesini, F.J.; Sawaya, A.C.H.F.; Cabral, E.C.; Da Silva Cunha, I.B.; Eberlin, M.N.; De Oliveira Carvalho, P. Enhancement of the antioxidant activity of orange and lime juices by flavonoid enzymatic de-glycosylation. Food Res. Int. 2013, 52, 308–314. [Google Scholar] [CrossRef]
- Sadłek, J.; Sadłek, W. Biodostępna kompozycja flawonoidów zawierających ramnozę oraz jej zastosowanie. PL patent (11) 229133, 15 June 2015. [Google Scholar]
- Zhu, Y.; Jia, H.; Xi, M.; Li, J.; Yang, L.; Li, X. Characterization of a naringinase from Aspergillus oryzae 11250 and its application in the debitterization of orange juice. Process Biochem. 2017, 62, 114–121. [Google Scholar] [CrossRef]
- Li, L.J.; Wu, Z.Y.; Yu, Y.; Zhang, L.J.; Zhu, Y.B.; Ni, H.; Chen, F. Development and characterization of an α-L-rhamnosidase mutant with improved thermostability and a higher efficiency for debittering orange juice. Food Chem. 2018, 245, 1070–1078. [Google Scholar] [CrossRef]
- Puri, M.; Marwaha, S.S.; Kothari, R.M.; Kennedy, J.F. Biochemical Basis of Bitterness in Citrus Fruit Juices and Biotech Approaches for Debittering. Crit. Rev. Biotechnol. 1996, 16, 145–155. [Google Scholar] [CrossRef]
- Jiang, D.S.; Long, S.Y.; Huang, J.; Xiao, H.Y.; Zhou, J.Y. Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 2005, 25, 15–23. [Google Scholar] [CrossRef]
- Magario, I.; Ma, X.; Neumann, A.; Syldatk, C.; Hausmann, R. Non-porous magnetic micro-particles: Comparison to porous enzyme carriers for a diffusion rate-controlled enzymatic conversion. J. Biotechnol. 2008, 134, 72–78. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, C.; Xia, H.; Mahmood, I.; Liu, C.; Liu, H. Magnetic nanoparticles supported ionic liquids for lipase immobilization: Enzyme activity in catalyzing esterification. J. Mol. Catal. B Enzym. 2009, 58, 103–109. [Google Scholar] [CrossRef]
- Jordan, J.; Kumar, C.S.S.R.; Theegala, C. Preparation and characterization of cellulase-bound magnetite nanoparticles. J. Mol. Catal. B Enzym. 2011, 68, 139–146. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Bordbar, A.K.; Zare, D.; Davoodi, D.; Noruzi, M.; Barkhi, M.; Tabatabaei, M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011, 171, 669–673. [Google Scholar] [CrossRef]
- Cao, M.; Li, Z.; Wang, J.; Ge, W.; Yue, T.; Li, R.; Colvin, V.L.; Yu, W.W. Food related applications of magnetic iron oxide nanoparticles: Enzyme immobilization, protein purification, and food analysis. Trends Food Sci. Technol. 2012, 27, 47–56. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Ellenrieder, G.; Oliveira, G.B.; Cabrera, M.; Carvalho, L.B. α-L-Rhamnosidase of Aspergillus terreus immobilized on ferromagnetic supports. Appl. Microbiol. Biotechnol. 2012, 93, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Bolibok, P.; Gembala, J.; Wujak, M.; Roszek, K.; Terzyk, A.; Wiśniewski, M. Immobilizacja enzymów na nośnikach sposobem na ukierunkowaną modyfikację właściwości biokatalizatorów. Przem. Chem. 2016, 11, 2254–2258. [Google Scholar]
- Synowiecki, J.; Wołosowska, S. Otrzymywanie i niektóre zastosowania unieruchomionych enzymów. Biotechnologia 2007, 2, 7–26. [Google Scholar]
- Tarnowska, K.; Gryczyńska, E.; Kowalski, B. Immobilizacja kowalencyjna lipaz. Postępy Tech. Przetwórstwa Spożywczego 2008, 1, 72–78. [Google Scholar]
- Tsen, H.-Y.; Tsai, S.-Y. Comparison of the kinetics and factors affecting the stabilities of chitin-immobilized naringinases from two fungal sources. J. Ferment. Technol. 1998, 66, 193–198. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of Naringinase from Penicillium decumbens on Chitosan Microspheres for Debittering Grapefruit Juice. Molecules 2019, 24, 4234. [Google Scholar] [CrossRef]
- Puri, M.; Marwaha, S.; Kothari, R. Studies on the applicability of alginate-entrapped naringiase for the debittering of kinnow juice. Enzyme Microb. Technol. 1996, 18, 281–285. [Google Scholar] [CrossRef]
- Norouzian, D.; Hosscinzadeh, A.; Inanlou, D.N.; Moazami, N. Various techniques used to immobilize naringinase produced by Penicillium decombens PTCC 5248. World, J. Microbiol. Biotechnol. 1999, 15, 501–502. [Google Scholar] [CrossRef]
- Mishra, P.; Kar, R. Treatment of Grapefruit Juice for Bitterness Removal by Amberlite IR 120 and Amberlite IR 400 and Alginate Entrapped Naringinase Enzyme. J. Food Sci. 2003, 68, 1229–1233. [Google Scholar] [CrossRef]
- Pedro, H.A.L.; Alfaia, A.J.; Marques, J.; Vila-Real, H.J.; Calado, A.; Ribeiro, M.H.L. Design of an immobilized enzyme system for naringin hydrolysis at high-pressure. Enzyme Microb. Technol. 2007, 40, 442–446. [Google Scholar] [CrossRef]
- Ferreira, L.; Afonso, C.; Vila-real, H.; Alfaia, A. Evaluation of the Effect of High Pressure on Naringin Hydrolysis in Grapefruit Juice with Naringinase Immobilised in Calcium Alginate Beads. Food Technol. Biotechnol. 2008, 46, 146–150. [Google Scholar]
- Ribeiro, M.H.L.; Afonso, C.; Vila-Real, H.J.; Alfaia, A.J.; Ferreira, L. Contribution of response surface methodology to the modeling of naringin hydrolysis by naringinase Ca-alginate beads under high pressure. LWT Food Sci. Technol. 2010, 43, 482–487. [Google Scholar] [CrossRef]
- Ribeiro, I.A.C.; Ribeiro, M.H.L. Kinetic modelling of naringin hydrolysis using a bitter sweet alfa-rhamnopyranosidase immobilized in k-carrageenan. J. Mol. Catal. B Enzym. 2008, 51, 10–18. [Google Scholar] [CrossRef]
- Esmaeilnejad-Ahranjani, P.; Kazemeini, M.; Singh, G.; Arpanaei, A. Amine-functionalized magnetic nanocomposite particles for efficient immobilization of lipase: Effects of functional molecule size on properties of the immobilized lipase. RSC Adv. 2015, 5, 33313–33327. [Google Scholar] [CrossRef]
- Rajdeo, K.; Harini, T.; Lavanya, K.; Fadnavis, N.W. Immobilization of pectinase on reusable polymer support for clarification of apple juice. Food Bioprod. Process. 2016, 99, 12–19. [Google Scholar] [CrossRef]
- Roitner, M.; Schalkhammer, T.; Pittner, F. Preparation of prunin with the help of immobilized naringinase pretreated with alkaline buffer. Appl. Biochem. Biotechnol. 1984, 9, 483–488. [Google Scholar] [CrossRef]
- Puri, M.; Seth, M.; Marwaha, S.S.; Kothari, R.M. Debittering of Kinnow mandarin juice by covalently bound naringinase on hen egg white. Food Biotechnol. 2001, 15, 13–23. [Google Scholar] [CrossRef]
- Puri, M.; Kaur, H.; Kennedy, J.F. Covalent immobilization of naringinase for the transformation of a flavonoid. J. Chem. Technol. Biotechnol. 2005, 80, 1160–1165. [Google Scholar] [CrossRef]
- Inês Amaro, M.; Rocha, J.; Vila-Real, H.; Eduardo-Figueira, M.; Mota-Filipe, H.; Sepodes, B.; Ribeiro, M.H. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res. Int. 2009, 42, 1010–1017. [Google Scholar] [CrossRef]
- Lei, S.; Xu, Y.; Fan, G.; Xiao, M.; Pan, S. Immobilization of naringinase on mesoporous molecular sieve MCM-41 and its application to debittering of white grapefruit. Appl. Surf. Sci. 2011, 257, 4096–4099. [Google Scholar] [CrossRef]
- Orrego, A.H.; Ghobadi, R.; Moreno-Perez, S.; Mendoza, A.J.; Fernandez-Lorente, G.; Guisan, J.M.; Rocha-Martin, J. Stabilization of immobilized lipases by intense intramolecular cross-linking of their surfaces by using aldehyde-dextran polymers. Int. J. Mol. Sci. 2018, 19, 553. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; You, H.; Wu, C.; Cai, H. Immobilization and characterization of naringinase from Aspergillus aculeatus onto magnetic Fe3O4 nanoparticles. Nanosci. Nanotechnol. Lett. 2015, 7, 770–778. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Mikani, M. Kinetic and thermodynamic features of nanomagnetic cross-linked enzyme aggregates of naringinase nanobiocatalyst in naringin hydrolysis. Int. J. Biol. Macromol. 2018, 119, 717–725. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Mikani, M. Nano-magnetic cross-linked enzyme aggregates of naringinase an efficient nanobiocatalyst for naringin hydrolysis. Int. J. Biol. Macromol. 2018, 117, 134–143. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J. Badania nad otrzymywaniem i unieruchamianiem naringinazy z Aspergillus niger. Ph.D. Thesis, Uniwersytet Ekonomiczny we Wrocławiu, Wrocław, Poland, 2019. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Silva, M.J.A.; Klein, M.; Feddern, V.; Feltes, M.M.C.; Oliveira, J.V.; Ninow, J.L.; De Oliveira, D. Current status and trends in enzymatic nanoimmobilization. J. Mol. Catal. B Enzym. 2013, 99, 56–67. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of iron magnetic nanoparticles in protein immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Pessela, B.C.C.; Fuentes, M.; Mateo, C.; Munilla, R.; Fernandez-Lafuente, R.; Guisán, J.M. Supports coated with PEI as a new tool in chromatography. Enzyme Microb. Technol. 2006, 39, 711–716. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Liu, H.; Liu, C. Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J. Chem. Technol. Biotechnol. 2008, 104, 97–104. [Google Scholar] [CrossRef]
- Vieira, M.F.; Vieira, A.M.S.; Zanin, G.M.; Tardioli, P.W.; Mateo, C.; Guisán, J.M. β-Glucosidase immobilized and stabilized on agarose matrix functionalized with distinct reactive groups. J. Mol. Catal. B Enzym. 2011, 69, 47–53. [Google Scholar] [CrossRef]
- Luo, J.; Li, Q.; Sun, X.; Tian, J.; Fei, X.; Shi, F.; Zhang, N.; Liu, X. The study of the characteristics and hydrolysis properties of naringinase immobilized by porous silica material. RSC Adv. 2019, 9, 4514–4520. [Google Scholar] [CrossRef]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual salt and pH induced changes in polyethylenimine solutions. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef]
- Busto, M.D.; Meza, V.; Ortega, N.; Perez-Mateos, M. Immobilization of naringinase from Aspergillus niger CECT 2088 in poly(vinyl alcohol) cryogels for the debittering of juices. Food Chem. 2007, 104, 1177–1182. [Google Scholar] [CrossRef]
- Vila-Real, H.; Alfaia, A.J.; Rosa, M.E.; Calado, A.R.; Ribeiro, M.H.L. An innovative sol-gel naringinase bioencapsulation process for glycosides hydrolysis. Process Biochem. 2010, 45, 841–850. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Wagner, H.; Rapp, B.E.; Franzreb, M. Optimization of enzyme immobilization on magnetic microparticles using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) as a crosslinking agent. Anal. Methods 2015, 7, 10291–10298. [Google Scholar] [CrossRef]
- Martino, A.; Durante, M.; Pifferi, P.G.; Spagna, G.; Bianchi, G. Immobilization of β-glucosidase from a commercial preparation. Part 1. A comparative study of natural supports. Process Biochem. 2002, 31, 281–285. [Google Scholar] [CrossRef]
- Huo, Y.; Li, Y.; Yuan, Z.; Huang, J. Immobilization of glucoamylase onto novel porous polymer supports of vinylene carbonate and 2-hydroxyethyl methacrylate. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2004, 119, 121–131. [Google Scholar] [CrossRef]
- Sugahara, V.H.; Varéa, G.D.S. Immobilization of Beauveria bassiana lipase on silica gel by physical adsorption. Brazilian Arch. Biol. Technol. 2014, 57, 842–850. [Google Scholar] [CrossRef]
- De Araújo, J.H.B.; Uemura, V.O.; De Moraes, F.F.; De Barbosa, A.M.; Zanin, G.M. A comparative study on fungal laccases immobilized on chitosan. Brazilian Arch. Biol. Technol. 2005, 48, 1–6. [Google Scholar]
- Vila-Real, H.; Alfaia, A.J.; Rosa, J.N.; Gois, P.M.P.; Rosa, M.E.; Calado, A.R.T.; Ribeiro, M.H. α-Rhamnosidase and β-glucosidase expressed by naringinase immobilized on new ionic liquid sol-gel matrices: Activity and stability studies. J. Biotechnol. 2011, 152, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Ortiz, C.; Rueda, N.; Berenguer-Murcia, Á.; Acosta, N.; Aranaz, I.; Civera, C.; Fernandez-Lafuente, R.; Alcántara, A.R. Dextran aldehyde in biocatalysis: More than a mere immobilization system. Catalysts 2019, 9, 622. [Google Scholar] [CrossRef]
- Rocchietti, S.; Ubiali, D.; Terreni, M.; Albertini, A.M.; Fernández-Lafuente, R.; Guisán, J.M.; Pregnolato, M. Immobilization and stabilization of recombinant multimeric uridine and purine nucleoside phosphorylases from Bacillus subtilis. Biomacromolecules 2004, 5, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Serra, C.D.; Rocchietti, S.; Ubiali, D.; Terreni, M. Stabilization of thymidine phosphorylase from Escherichia coli by immobilization and post immobilization techniques. Enzyme Microb. Technol. 2011, 49, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fresco-Taboada, A.; Serra, I.; Arroyo, M.; Fernández-Lucas, J.; De La Mata, I.; Terreni, M. Development of an immobilized biocatalyst based on Bacillus psychrosaccharolyticus NDT for the preparative synthesis of trifluridine and decytabine. Catal. Today 2016, 259, 197–204. [Google Scholar] [CrossRef]
- Mateo, C.; Grazu, V.; Abian, O.; Fuentes, M.; Fernandez-Lafuente, R.; Palomo, J.M.; Fernandez-Lafuente, G.; Guisan, J.M. Preparation of an Industrial Biocatalyst of Penicillin G Acylase on Sepabeads. In Microbial Enzymes and Biotransformations; Humana Press: Totowa, NJ, USA, 2005; pp. 273–288. [Google Scholar]
- Awad, G.E.A.; Abd El Aty, A.A.; Shehata, A.N.; Hassan, M.E.; Elnashar, M.M. Covalent immobilization of microbial naringinase using novel thermally stable biopolymer for hydrolysis of naringin. 3 Biotech 2016, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Ondul, E.; Dizge, N.; Albayrak, N. Immobilization of Candida antarctica A and Thermomyces lanuginosus lipases on cotton terry cloth fibrils using polyethyleneimine. Colloids Surfaces B Biointerfaces 2012, 95, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nie, Y.; Luo, Y.; Lin, F.; Zheng, Y.; Cheng, G.; Wu, H.; Zhang, K.; Su, W.; Shen, J. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem. Toxicol. 2013, 58, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Manjon, A.; Romojaro, F.; Iborra, J.L. Properties of pectolytic enzymes covalently bound to nylon for apricot juice clarification. Process Biochem. 1988, 23, 75–78. [Google Scholar]
- Puri, M.; Kalra, S. Purification and characterization of naringinase from a newly isolated strain of Aspergillus niger 1344 for the transformation of flavonoids. World J. Microbiol. Biotechnol. 2005, 21, 753–758. [Google Scholar] [CrossRef]
- Ni, H.; Chen, F.; Cai, H.; Xiao, A.; You, Q.; Lu, Y. Characterization and preparation of Aspergillus niger naringinase for debittering citrus juice. J. Food Sci. 2012, 77, C1–C7. [Google Scholar] [CrossRef]
- Igbonekwu, A.; Ko, O.; Al, E.; Soo, E.; Ou, N.; Fc, C. Characterization of Nariginase Obtained from Aspergillus niger by Submerged Fermentation Using Naringin Extracted from Lemon Peels. Res. Mater. Sci. 2018, 4, 1–5. [Google Scholar]
- Shanmugaprakash, M.; Vinothkumar, V.; Ragupathy, J.; Reddy, D.A. Biochemical characterization of three phase partitioned naringinase from Aspergillus brasiliensis MTCC 1344. Int. J. Biol. Macromol. 2015, 80, 418–423. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Lee, Y.-B.; Bae, H.-A.; Huh, J.-Y.; Nam, S.-H.; Sohn, H.-S.; Lee, H.J.; Lee, S.-B. Purification and characterisation of Aspergillus sojae naringinase: The production of prunin exhibiting markedly enhanced solubility with in vitro inhibition of HMG-CoA reductase. Food Chem. 2011, 124, 234–241. [Google Scholar] [CrossRef]
- Elnashar, M.M.M.; Hassan, M.E. Novel Epoxy Activated Hydrogels for Solving Lactose Intolerance. BioMed Researh Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Leonowicz, A.; Sarkar, J.M.; Bollag, J. Improvement instability of an immobilized fungal laccase. Appl. Microbiol. Biotechnol. 1988, 29, 129–135. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Oliveira, M.G.; Maugeri, F. Modelling thermal stability and activity of free and immobilized enzymes as a novel tool for enzyme reactor design. Bioresour. Technol. 2007, 98, 3142–3148. [Google Scholar] [CrossRef]
- Vila-Real, H.; Alfaia, A.J.; Calado, A.R.; Ribeiro, M.H.L. Enzymatic Improvement of activity and stability of soluble and sol—Gel immobilized naringinase in co-solvent systems. J. Mol. Catal. B Enzym. 2010, 65, 91–101. [Google Scholar] [CrossRef]
- Nunes, M.A.P.; Martins, S.; Rosa, M.E.; Gois, P.M.P.; Fernandes, P.C.B.; Ribeiro, M.H.L. Improved thermostable polyvinyl alcohol electrospun nanofibers with entangled naringinase used in a novel mini-packed bed reactor. Bioresour. Technol. 2016, 213, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.P.; Vila-Real, H.; Fernandes, P.C.B.; Ribeiro, M.H.L. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Biochem. Biotechnol. 2010, 160, 2129–2147. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J. Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Davis, W.B. Determination of Flavanones in Citrus Fruits. Anal. Chem. 1947, 19, 476–478. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, L.; Randall, R.J. Protein measurment with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilizacja naringinazy z Penicillium decumbens na magnetycznych polisacharydowych nośnikach. Pr. Nauk. Uniw. Ekon. we Wrocławiu 2019, 524, 9–24. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Desorption Method | Before Desorption | After Desorption | |
|---|---|---|---|
| Activity (µmol·min−1·g−1 of a Carrier) | Activity (µmol·min−1·g−1 of a Carrier) | % of Initial Activity | |
| 10% (w/v) aqueous solution of NaCl | 3.160 ± 0.140 | 0.239 ± 0.015 | 7.6 |
| 4% (v/v) aqueous surfactant solution | 3.160 ± 0.140 | 1.345 ± 0.052 | 42.6 |
| Process Variant | Parameters of the Immobilization Process | Naringinase Activity (µmol·min−1·g−1 of the Carrier) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | ||||||
| pH | Immobilization Time | Concentration of Naringinase Preparation | Temperature | ||||||
| Coded Levels | (–) | Coded Levels | (h) | Coded Levels | (g·100 mL−1) | Coded Levels | (°C) | ||
| 1 | 1 | 8.0 | 1 | 5.0 | 1 | 0.53 | −1 | 25.0 | 5.18 |
| 2 | 1 | 8.0 | 1 | 5.0 | −1 | 0.27 | −1 | 25.0 | 3.27 |
| 3 | 1 | 8.0 | −1 | 3.0 | 1 | 0.53 | 1 | 35.0 | 4.12 |
| 4 | −1 | 6.0 | 1 | 5.0 | −1 | 0.27 | 1 | 35.0 | 11.79 |
| 5 | 1 | 8.0 | −1 | 3.0 | −1 | 0.27 | 1 | 35.0 | 4.25 |
| 6 | −1 | 6.0 | −1 | 3.0 | 1 | 0.53 | −1 | 25.0 | 4.57 |
| 7 | −1 | 6.0 | 1 | 5.0 | 1 | 0.53 | 1 | 35.0 | 10.51 |
| 8 | −1 | 6.0 | −1 | 3.0 | −1 | 0.27 | −1 | 25.0 | 4.95 |
| 9 (C) | 0 | 7.0 | 0 | 4.0 | 0 | 0.40 | 0 | 30.0 | 9.50 |
| 10 | −2 | 5.0 | 0 | 4.0 | 0 | 0.40 | 0 | 30.0 | 10.00 |
| 11 | 2 | 9.0 | 0 | 4.0 | 0 | 0.40 | 0 | 30.0 | 5.78 |
| 12 | 0 | 7.0 | −2 | 2.0 | 0 | 0.40 | 0 | 30.0 | 0.30 |
| 13 | 0 | 7.0 | 2 | 6.0 | 0 | 0.40 | 0 | 30.0 | 7.92 |
| 14 | 0 | 7.0 | 0 | 4.0 | −2 | 0.13 | 0 | 30.0 | 8.41 |
| 15 | 0 | 7.0 | 0 | 4.0 | 2 | 0.67 | 0 | 30.0 | 8.62 |
| 16 | 0 | 7.0 | 0 | 4.0 | 0 | 0.40 | −2 | 20.0 | 10.07 |
| 17 | 0 | 7.0 | 0 | 4.0 | 0 | 0.40 | 2 | 40.0 | 0.15 |
| 18 (C) | 0 | 7.0 | 0 | 4.0 | 0 | 0.40 | 0 | 30.0 | 8.81 |
| Activity (µmol·min−1·g−1 of Carrier) | Mass of Bound Protein (mg 150·mg−1 of Carrier) | Specific Activity (µmol·min−1·mg−1 of Protein) | |
|---|---|---|---|
| Naringinase immobilized by adsorption (IMNA) | Determination before addition of dextran aldehyde | ||
| 17.06 ± 0.20 a | 2.528 ± 0.006 b | 1.012 ± 0.014 c | |
| Immobilized naringinase after crosslinking | Determination after addition of dextran aldehyde | ||
| 17.04 ± 0.16 a | 2.528 ± 0.008 b | 1.011 ± 0.006 c | |
| Type of Enzyme | Activation Energy (Ea) (kJ·mol−1) |
|---|---|
| Free naringinase | 32.5 |
| Naringinase immobilized by adsorption | 28.1 |
| Adsorbed naringinase crosslinked with dextran aldehyde | 28.6 |
| Type of Enzyme | Activation Energy of the Thermal Deactivation Process (Ed) (kJ mol−1) |
|---|---|
| Free naringinase | 83.0 |
| Naringinase immobilized by adsorption Adsorbed naringinase crosslinked with dextran aldehyde | 264.8 265.1 |
| Temperature (°C) | Half-Life Times (h) | |
|---|---|---|
| Type of Enzyme | ||
| Free Naringinase | Naringinase Crosslinked With Dextran Aldehyde | |
| 35 | 47.23 | 68.31 |
| 40 | 24.15 | 63.00 |
| 45 | 16.00 | 55.89 |
| 50 | 4.11 | 45.89 |
| 55 | 1.82 | 34.65 |
| 60 | 1.62 | 8.66 |
| 62 | 1.27 | 4.39 |
| 65 | 1.03 | 1.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of Naringinase from Aspergillus Niger on a Magnetic Polysaccharide Carrier. Molecules 2020, 25, 2731. https://doi.org/10.3390/molecules25122731
Bodakowska-Boczniewicz J, Garncarek Z. Immobilization of Naringinase from Aspergillus Niger on a Magnetic Polysaccharide Carrier. Molecules. 2020; 25(12):2731. https://doi.org/10.3390/molecules25122731
Chicago/Turabian StyleBodakowska-Boczniewicz, Joanna, and Zbigniew Garncarek. 2020. "Immobilization of Naringinase from Aspergillus Niger on a Magnetic Polysaccharide Carrier" Molecules 25, no. 12: 2731. https://doi.org/10.3390/molecules25122731
APA StyleBodakowska-Boczniewicz, J., & Garncarek, Z. (2020). Immobilization of Naringinase from Aspergillus Niger on a Magnetic Polysaccharide Carrier. Molecules, 25(12), 2731. https://doi.org/10.3390/molecules25122731





