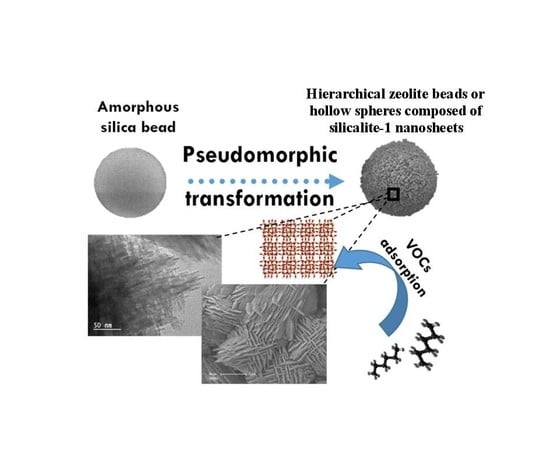
Synthesis of Hierarchical Zeolites with Morphology Control: Plain and Hollow Spherical Beads of Silicalite-1 Nanosheets
Abstract
1. Introduction
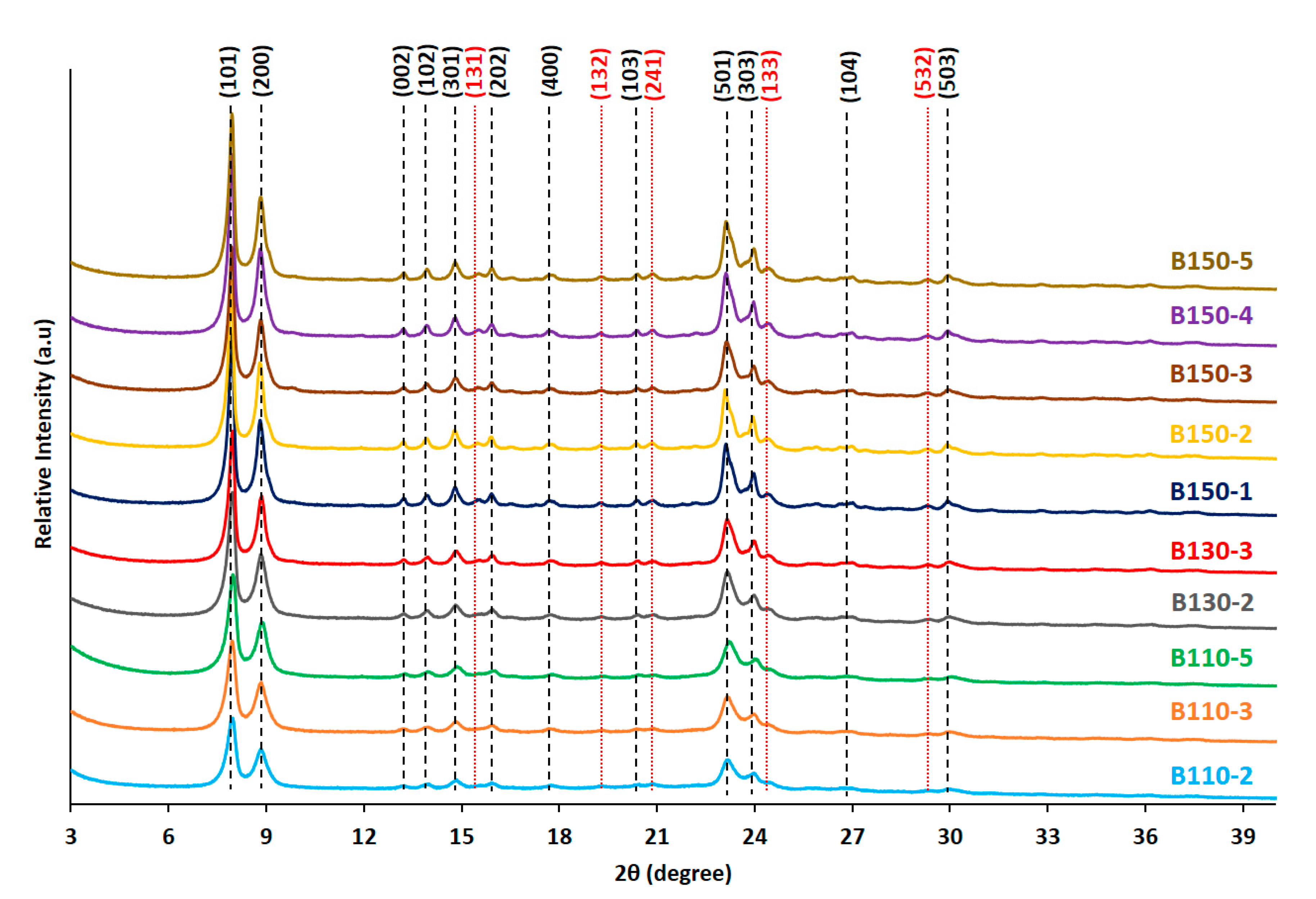
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Silicalite-1 Beads
3.1.1. Structure Directing Agent
3.1.2. Pseudomorphic Synthesis of Silicalite-1 Nanosheet Spheres
3.2. Characterization of Zeosils
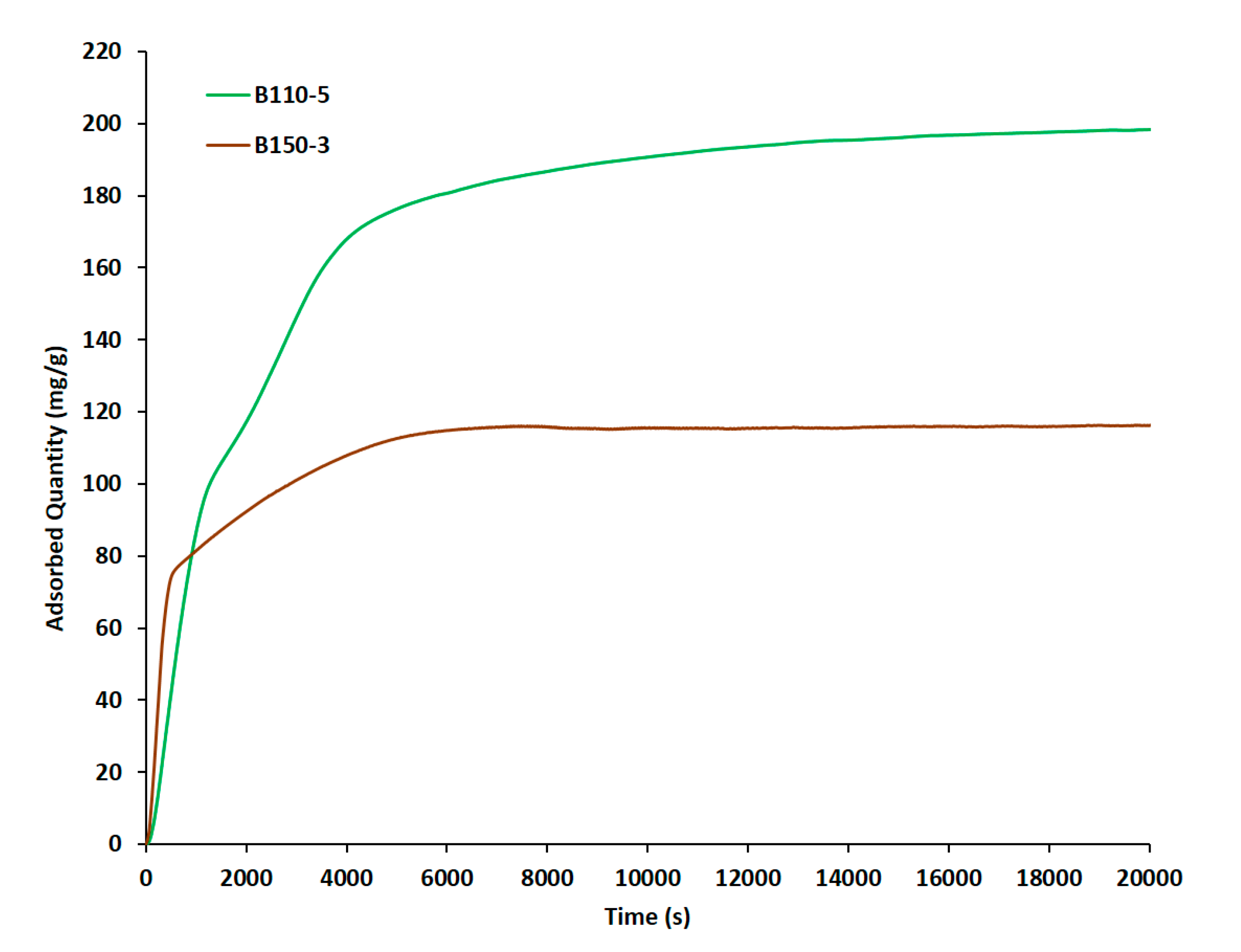
3.3. Dynamic n-Hexane Adsorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cosseron, A.-F.; Daou, T.J.; Tzanis, L.; Nouali, H.; Deroche, I.; Coasne, B.; Tchamber, V. Adsorption of volatile organic compounds in pure silica CHA, ∗BEA, MFI and STT-type zeolites. Micro. Meso. Mater. 2013, 173, 147–154. [Google Scholar] [CrossRef]
- Kabalan, I.; Rioland, G.; Nouali, H.; Lebeau, B.; Rigolet, S.; Fadlallah, M.-B.; Toufaily, J.; Hamiyeh, T.; Daou, T.J. Synthesis of purely silica MFI-type nanosheets for molecular decontamination. RSC Adv. 2014, 4, 37353–37358. [Google Scholar] [CrossRef]
- Kabalan, I.; Khay, I.; Nouali, H.; Ryzhikov, A.; Lebeau, B.; Albrecht, S.; Rigolet, S.; Fadlallah, M.-B.; Toufaily, J.; Hamiyeh, T.; et al. Influence of the particle sizes on the energetic performances of MFI-type zeolites. J. Phys. Chem. C 2015, 119, 18074–18083. [Google Scholar] [CrossRef]
- Eroshenko, V.; Regis, R.C.; Soulard, M.; Patarin, J. Energetics: A new feld of applications for hydrophobic zeolites. J. Am. Chem. Soc. 2001, 123, 8129–8130. [Google Scholar] [CrossRef]
- Tzanis, L.; Nouali, H.; Daou, T.J.; Soulard, M.; Patarin, J. Influence of the aqueous medium on the energetic performances of Silicalite-1. Mater. Letter. 2014, 115, 229–232. [Google Scholar] [CrossRef]
- Deroche, I.; Daou, T.J.; Picard, C.; Coasne, B. Reminiscent capillarity in subnanopores. Nature Com. 2019, 10. [Google Scholar] [CrossRef]
- Pascual, P.; Ungerer, P.; Tavitian, P.; Pernot, P.; Boutin, A. Development of a transferable guest–host force field for adsorption of hydrocarbons in zeolites. Phys. Chem. Chem. Phys. 2003, 5, 3684–3693. [Google Scholar] [CrossRef]
- Rioland, G.; Daou, T.J.; Faye, D.; Patarin, J. A new generation of MFI-type zeolite pellets with very high mechanical performance for space decontamination. Micro. Meso. Mater. 2016, 221, 167–174. [Google Scholar] [CrossRef]
- Bingre, R.; Louis, B.; Nguyen, P. An Overview on Zeolite Shaping Technology and Solutions to Overcome Diffusion Limitations. Catalysts. 2018, 8, 163. [Google Scholar] [CrossRef]
- Lauridant, N.; Daou, T.J.; Arnold, G.; Soulard, M.; Nouali, H.; Patarin, J.; Faye, D. Key steps influencing the formation of ZSM-5 films on aluminum substrates. Micro. Meso. Mater. 2012, 152, 1–8. [Google Scholar] [CrossRef]
- Schumann, K.; Unger, B.; Brandt, A.; Scheffler, F. Investigation on the pore structure of binderless zeolite 13× shapes. Micro. Meso. Mater. 2012, 154, 119–123. [Google Scholar] [CrossRef]
- Rioland, G.; Bullot, L.; Daou, T.J.; Simon-Masseron, A.; Chaplais, G.; Faye, D.; Fiani, E.; Patarin, J. Elaboration of FAU-type zeolite beads with good mechanical performances for molecular decontamination. RSC Adv. 2016, 6, 2470–2478. [Google Scholar] [CrossRef]
- Lauridant, N.; Daou, T.J.; Arnold, G.; Patarin, J.; Faye, D. MFI/∗BEA hybrid coating on aluminum alloys. Micro. Meso. Mater. 2013, 166, 79–85. [Google Scholar] [CrossRef]
- Daou, T.J.; Lauridant, N.; Josien, L.; Arnold, G.; Faye, D.; Patarin, J. Synthesis of MFI/EMT zeolite bi-layer films for molecular decontamination. Chem. Eng. J. 2013, 234, 66–73. [Google Scholar] [CrossRef]
- Said, A.; Lionel, L.; Nouali, H.; Michelin, L.; Halawani, J.; Toufaily, J.; Hamiyeh, T.; Dutournié, P.; Daou, T.J. Synthesis of mono- and bi-layer MFI zeolite films on macroporous alumina tubular supports: Application to nanofiltration. J. Cryst. Growth. 2015, 428, 71–79. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A.; Nouali, H.; Deroche, I.; Rigolet, S.; Lebeau, B.; Daou, T.J. Synthesis of binderless ZK-4 zeolite microspheres at high temperature. Molecules 2018, 23, 2647. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Shi, B.; Ren, J.; Liu, X.; Wang, Y.; Gua, Y.; Gua, Y.; Lu, G. Synthesis of hierarchical MFI zeolite microspheres with stacking nanocrystals. Micro. Meso. Mater. 2009, 117, 104–110. [Google Scholar] [CrossRef]
- Manko, M.; Vittenet, J.; Rodriguez, J.; Cot, D.; Mendret, J.; Brosillon, S.; Makowskia, W.; Galarneau, A. Synthesis of binderless zeolite aggregates (SOD, LTA, FAU) beads of 10, 70 µm and 1 mm by direct pseudomorphic transformation. Micro. Meso. Mater. 2013, 176, 145–154. [Google Scholar] [CrossRef]
- Yue, N.; Xue, M.; Qiu, S. Fabrication of hollow zeolite spheres using oil/water emulsions as templates. Inorg. Chem. Commun. 2011, 14, 1233–1236. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Ma, X. Confined synthesis of silicalite-1 hollow spheres with a lamellar shell. Scripta Materialia 2015, 95, 31–34. [Google Scholar] [CrossRef]
- Pashkova, V.; Tokarova, V.; Brabec, L.; Dedecek, J. Self-templating synthesis of hollow spheres of zeolite ZSM-5 from spray-dried aluminosilicate precursor. Micro. Meso. Mater. 2016, 228, 59–63. [Google Scholar] [CrossRef]
- Yan, B.; Zeng, C.; Yu, L.; Wang, C.; Zhang, L. Preparation of hollow zeolite NaA/chitosan composite microspheres via in situ hydrolysis-gelation-hydrothermal synthesis of TEOS. Micro. Meso. Mater. 2018, 257, 262–271. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, J.; Wu, S.; Xu, C.; Ma, Y.; Lei, J.; Kan, Q. Preparation of uniform Silicalite-1 microspheres with large secondary pore architecture using monodisperse porous polystyrene particles as template. Mater. Lett. 2010, 64, 1325–1327. [Google Scholar] [CrossRef]
- Yin, C.; Tian, D.; Xu, M.; Wei, Y.; Bao, X.; Chen, Y.; Wang, F. One-step synthesis of hierarchical mesoporous zeolite beta microspheres from assembly of nanocrystals. J. Colloid Interface Sci. 2013, 397, 108–113. [Google Scholar] [CrossRef]
- Tao, H.; Yang, H.; Zhang, Y.; Ren, J.; Liu, X.; Wang, Y.; Lu, G. Space-confined synthesis of nanorod oriented-assembled hierarchical MFI zeolite microspheres. J. Mater. Chem. A 2013, 1, 13821–13827. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Ren, F.; Du, J.; Li, R. Hierarchical architectures of ZSM-5 nanocrystalline aggregates with particular catalysis for lager molecule reaction. Micro. Meso. Mater. 2017, 240, 22–30. [Google Scholar] [CrossRef]
- Tosheva, L.; Valtchev, V.; Sterte, J. Silicalite-1 containing microspheres prepared using shape-directing macro-templates. Micro. Meso. Mater. 2000, 35–36, 621–629. [Google Scholar] [CrossRef]
- Tosheva, L.; Mihailova, B.; Valtchev, V.; Sterte, J. Zeolite beta spheres. Micro. Meso. Mater. 2001, 48, 31–37. [Google Scholar] [CrossRef]
- Yin, C.; Wei, Y.; Wang, F.; Chen, Y. Introduction of mesopority in zeolite ZSM-5 using resin as templates. Mater. Lett. 2013, 98, 194–196. [Google Scholar] [CrossRef]
- Galarneau, A.; Sachse, A.; Said, B.; Pelisson, C.-H.; Boscaro, P.; Brun, N.; Courtheoux, L.; Olivi-Tran, N.; Coasne, B.; Fajula, F. Hierarchical porous silica monoliths: A novel class of microreactors for process intensification in catalysis and adsorption. C. R. Chimie 2016, 19, 231–247. [Google Scholar] [CrossRef]
- Didi, Y.; Said, B.; Micolle, M.; Cacciaguerra, T.; Cot, D.; Geneste, A.; Fajula, F.; Galarneau, A. Nanocrystals FAU-X monoliths as highly efficient microreactors for cesium capture in continuous flow. Microporous Mesoporous Mater. 2019, 285, 185–194. [Google Scholar] [CrossRef]
- Said, B.; Grandjean, A.; Barre, Y.; Tancret, F.; Fajula, F.; Galarneau, A. LTA zeolite monoliths with hierarchical trimodal porosity as highly efficient microreactors for strontium capture in continuous flow. Microporous Mesoporous Mater. 2016, 232, 39–52. [Google Scholar] [CrossRef]
- Moukahhal, K.; Daou, T.J.; Josien, L.; Nouali, H.; Toufaily, J.; Hamieh, T.; Galarneau, A.; Lebeau, B. Hierarchical ZSM-5 beads composed of zeolite nanosheets obtained by pseudomorphic transformation. Microporous Mesoporous Mater. 2019, 288. [Google Scholar] [CrossRef]
- Database of Zeolite Structures, version 2017, Framework chemical composition included for all materials in the database; Structure Commission of the International Zeolite Association (IZA-SC): Switzerland. 2017. Available online: http://www.iza-structure.org/databases/ (accessed on 20 March 2020).
- El Hanache, L.; Sundermann, L.; Lebeau, B.; Toufaily, J.; Hamieh, T.; Daou, T.J. Surfactant-modified MFI-type nanozeolites: Super-adsorbents for nitrate removal from contaminated water. Microporous Mesoporous Mater. 2009, 117, 104–110. [Google Scholar] [CrossRef]
- Kabalan, I.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamiyeh, T.; Daou, T.J. New Generation of Zeolite Materials for Environmental Applications. J. Phys. Chem. C. 2016, 120, 2688–2697. [Google Scholar] [CrossRef]
- Galarneau, A.; Mehlhorn, D.; Guenneau, F.; Coasne, B.; Villemot, F.; Minoux, D.; Aquino, C.; Dath, J.P. Specific Surface Area Determination for Microporous/Mesoporous Materials: The Case of Mesoporous FAU-Y Zeolites. Langmuir. 2018, 34, 14134–14142. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Vlugt, T.J.H.; Krishna, R.; Smit, B. Molecular Simulations of Adsorption Isotherms for Linear and Branched Alkanes and Their Mixtures in Silicalite. J. Phys. Chem. B. 1999, 103, 1102–1118. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature. 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
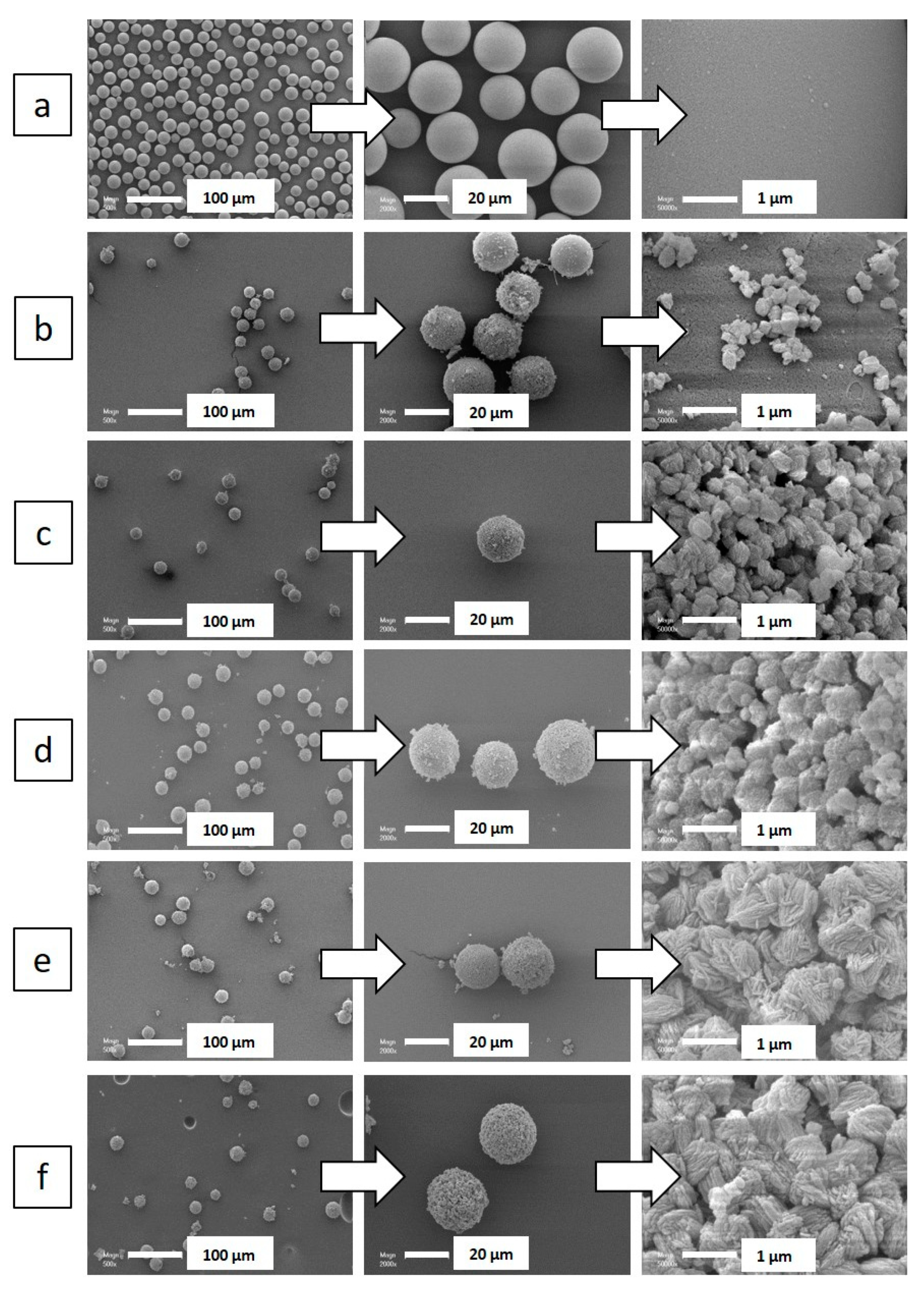
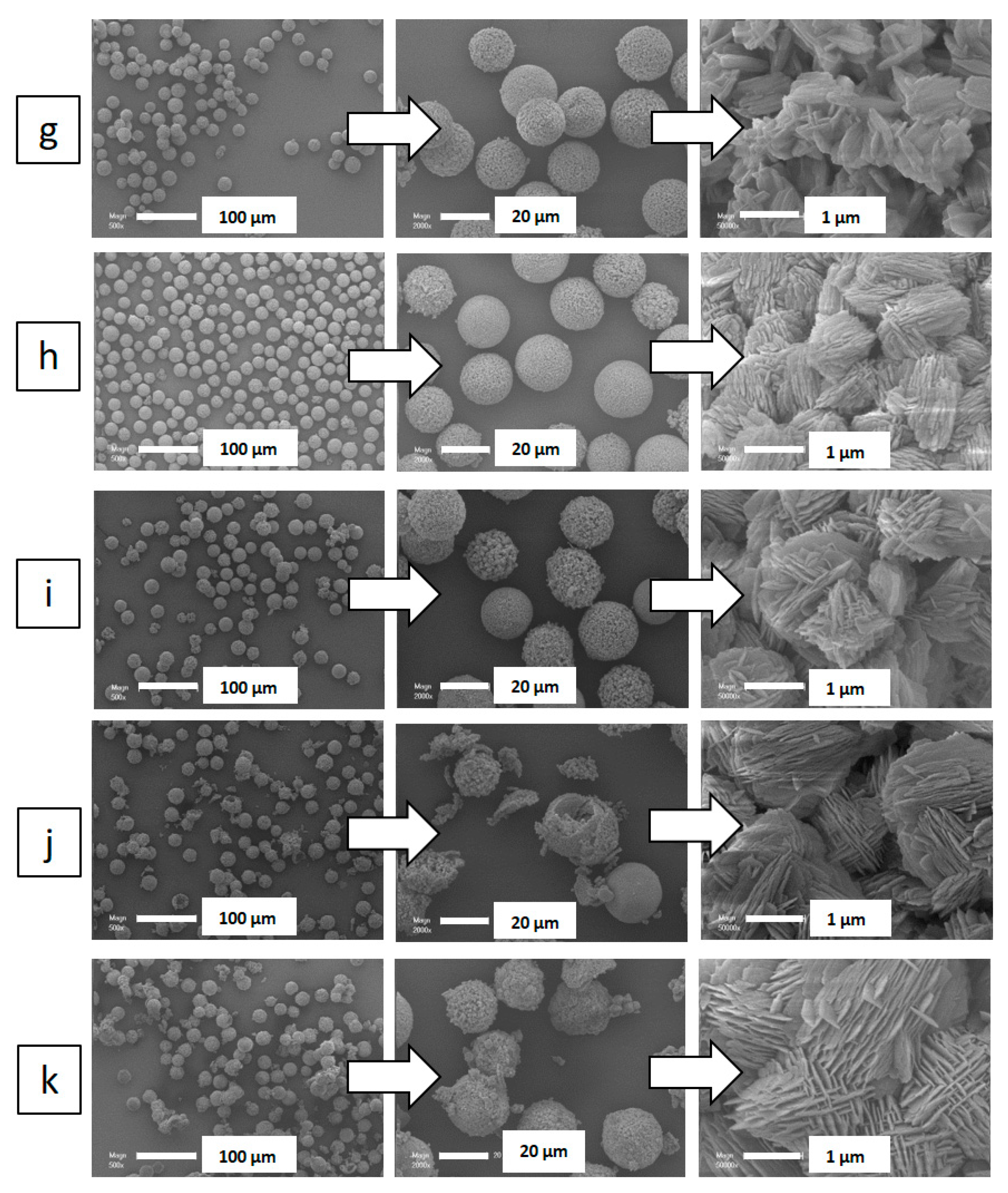
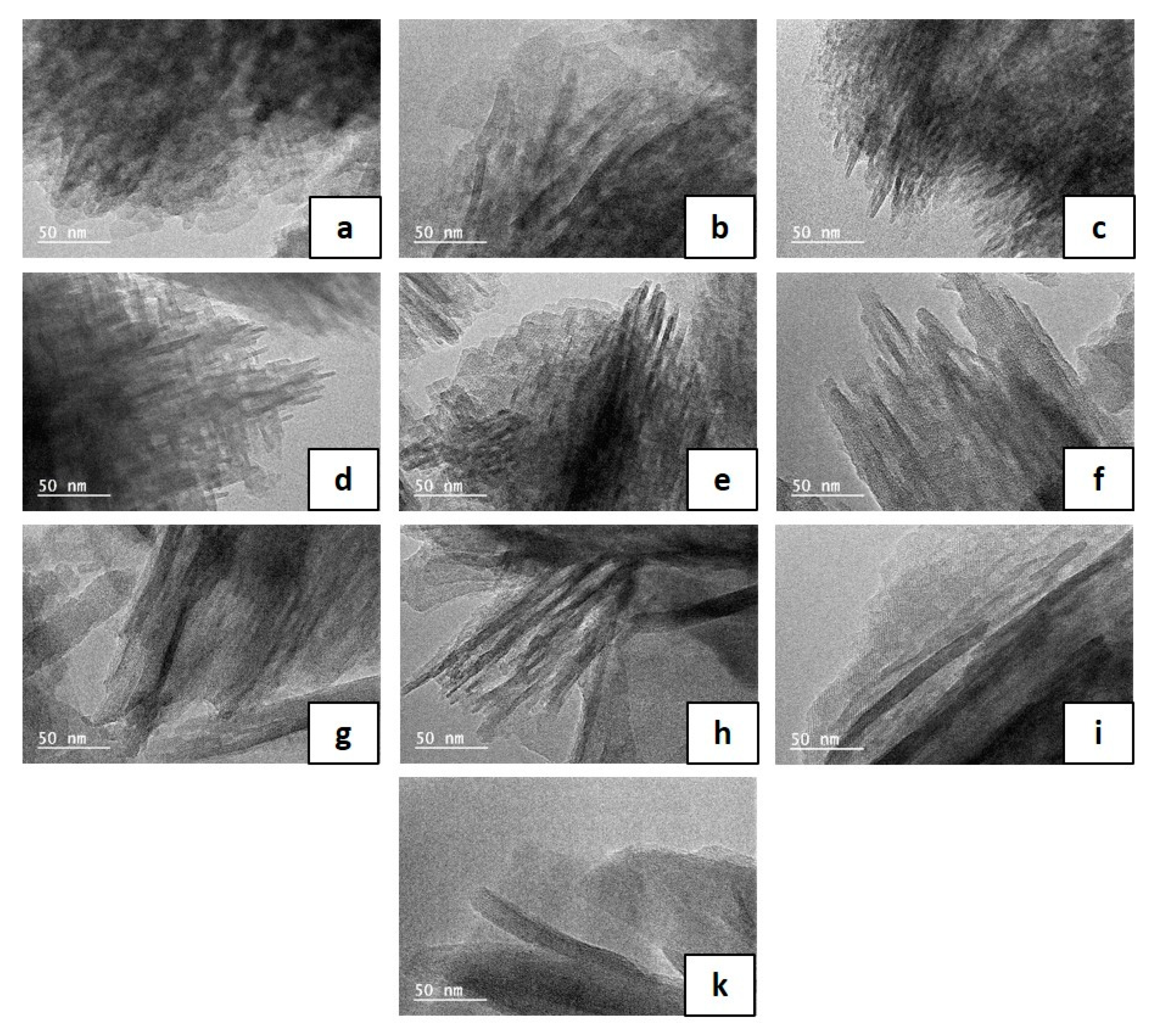


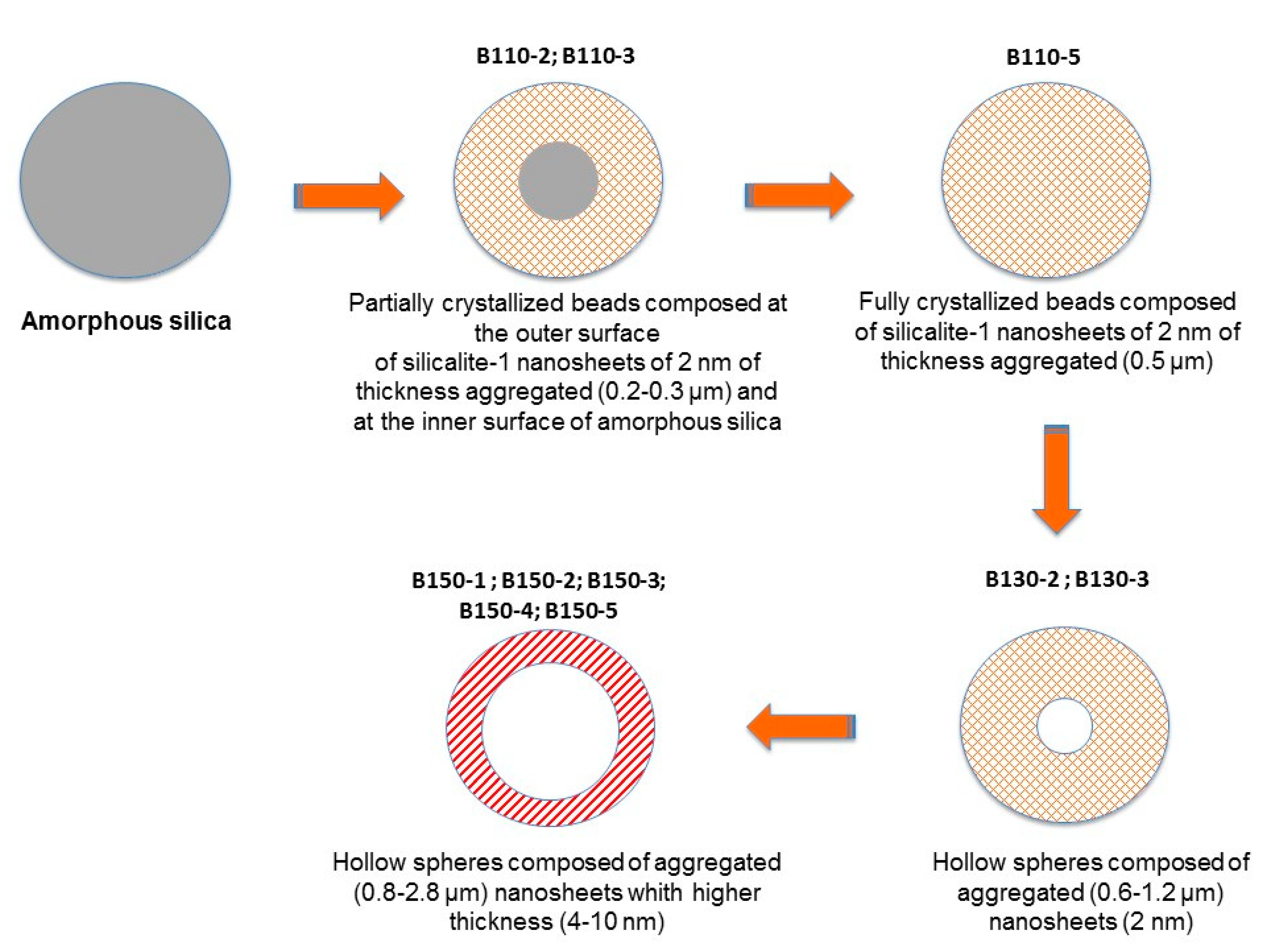
| Particle Diametera (µm) | Nanosheet Thickness (nm) | |
|---|---|---|
| B110-2 | 0.2 | 2 |
| B110-3 | 0.3 | 2 |
| B110-5 | 0.5 | 2 |
| B130-2 | 0.6 | 2 |
| B130-3 | 1.2 | 2 |
| B150-1 | 0.8 | 4 |
| B150-2 | 1.4 | 5 |
| B150-3 | 1.8 | 7 |
| B150-4 | 2.0 | 7 |
| B150-5 | 2.8 | 10 |
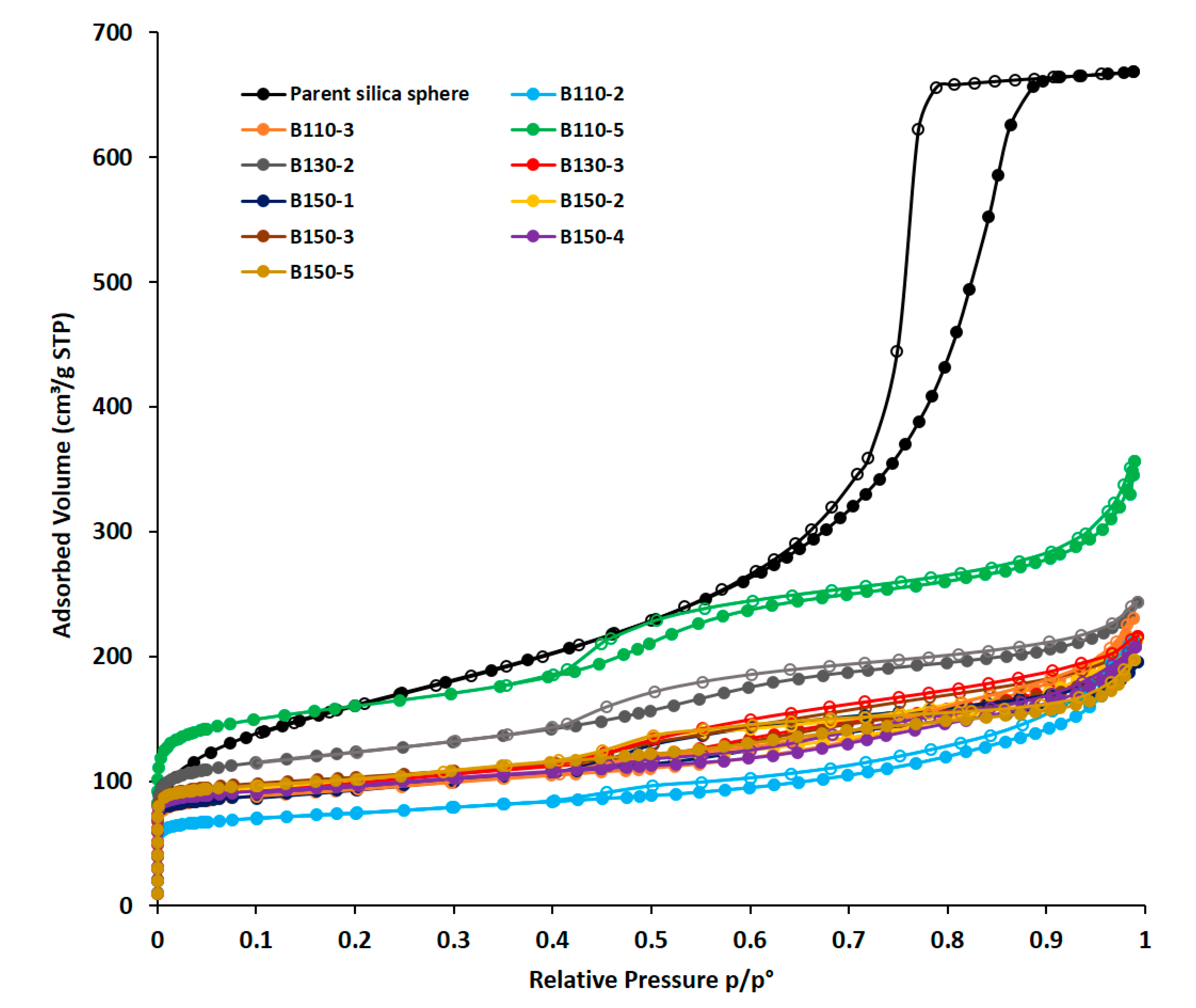
| SBET a (m2/g) | Smic b (m2/g) | Smes b (m2/g) | Sext (m2/g) | Vtot c (cm3/g) | Vmicro b (cm3/g) | Vmeso b (cm3/g) | Mesopore Diameter d (nm) | Crystallization Degree (%)e | |
|---|---|---|---|---|---|---|---|---|---|
| Parent silica sphere | 545 | 1.04 | 3.4–6.4–10.7 | 0 | |||||
| B110-2 | 282 | 191 | 0 | 91 | 0.329 | 0.106 | 0 | 3.3–6.6–9.1 | 58 |
| B110-3 | 354 | 246 | 0 | 108 | 0.357 | 0.137 | 0 | 2.3–3.3–8.6 | 76 |
| B110-5 | 595 | 398 | 45 | 152 | 0.552 | 0.189 | 0.184 | 4.3 | 100 |
| B130-2 | 458 | 327 | 31 | 100 | 0.377 | 0.155 | 0.135 | 4.7 | 86 |
| B130-3 | 374 | 246 | 0 | 128 | 0.335 | 0.134 | 0.105 | 5 | 74 |
| B150-1 | 352 | 251 | 0 | 101 | 0.302 | 0.138 | 0.087 | 3.4–5.4 | 76 |
| B150-2 | 381 | 296 | 0 | 85 | 0.330 | 0.162 | 0.014 | 3.2–6.4 | 90 |
| B150-3 | 395 | 284 | 0 | 111 | 0.326 | 0.157 | 0.078 | 3.4–5.5 | 87 |
| B150-4 | 370 | 283 | 0 | 87 | 0.321 | 0.155 | 0.080 | 3.4–5.5 | 86 |
| B150-5 | 390 | 296 | 0 | 94 | 0.305 | 0.162 | 0.063 | 3.4–5.5 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moukahhal, K.; Lebeau, B.; Josien, L.; Galarneau, A.; Toufaily, J.; Hamieh, T.; Daou, T.J. Synthesis of Hierarchical Zeolites with Morphology Control: Plain and Hollow Spherical Beads of Silicalite-1 Nanosheets. Molecules 2020, 25, 2563. https://doi.org/10.3390/molecules25112563
Moukahhal K, Lebeau B, Josien L, Galarneau A, Toufaily J, Hamieh T, Daou TJ. Synthesis of Hierarchical Zeolites with Morphology Control: Plain and Hollow Spherical Beads of Silicalite-1 Nanosheets. Molecules. 2020; 25(11):2563. https://doi.org/10.3390/molecules25112563
Chicago/Turabian StyleMoukahhal, Kassem, Bénédicte Lebeau, Ludovic Josien, Anne Galarneau, Joumana Toufaily, Tayssir Hamieh, and T. Jean Daou. 2020. "Synthesis of Hierarchical Zeolites with Morphology Control: Plain and Hollow Spherical Beads of Silicalite-1 Nanosheets" Molecules 25, no. 11: 2563. https://doi.org/10.3390/molecules25112563
APA StyleMoukahhal, K., Lebeau, B., Josien, L., Galarneau, A., Toufaily, J., Hamieh, T., & Daou, T. J. (2020). Synthesis of Hierarchical Zeolites with Morphology Control: Plain and Hollow Spherical Beads of Silicalite-1 Nanosheets. Molecules, 25(11), 2563. https://doi.org/10.3390/molecules25112563