An Improved Nanoemulsion Formulation Containing Kojic Monooleate: Optimization, Characterization and In Vitro Studies
Abstract
1. Introduction
2. Results and Discussion
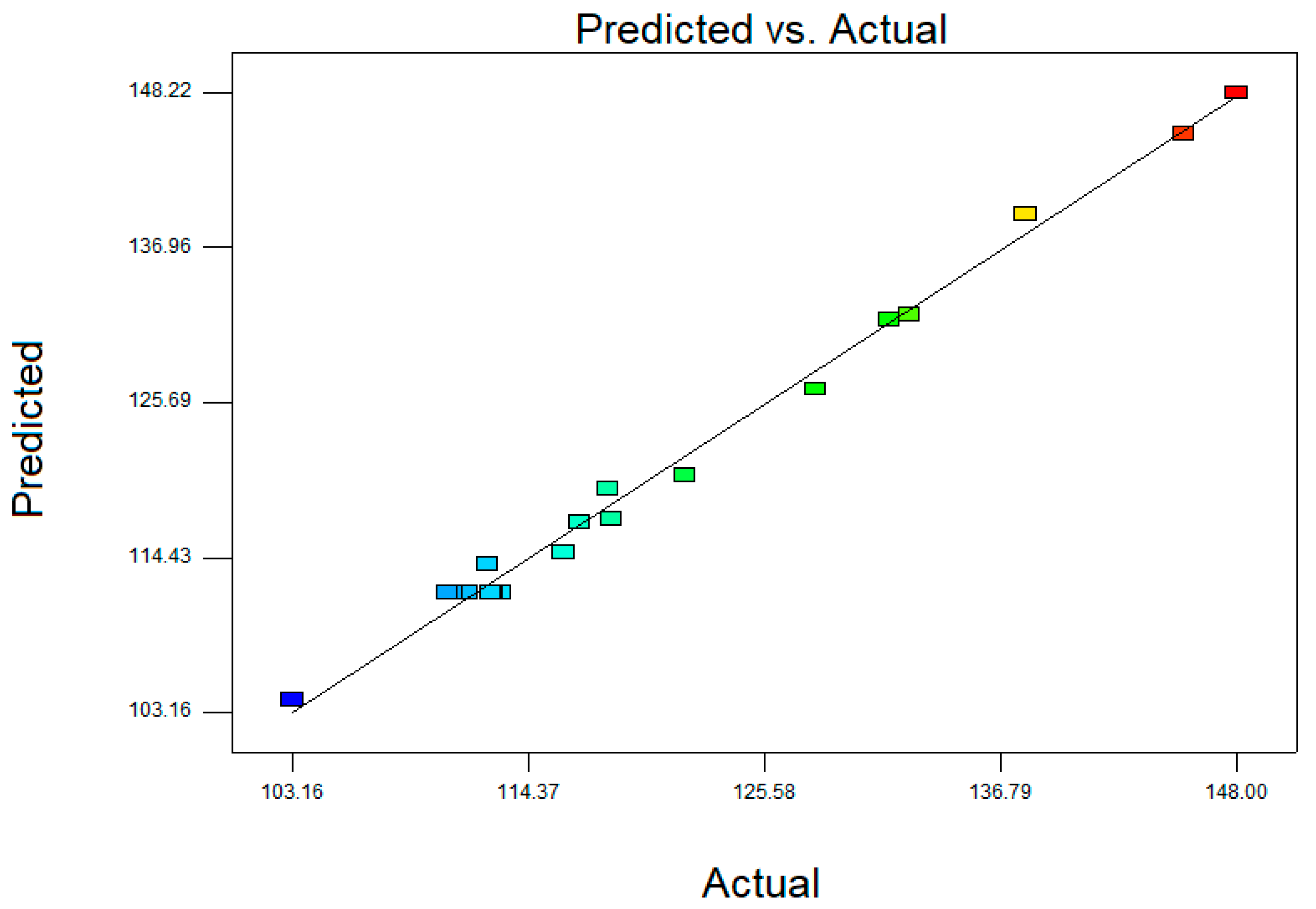
2.1. Fitting the Model
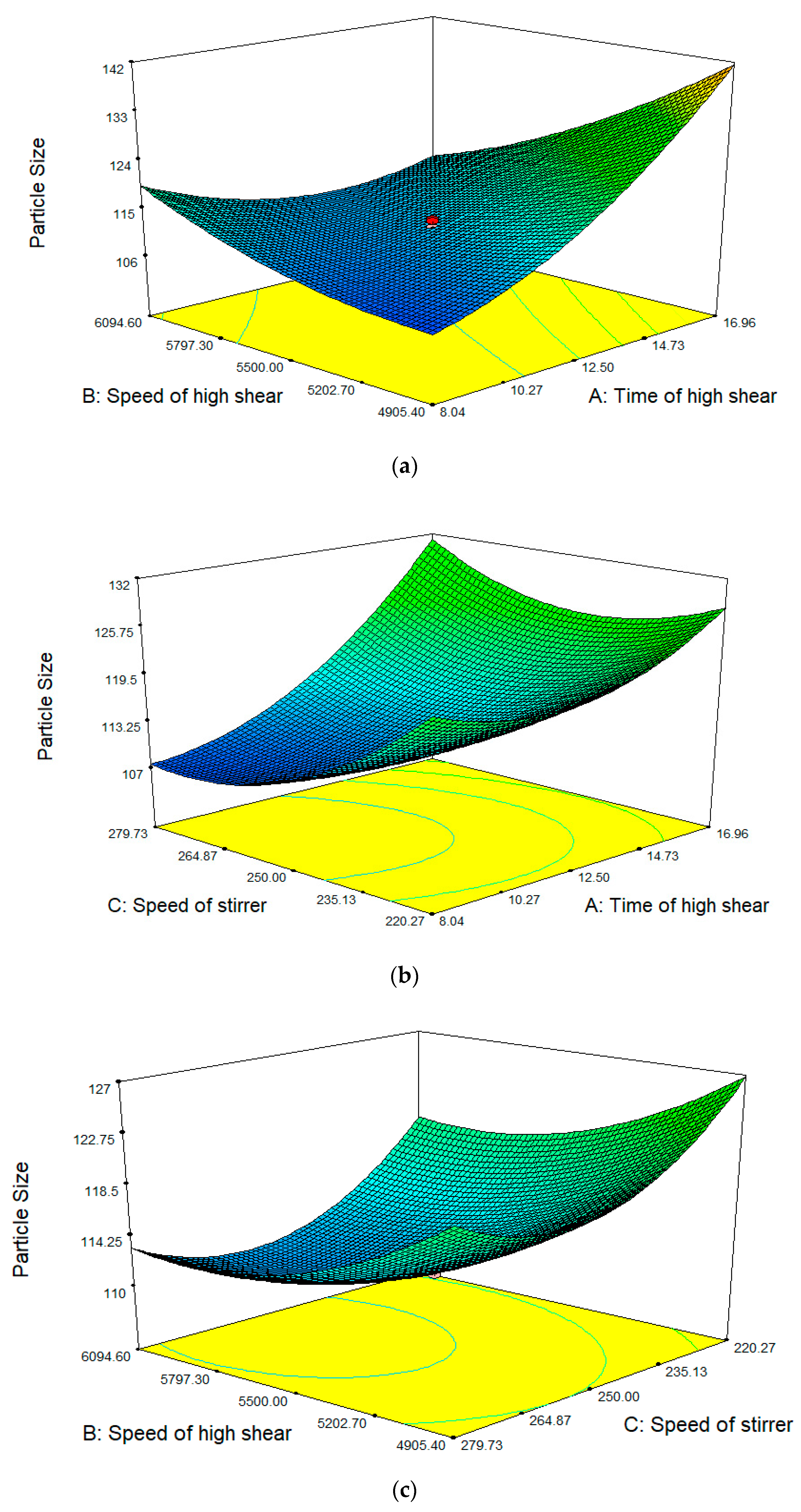
2.2. Response Surface Analysis
2.3. Verification of Model
2.4. Optimization of the Responses
2.5. Physicochemical Characterization of Optimized Nanoemulsion
2.5.1. Mean Droplet Size, Zeta Potential and Polydispersity Index (PDI) Analysis
2.5.2. Morphology
2.5.3. pH and Conductivity Measurement
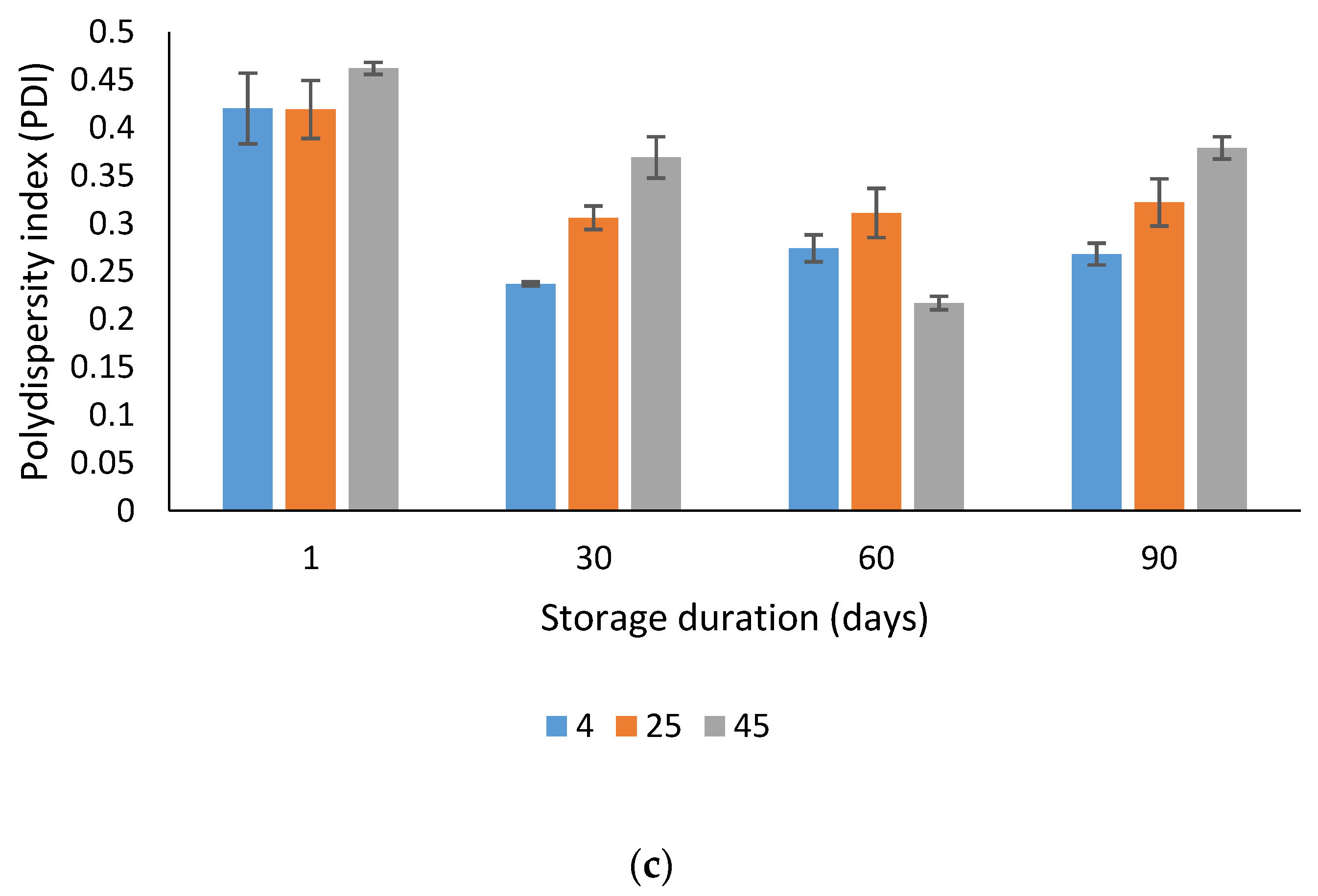
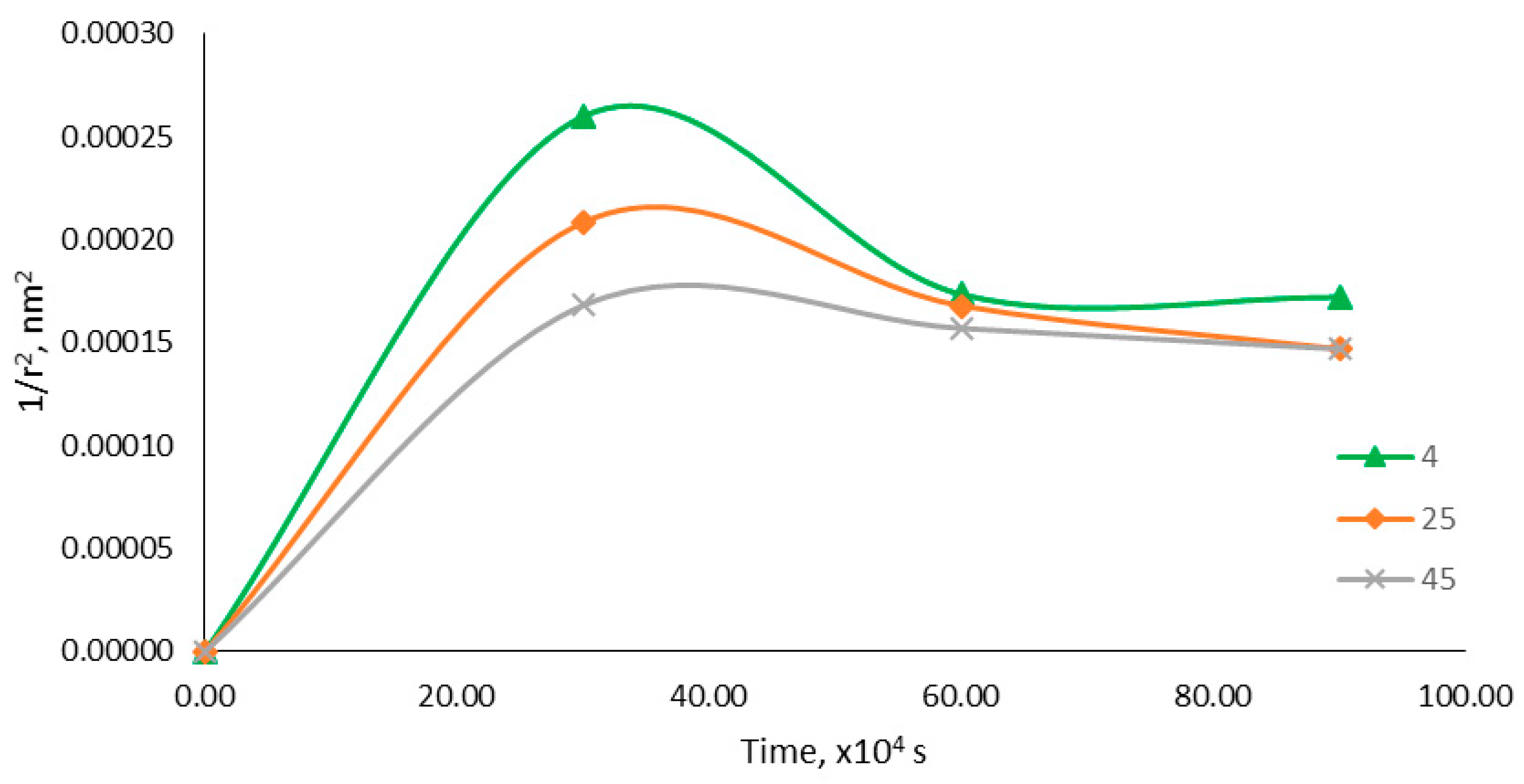
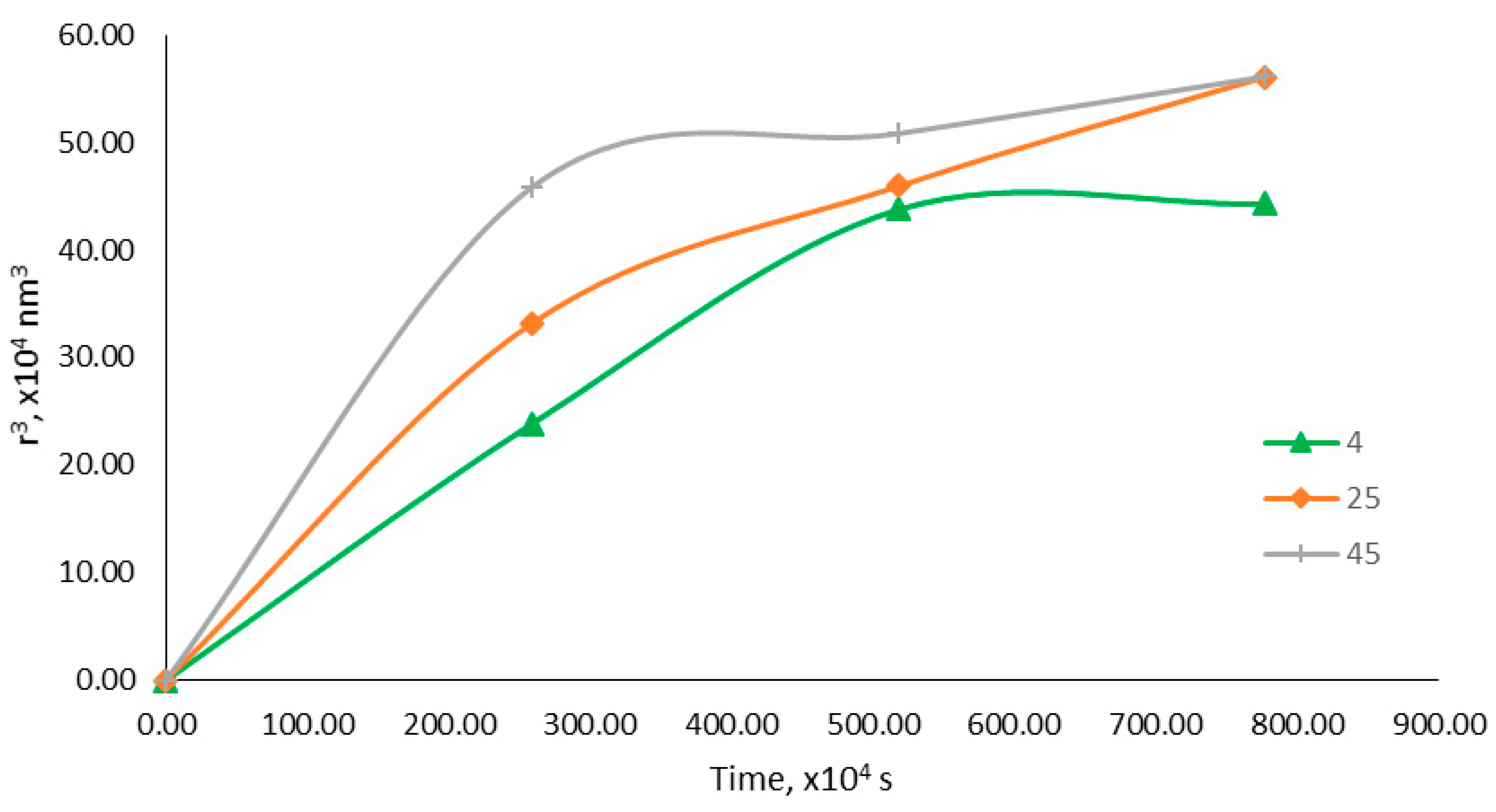
2.5.4. Accelerated Stability Study
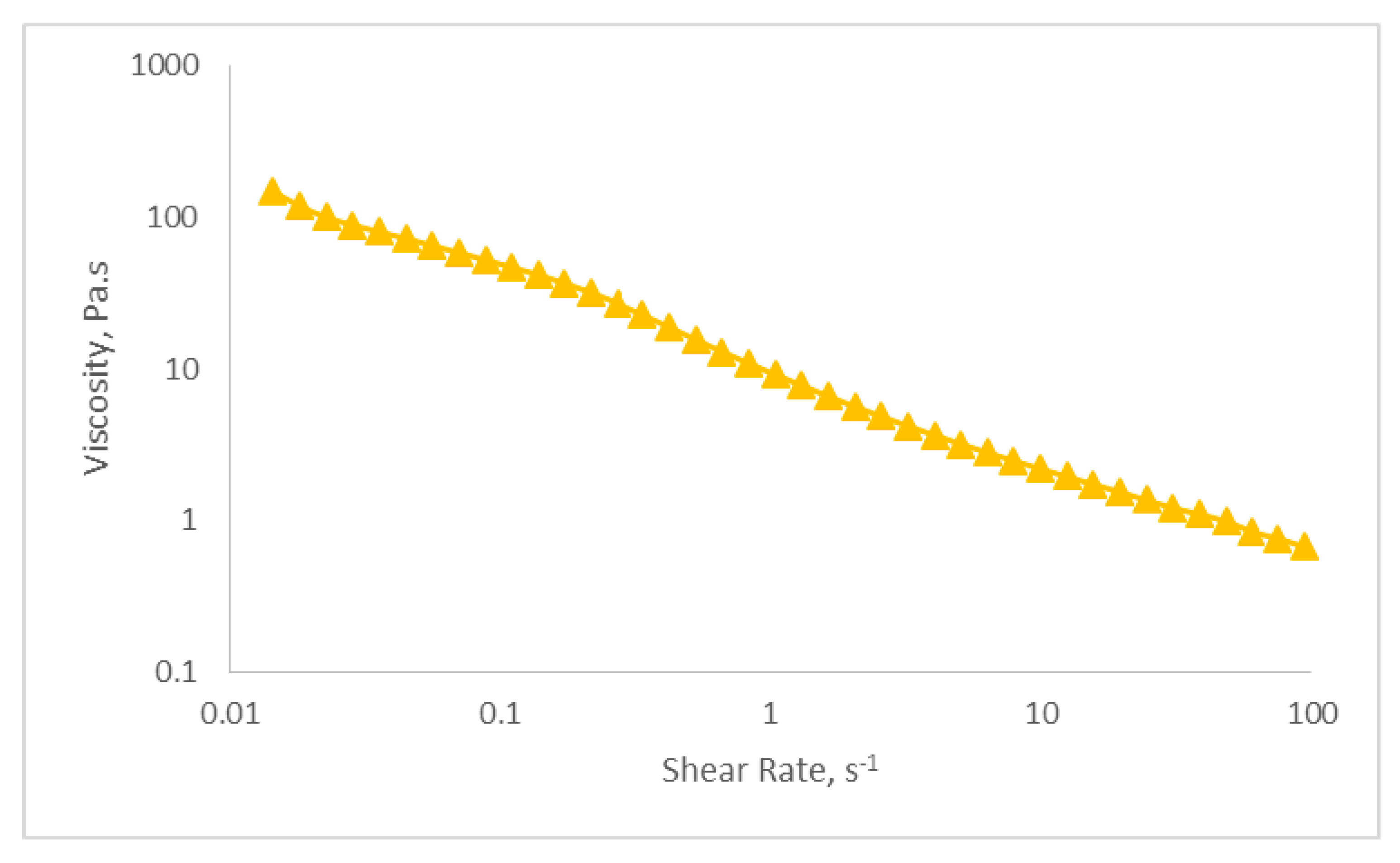
2.5.5. Viscosity Determination
2.6. In Vitro Assessments of Optimized Nanoemulsion Containing KMO
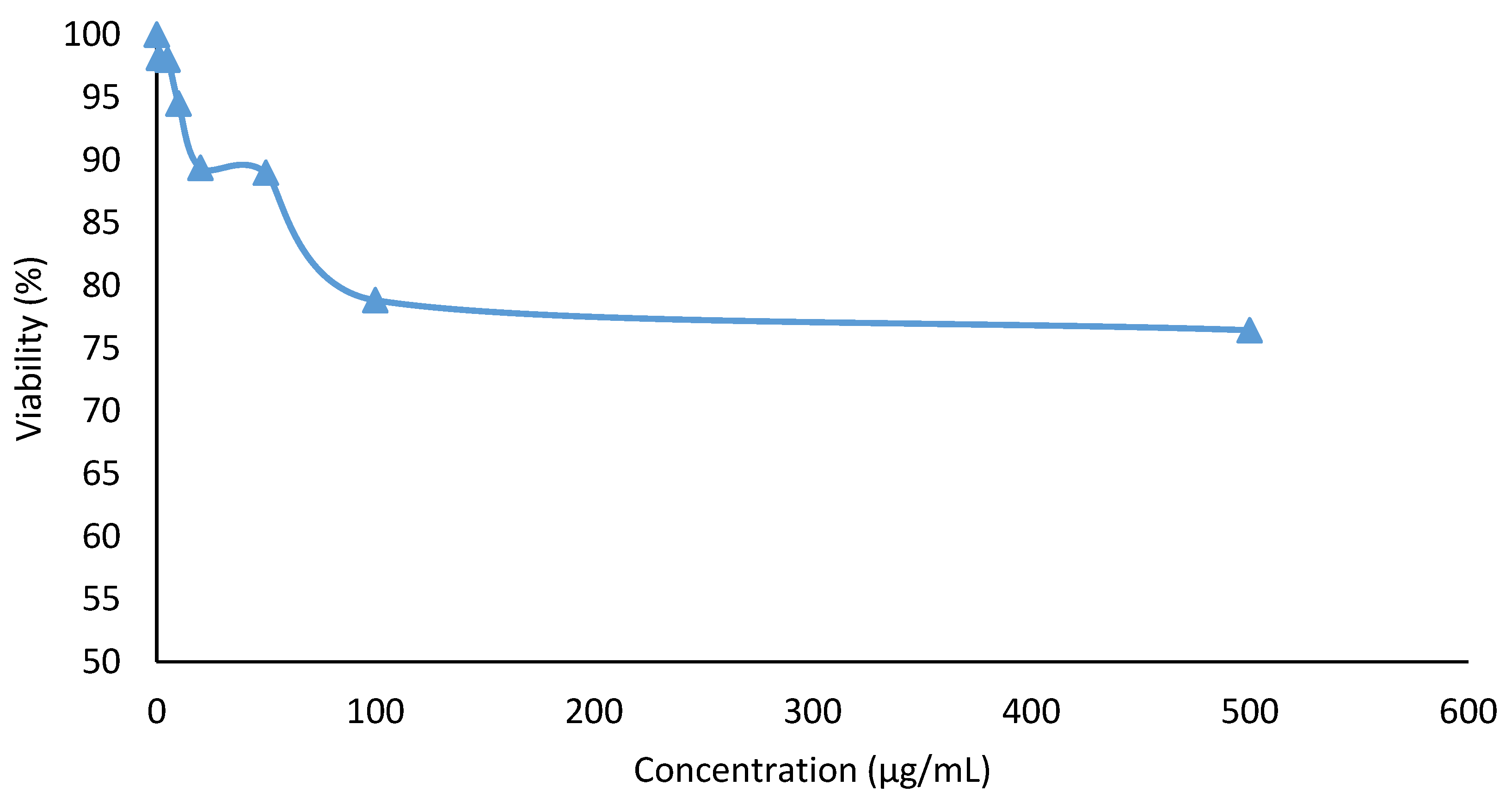
2.6.1. Cytotoxicity Assay
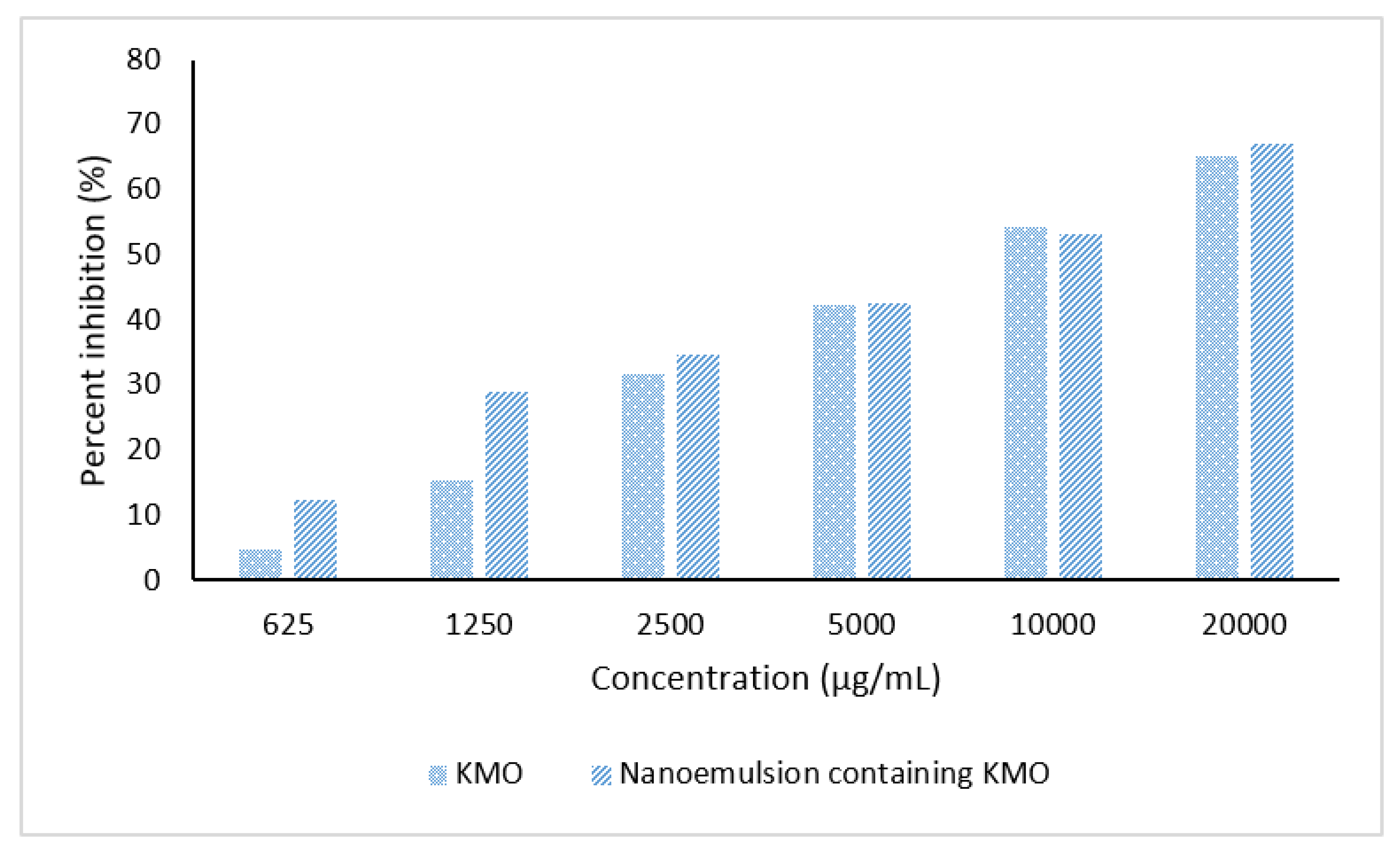
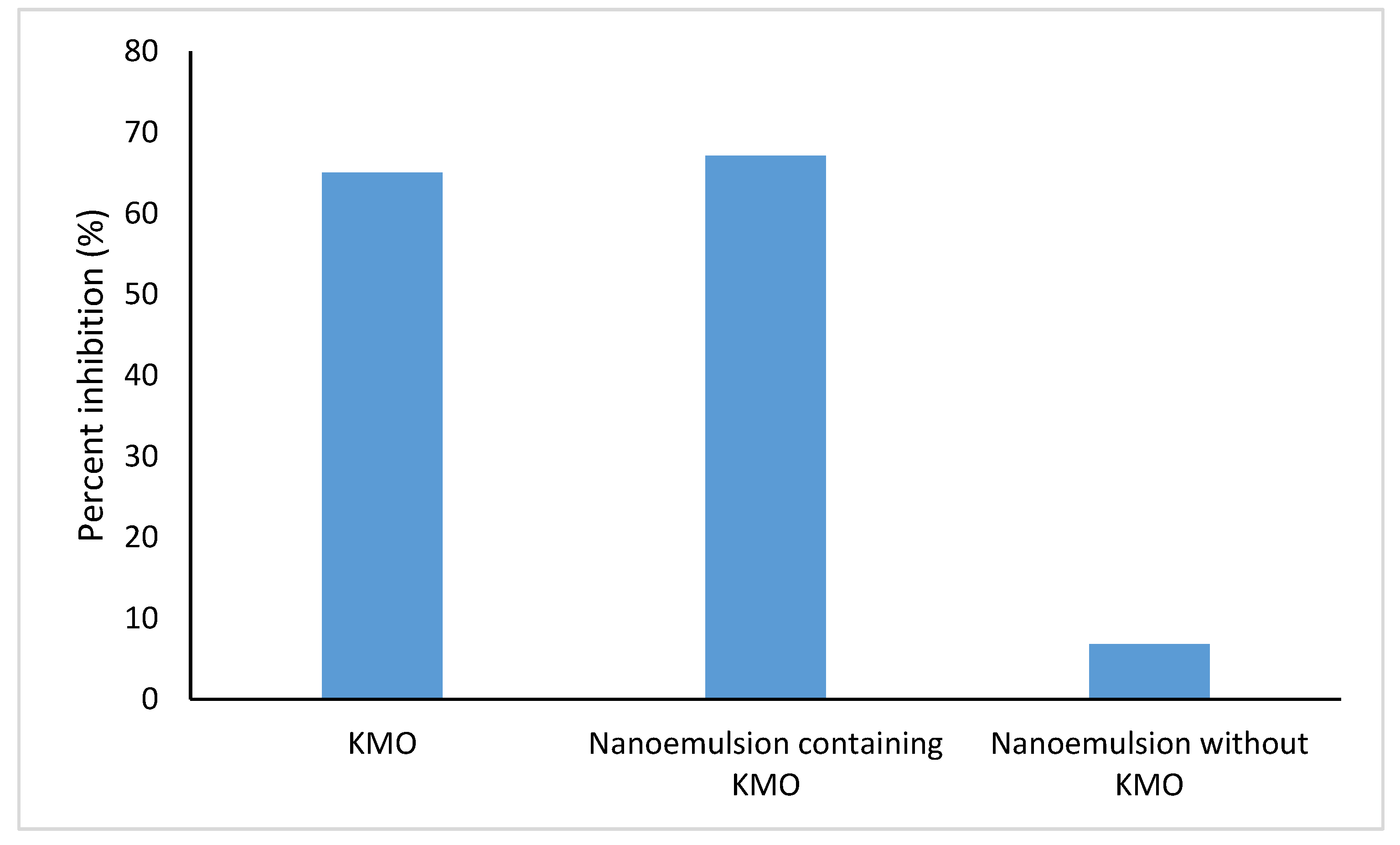
2.6.2. Tyrosinase Inhibition Assay
3. Materials and Methods
3.1. Materials
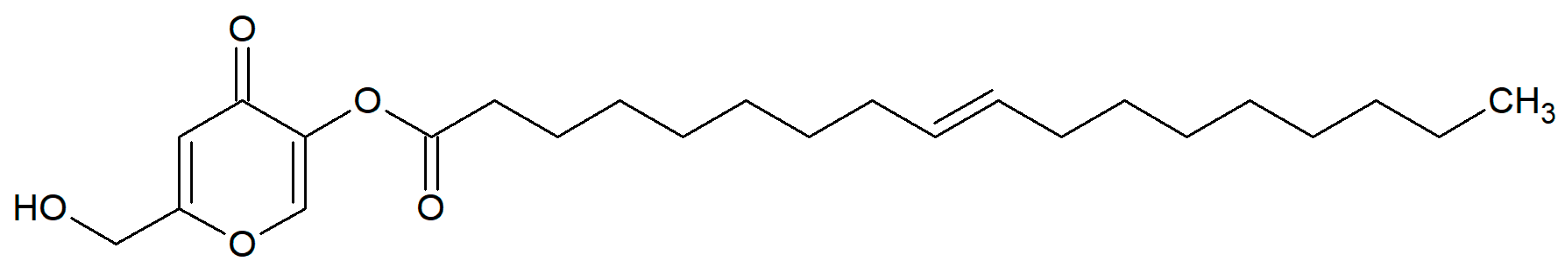
3.2. Preparation of KMO Nanoemulsion
3.3. Experimental Design
3.4. Statistical Analysis
3.5. Verification of Model
3.6. Physicochemical Characterization
3.6.1. Droplet Size, Zeta Potential and Polydispersity Index (PDI) Determination
3.6.2. Morphology Study
3.6.3. pH and Conductivity Measurement
3.6.4. Accelerated Stability Study
3.6.5. Viscosity Determination
3.7. In Vitro Assessments of Nanoemulsion Containing KMO
3.7.1. Cytotoxicity Assay
3.7.2. Tyrosinase Inhibition Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanlayavattanakul, M.; Lourith, N. Skin hyperpigmentation treatment using herbs: A review of clinical evidences. J. Cosmet. Laser Ther. 2017, 20, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-P.; Shih, Y.-W.; Chang, Y.-C.; Hung, C.-N.; Wang, C.-J. Chemoinhibitory Effect of Mulberry Anthocyanins on Melanoma Metastasis Involved in the Ras/PI3K Pathway. J. Agric. Food Chem. 2008, 56, 9286–9293. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2014, 91, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Kongtaphan, K.; Onkoksoong, T.; Jaemsak, K.; Phadungrakwittaya, R.; Thaworn, A.; Akarasereenont, P.; Wongkajornsilp, A. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Boil. Toxicol. 2009, 26, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Jung, H.J.; Moon, H.R. A Potent Tyrosinase Inhibitor, (E)-3-(2,4-Dihydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one, with Anti-Melanogenesis Properties in α-MSH and IBMX-Induced B16F10 Melanoma Cells. Molecules 2018, 23, 2725. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Adsul, L.K.; Chavan, H.V.; Shringare, S.N.; Korbad, B.L.; Jalde, S.S.; Lonikar, S.V.; Nile, S.H.; Shirfule, A.L. Synthesis, biological evaluation, and molecular docking of N-{3-[3-(9-methyl-9H-carbazol-3-yl)-acryloyl]-phenyl}-benzamide/amide derivatives as xanthine oxidase and tyrosinase inhibitors. Bioorg. Med. Chem. 2012, 20, 5649–5657. [Google Scholar] [CrossRef]
- Ha, S.K.; Koketsu, M.; Lee, K.; Choi, S.Y.; Park, J.-H.; Ishihara, H.; Kim, S.Y. Inhibition of tyrosinase activity by N,N-unsubstituted selenourea derivatives. Boil. Pharm. Bull. 2005, 28, 838–840. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting Effect of Kojic Acid Esters in Hyperpigmented B16F1 Melanoma Cells. J. Biomed. Biotechnol. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Jumbri, K.; Rozy, M.F.A.-H.; Ashari, S.E.; Mohamad, R.; Basri, M.; Masoumi, H.R.F. Optimisation and Characterisation of Lipase-Catalysed Synthesis of a Kojic Monooleate Ester in a Solvent-Free System by Response Surface Methodology. PLoS ONE 2015, 10, e0144664. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Cho, J.; Kim, S.Y.; Lee, Y.-S. Solid-phase synthesis of kojic acid-tripeptides and their tyrosinase inhibitory activity, storage stability, and toxicity. Bioorganic Med. Chem. Lett. 2004, 14, 2843–2846. [Google Scholar] [CrossRef] [PubMed]
- A Burdock, G.; Soni, M.G.; Carabin, I.G. Evaluation of Health Aspects of Kojic Acid in Food. Regul. Toxicol. Pharmacol. 2001, 33, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-M.; Kwak, S.-Y.; Seo, H.; Seo, J.-H.; Kim, B.-G.; Lee, J.-Y. Kojic acid–amino acid conjugates as tyrosinase inhibitors. Bioorganic Med. Chem. Lett. 2009, 19, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Carlotti, M.E.; Trotta, M.; E Grande, A.; Talarico, C. Photostability of naturally occurring whitening agents in cosmetic microemulsions. J. Cosmet. Sci. 2004, 55, 139–148. [Google Scholar] [PubMed]
- Chen, W.-C.; Tseng, T.-S.; Hsiao, N.-W.; Lin, Y.-L.; Wen, Z.-H.; Tsai, C.-C.; Lee, Y.-C.; Lin, H.-H.; Tsai, K.-C. Discovery of Highly Potent Tyrosinase Inhibitor, T1, with Significant Anti-Melanogenesis Ability by zebrafish in vivo Assay and Computational Molecular Modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef]
- Ashari, S.E.; Mohamad, R.; Ariff, A.; Basri, M.; Salleh, A.B. Optimization of enzymatic synthesis of palm-based kojic acid ester using response surface methodology. J. Oleo Sci. 2009, 58, 503–510. [Google Scholar] [CrossRef]
- Azhar, S.N.A.S.; Ashari, S.E.; Salim, N. Development of a kojic monooleate-enriched oil-in-water nanoemulsion as a potential carrier for hyperpigmentation treatment. Int. J. Nanomed. 2018, 13, 6465–6479. [Google Scholar] [CrossRef]
- Sole, I.; Maestro, A.; Pey, C.; Gonzalez, C.; Solans, C.; Gutierrez, J.M. Nano-emulsions preparation by low energy methods in an ionic surfactant system. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 138–143. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; A Rocha-Filho, P. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Pey, C.; Maestro, A.; Sole, I.; Gonzalez, C.; Solans, C.; Gutierrez, J.M. Optimization of nano-emulsions prepared by low-energy emulsification methods at constant temperature using a factorial design study. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 144–150. [Google Scholar] [CrossRef]
- Solè, I.; Maestro, A.; Gonzalez, C.; Solans, C.; Gutierrez, J.M. Corrigendum to “Influence of the phase behavior on the properties of ionic nanoemulsions prepared by the phase inversion composition method” [J. Colloid Interface Sci. 327 (2008) 433–439]. J. Colloid Interface Sci. 2009, 330, 493. [Google Scholar] [CrossRef]
- Teo, B.S.X.; Basri, H.; Zakaria, M.R.S.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Rahman, M.B.A. A potential tocopherol acetate loaded palm oil esters-in-water nanoemulsions for nanocosmeceuticals. J. Nanobiotechnol. 2010, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.A.; Guerreiro, C.M.; Maruno, M.; Ferrari, M.; A Rocha-Filho, P. Exotic Vegetable Oils for Cosmetic O/W Nanoemulsions: In Vivo Evaluation. Molecules 2016, 21, 248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bansal, M.; Visht, S.; Sharma, P.; Kulkarni, G. Nanoemulsion: A new concept of delivery system. Chron. Young Sci. 2010, 1, 2. [Google Scholar]
- Garcia-Bilbao, A.; Gómez-Fernández, P.; Larush, L.; Soroka, Y.; Suarez-Merino, B.; Frušić-Zlotkin, M.; Magdassi, S.; Goñi-De-Cerio, F. Preparation, characterization, and biological evaluation of retinyl palmitate and Dead Sea water loaded nanoemulsions toward topical treatment of skin diseases. J. Bioact. Compat. Polym. 2019, 35, 24–38. [Google Scholar] [CrossRef]
- Sarheed, O.; Shouqair, D.; Ramesh, K.; Khaleel, T.; Amin, M.; Boateng, J.; Drechsler, M. Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int. J. Pharm. 2020, 576, 118952. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Buheazaha, T.M.; Al-Homoud, H.S.; Al-Nasif, H.A.; Sarafroz; Mohammed, T. A comparative ex vivo permeation evaluation of a novel 5-Fluorocuracil nanoemulsion-gel by topically applied in the different excised rat, goat, and cow skin. Saudi J. Boil. Sci. 2020, 27, 1024–1040. [Google Scholar] [CrossRef]
- Fasolo, D.; Pippi, B.; Meirelles, G.; Zorzi, G.; Fuentefria, A.M.; Von Poser, G.; Teixeira, H.F. Topical delivery of antifungal Brazilian red propolis benzophenones-rich extract by means of cationic lipid nanoemulsions optimized by means of Box-Behnken Design. J. Drug Deliv. Sci. Technol. 2020, 56, 101573. [Google Scholar] [CrossRef]
- Nastiti, C.M.; Ponto, T.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Novel Nanocarriers for Targeted Topical Skin Delivery of the Antioxidant Resveratrol. Pharmaceutics 2020, 12, 108. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Gonzalez, C.; Maestro, A.; Solè, I.; Pey, C.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Abou-Taleb, H.A. Optimization of nano-emulsion formulations for certain emollient effect. World J. Pharm. Pharm. Sci. 2015, 4, 1314–1328. [Google Scholar]
- Norddin, F.A.A.; Azhar, S.N.A.S.; Ashari, S.E. Evaluation of Direct Esterification of Fatty Acid Derivative of Kojic Acid in Co-solvent System: A Statistical Approach. J. Biosens. Bioelectron. 2017, 8, 331. [Google Scholar] [CrossRef]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Basri, M.; Masoumi, H.R.F.; Karjiban, R.A.; Salim, N.; Shameli, K. Modeling and optimization of nanoemulsion containing Sorafenib for cancer treatment by response surface methodology. Chem. Central J. 2017, 11, 21. [Google Scholar] [CrossRef]
- Song, M.-M.; Branford-White, C.; Nie, H.; Zhu, L.-M. Optimization of adsorption conditions of BSA on thermosensitive magnetic composite particles using response surface methodology. Colloids Surf. B Biointerfaces 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Tan, S.F.; Masoumi, H.R.F.; Karjiban, R.A.; Stanslas, J.; Kirby, B.; Basri, H.; Basri, H. Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability. Ultrason. Sonochemistry 2016, 29, 299–308. [Google Scholar] [CrossRef]
- Lee, A.; Chaibakhsh, N.; Rahman, M.B.A.; Basri, M.; Tejo, B. Optimized enzymatic synthesis of levulinate ester in solvent-free system. Ind. Crop. Prod. 2010, 32, 246–251. [Google Scholar] [CrossRef]
- Musa, S.H.; Basri, M.; Masoumi, H.R.F.; Karjiban, R.A.; Malek, E.A.; Basri, H.; Shamsuddin, A.F. Formulation optimization of palm kernel oil esters nanoemulsion-loaded with chloramphenicol suitable for meningitis treatment. Colloids Surf. B Biointerfaces 2013, 112, 113–119. [Google Scholar] [CrossRef]
- Masoumi, H.R.F.; Basri, M.; Kassim, A.; Abdullah, D.K.; Abdollahi, Y.; Gani, S.S.A.; Rezaee, M. Statistical Optimization of Process Parameters for Lipase-Catalyzed Synthesis of Triethanolamine-Based Esterquats Using Response Surface Methodology in 2-Liter Bioreactor. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Mousavi, M.; Emam-Djomeh, Z.; Hadinezhad, M.; Gharibzahedi, S.M.T. Formulation Optimization of Pistachio Oil Spreads by Characterization of the Instrumental Textural Attributes. Int. J. Food Prop. 2014, 17, 1355–1368. [Google Scholar] [CrossRef]
- A Rocha-Filho, P.; Ferrari, M.; Maruno, M.; Souza, O.; Gumiero, V. In Vitro and In Vivo Evaluation of Nanoemulsion Containing Vegetable Extracts. Cosmetics 2017, 4, 32. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Weiss, M.; Kunzek, H. Influence of the emulsion components and preparation method on the laboratory-scale preparation of o/w emulsions containing different types of dispersed phases and/or emulsifiers. Food/Nahrung 2002, 46, 294–301. [Google Scholar] [CrossRef]
- Rebolleda, S.; Sanz, M.T.; Benito, J.M.; Beltrán, S.; Escudero, I.; González-Sanjosé, M.L. Formulation and characterisation of wheat bran oil-in-water nanoemulsions. Food Chem. 2015, 167, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Wang, B. Optimization of encapsulation efficiency and average particle size of Hohenbuehelia serotina polysaccharides nanoemulsions using response surface methodology. Food Chem. 2017, 229, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Wahgiman, N.A.; Salim, N.; Rahman, M.B.A.; Ashari, S.E. Optimization of nanoemulsion containing gemcitabine and evaluation of its cytotoxicity towards human fetal lung fibroblast (MRC5) and human lung carcinoma (A549) cells. Int. J. Nanomed. 2019, 14, 7323–7338. [Google Scholar] [CrossRef]
- Ngan, C.L.; Basri, M.; Lye, F.F.; Masoumi, H.R.F.; Tripathy, M.; Karjiban, R.A.; Abdulmalek, E. Comparison of Box–Behnken and central composite designs in optimization of fullerene loaded palm-based nano-emulsions for cosmeceutical application. Ind. Crop. Prod. 2014, 59, 309–317. [Google Scholar] [CrossRef]
- Ribeiro, R.C.D.A.; Barreto, S.M.A.G.; Ostrosky, E.A.; A Rocha-Filho, P.; Veríssimo, L.M.; Ferrari, M. Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef]
- Tang, S.Y.; Manickam, S.; Wei, T.K.; Nashiru, B. Formulation development and optimization of a novel Cremophore EL-based nanoemulsion using ultrasound cavitation. Ultrason. Sonochemistry 2012, 19, 330–345. [Google Scholar] [CrossRef]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason. Sonochemistry 2019, 60, 104604. [Google Scholar] [CrossRef]
- Abd Gani, S.S.; Basri, M.; Abdul Rahman, M.B.; Kassim, A.; Raja Abd Rahman, R.N.Z.; Salleh, A.B.; Ismail, Z. Engkabang fat as a base in preparing encapsulated titanium dioxide for cosmetics purpose. Asian J. Chem. 2011, 23, 380–384. [Google Scholar]
- Martínez-Pla, J.J.; Martín-Biosca, Y.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Evaluation of the pH effect of formulations on the skin permeability of drugs by biopartitioning micellar chromatography. J. Chromatogr. A 2004, 1047, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Mei, Z.; Xu, J.; Sun, D. Effect of inorganic electrolytes on the formation and the stability of water-in-oil (W/O) emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 82–90. [Google Scholar] [CrossRef]
- Hadzir, N.M.; Basri, H.; Rahman, M.B.A.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Basri, H. Phase Behaviour and Formation of Fatty Acid Esters Nanoemulsions Containing Piroxicam. AAPS PharmSciTech 2013, 14, 456–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pund, S.; Borade, G.; Rasve, G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine 2014, 21, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.; Basri, H.; Masoumi, H.R.F.; Malek, E.A.; Karjiban, R.A. An Artificial Neural Network Based Analysis of Factors Controlling Particle Size in a Virgin Coconut Oil-Based Nanoemulsion System Containing Copper Peptide. PLoS ONE 2016, 11, e0157737. [Google Scholar] [CrossRef]
- Itharat, A.; Houghton, P.J.; Eno-Amooquaye, E.; Burke, P.J.; Sampson, J.H.; Raman, A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharmacol. 2004, 90, 33–38. [Google Scholar] [CrossRef]
- Cui, H.-X.; Duan, F.-F.; Jia, S.-S.; Cheng, F.-R.; Yuan, K. Antioxidant and Tyrosinase Inhibitory Activities of Seed Oils from Torreya grandis Fort. ex Lindl. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Shahidan, N.S.; Salim, N.; Ashari, S.E. Preparation and Optimization of Ibuprofen-Loaded Nanoemulsion Formulation. J. Multidiscip. Eng. Sci. Technol. 2019, 6, 89–96. [Google Scholar]
Sample Availability: Samples of the nanoemulsions are available from the authors. |


| Standard Order | Run Order | Independent Variables | Droplet Size (nm) | |||
|---|---|---|---|---|---|---|
| Time of High Shear (min) | Speed of High Shear (rpm) | Speed of Stirrer (rpm) | Actual | Predicted | ||
| 1 | 1 | 8.04 | 4905.40 | 220.27 | 118.14 | 119.40 |
| 2 | 16 | 16.96 | 4905.40 | 220.27 | 145.50 | 145.22 |
| 3 | 3 | 8.04 | 6094.60 | 220.27 | 132.47 | 132.11 |
| 4 | 20 | 16.96 | 6094.60 | 220.27 | 118.30 | 117.24 |
| 5 | 13 | 8.04 | 4905.40 | 279.73 | 103.16 | 104.08 |
| 6 | 11 | 16.96 | 4905.40 | 279.73 | 148.00 | 148.22 |
| 7 | 10 | 8.04 | 6094.60 | 279.73 | 116.80 | 116.94 |
| 8 | 15 | 16.96 | 6094.60 | 279.73 | 121.79 | 120.39 |
| 9 | 17 | 5.00 | 5500.00 | 250.00 | 116.03 | 114.80 |
| 10 | 18 | 20.00 | 5500.00 | 250.00 | 138.00 | 139.42 |
| 11 | 8 | 12.50 | 4500.00 | 250.00 | 128.00 | 126.67 |
| 12 | 12 | 12.50 | 6500.00 | 250.00 | 112.43 | 113.96 |
| 13 | 9 | 12.50 | 5500.00 | 200.00 | 131.50 | 131.70 |
| 15 | 19 | 12.50 | 5500.00 | 250.00 | 113.00 | 111.82 |
| 16 | 2 | 12.50 | 5500.00 | 250.00 | 112.67 | 111.82 |
| 17 | 4 | 12.50 | 5500.00 | 250.00 | 111.45 | 111.82 |
| 18 | 14 | 12.50 | 5500.00 | 250.00 | 110.73 | 111.82 |
| 19 | 5 | 12.50 | 5500.00 | 250.00 | 112.60 | 111.82 |
| 20 | 6 | 12.50 | 5500.00 | 250.00 | 110.53 | 111.82 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 2850.06 | 9 | 316.67 | 148.79 | <0.0001 | significant |
| A | 731.78 | 1 | 731.78 | 343.82 | <0.0001 | |
| B | 195.15 | 1 | 195.15 | 91.69 | <0.0001 | |
| C | 82.68 | 1 | 82.68 | 38.85 | 0.0002 | |
| AB | 827.84 | 1 | 827.84 | 388.95 | <0.0001 | |
| AC | 167.81 | 1 | 167.81 | 78.85 | <0.0001 | |
| BC | 0.011 | 1 | 0.011 | 5.286 × 10−3 | 0.9436 | |
| A2 | 400.66 | 1 | 400.66 | 188.25 | <0.0001 | |
| B2 | 123.53 | 1 | 123.53 | 58.04 | <0.0001 | |
| C2 | 228.97 | 1 | 228.97 | 107.58 | <0.0001 | |
| Residual | 19.16 | 9 | 2.13 | |||
| Lack of Fit | 13.44 | 4 | 3.36 | 2.94 | 0.1337 | not significant |
| Pure Error | 5.71 | 5 | 1.14 | |||
| Corrected Total | 2869.22 | 18 |
| SD | 1.46 | R2 | 0.9933 |
| Mean | 121.11 | Adjusted R2 | 0.9866 |
| CV% | 1.20 | Predicted R2 | 0.9494 |
| PRESS | 145.07 | Adequate Precision | 41.710 |
| Independent Variable | Droplet Size (nm) | RSE (%) | |||
|---|---|---|---|---|---|
| A (min) | B (rpm) | C (rpm) | Actual | Predicted | |
| 10.00 | 6000 | 250.00 | 115.31 | 113.16 | 1.90 |
| 12.00 | 5600 | 225.00 | 116.08 | 117.39 | 1.16 |
| 10.00 | 5800 | 300.00 | 117.82 | 116.51 | 1.12 |
| 13.00 | 5600 | 250.00 | 113.06 | 111.97 | 0.97 |
| Constraints | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| Time of high shear (A) | In range | 8.04 | 16.96 |
| Speed of high shear (B) | In range | 4905.40 | 6094.60 |
| Speed of stirrer (C) | In range | 220.27 | 279.73 |
| Droplet size | Minimize | 102.60 | 183.02 |
| Independent Variable | Droplet Size (nm) | Desirability | |||
|---|---|---|---|---|---|
| A (min) | B (rpm) | C (rpm) | Actual | Predicted | |
| 8.04 | 4905.41 | 271.82 | 103.97 | 103.71 | 0.988 |
| Storage Temperature (°C) | Storage Stability (days) | Centrifugation | |||
|---|---|---|---|---|---|
| 1 | 30 | 60 | 90 | ||
| 4 | / | / | / | / | |
| 25 | / | / | / | / | / |
| 45 | / | / | / | / | |
| Sample | k | n | R2 |
|---|---|---|---|
| Optimized nanoemulsion containing KMO | 10.227 | 0.366 | 0.996 |
| Independent Variables | Unit | Coded Level | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | ||
| A | min | 5.00 | 8.04 | 12.50 | 16.96 | 20.00 |
| B | rpm | 4500.00 | 4905.40 | 5500.00 | 6094.60 | 6500.00 |
| C | rpm | 200.00 | 220.27 | 250.00 | 279.73 | 300.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roselan, M.A.; Ashari, S.E.; Faujan, N.H.; Mohd Faudzi, S.M.; Mohamad, R. An Improved Nanoemulsion Formulation Containing Kojic Monooleate: Optimization, Characterization and In Vitro Studies. Molecules 2020, 25, 2616. https://doi.org/10.3390/molecules25112616
Roselan MA, Ashari SE, Faujan NH, Mohd Faudzi SM, Mohamad R. An Improved Nanoemulsion Formulation Containing Kojic Monooleate: Optimization, Characterization and In Vitro Studies. Molecules. 2020; 25(11):2616. https://doi.org/10.3390/molecules25112616
Chicago/Turabian StyleRoselan, Muhammad Azimuddin, Siti Efliza Ashari, Nur Hana Faujan, Siti Munirah Mohd Faudzi, and Rosfarizan Mohamad. 2020. "An Improved Nanoemulsion Formulation Containing Kojic Monooleate: Optimization, Characterization and In Vitro Studies" Molecules 25, no. 11: 2616. https://doi.org/10.3390/molecules25112616
APA StyleRoselan, M. A., Ashari, S. E., Faujan, N. H., Mohd Faudzi, S. M., & Mohamad, R. (2020). An Improved Nanoemulsion Formulation Containing Kojic Monooleate: Optimization, Characterization and In Vitro Studies. Molecules, 25(11), 2616. https://doi.org/10.3390/molecules25112616





