Determination and Identification of Antibiotic Drugs and Bacterial Strains in Biological Samples
Abstract
1. Introduction
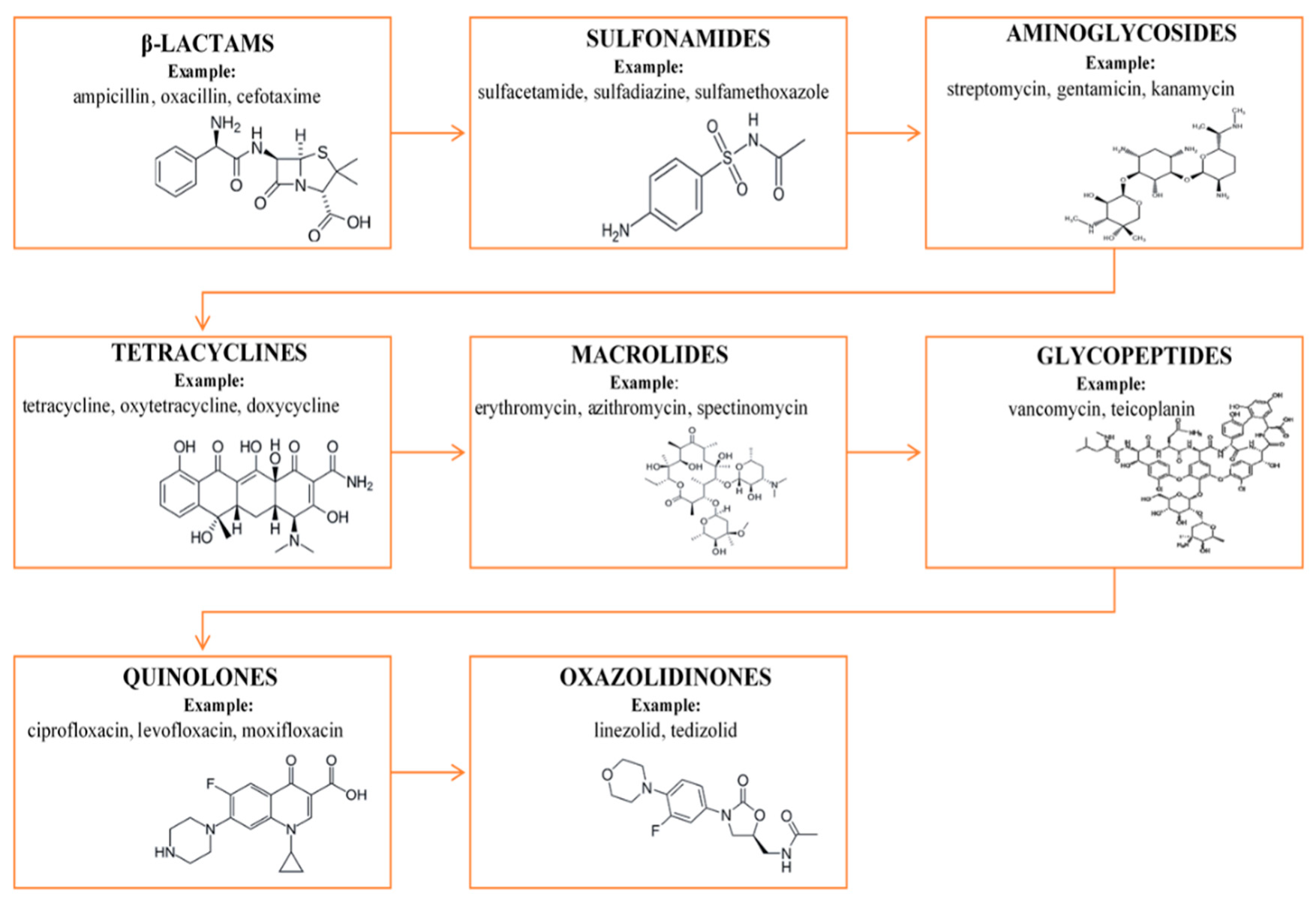
2. Antibiotic Drugs
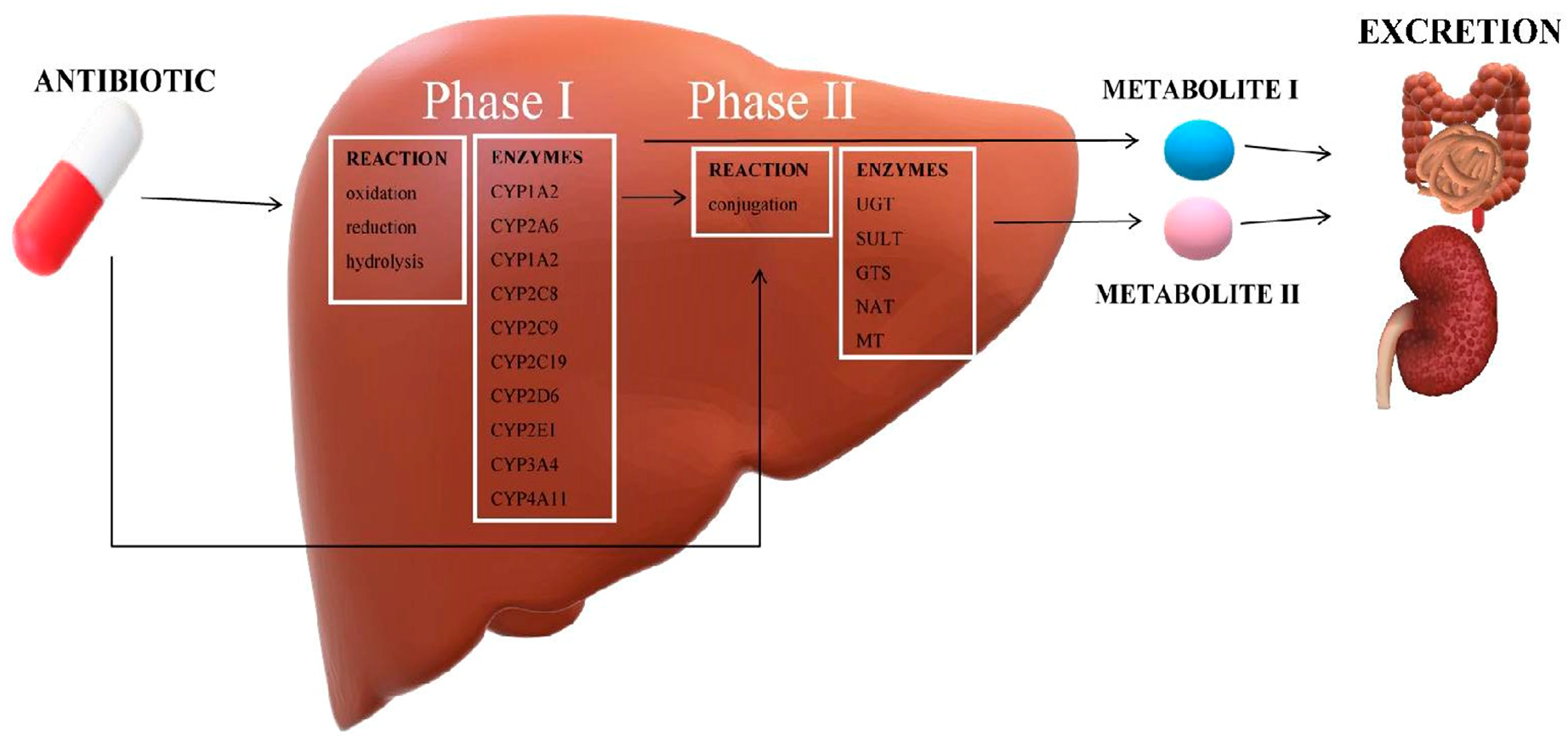
3. Drug Metabolism
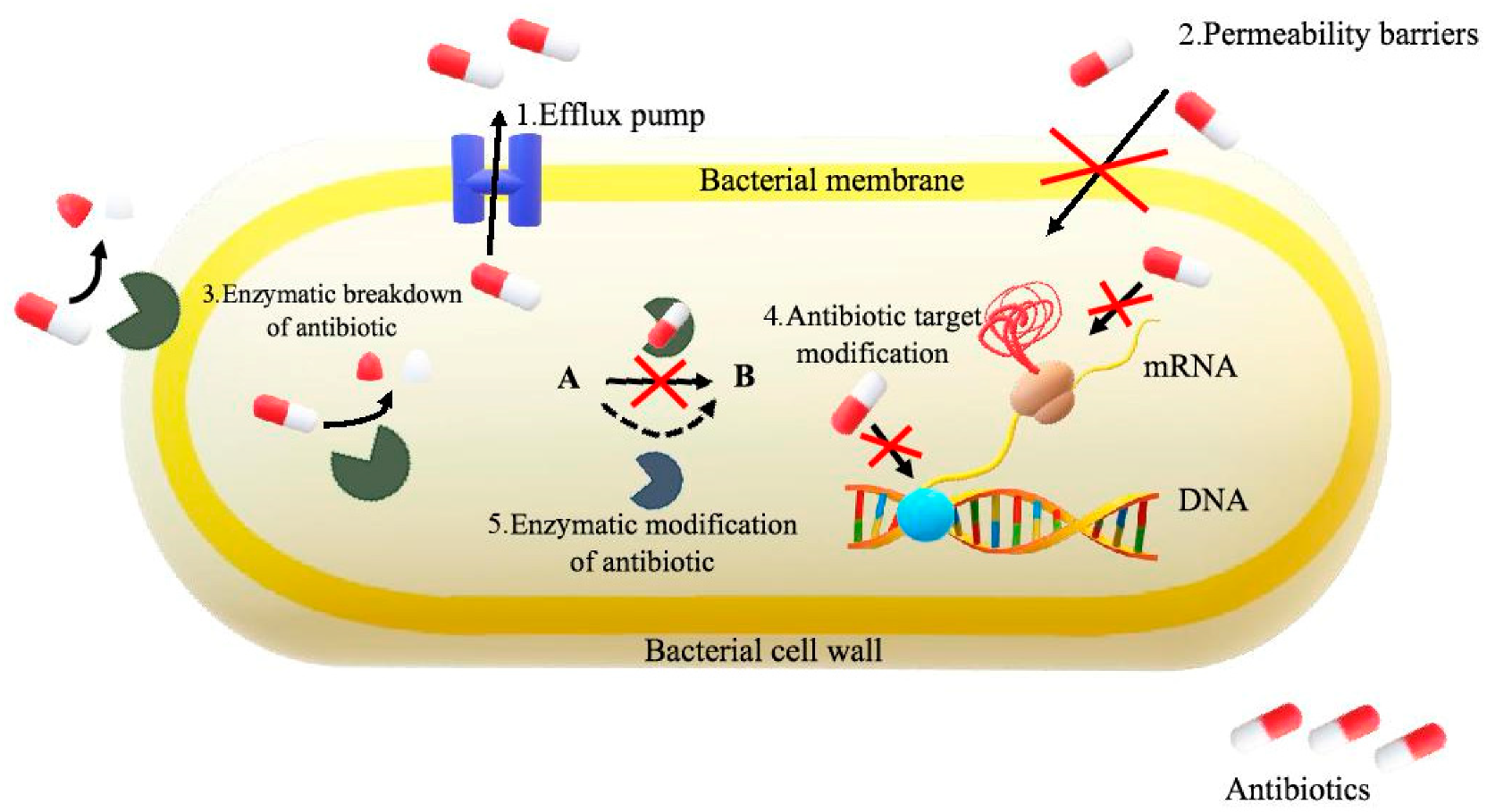
4. Antibiotic Resistance
- The active efflux, which prevents the achievement of the antibiotic target, i.e., the place where the function of the bacterial cell is damaged;
- The reduction of the permeability of the bacterial cell membrane, which occurs when its composition and function are modified;
- The modification of an antibiotic in its inactive form with the participation of enzymes produced by bacteria; they may change the antibiotic inside or outside the bacterial cell, removing its antibacterial effect;
- The change of the target of the antibiotic, reducing its affinity to it;
- Bacterial mutations resulting in the elimination of bacteria resistant by the antibiotic;
- The occurrence of a mixed population of sensitive and resistant bacteria at antibiotic concentrations on the selection of resistant cells [31].
5. Determination and Identification of Antibiotic Drugs and Their Metabolites
5.1. Microbiological Assay
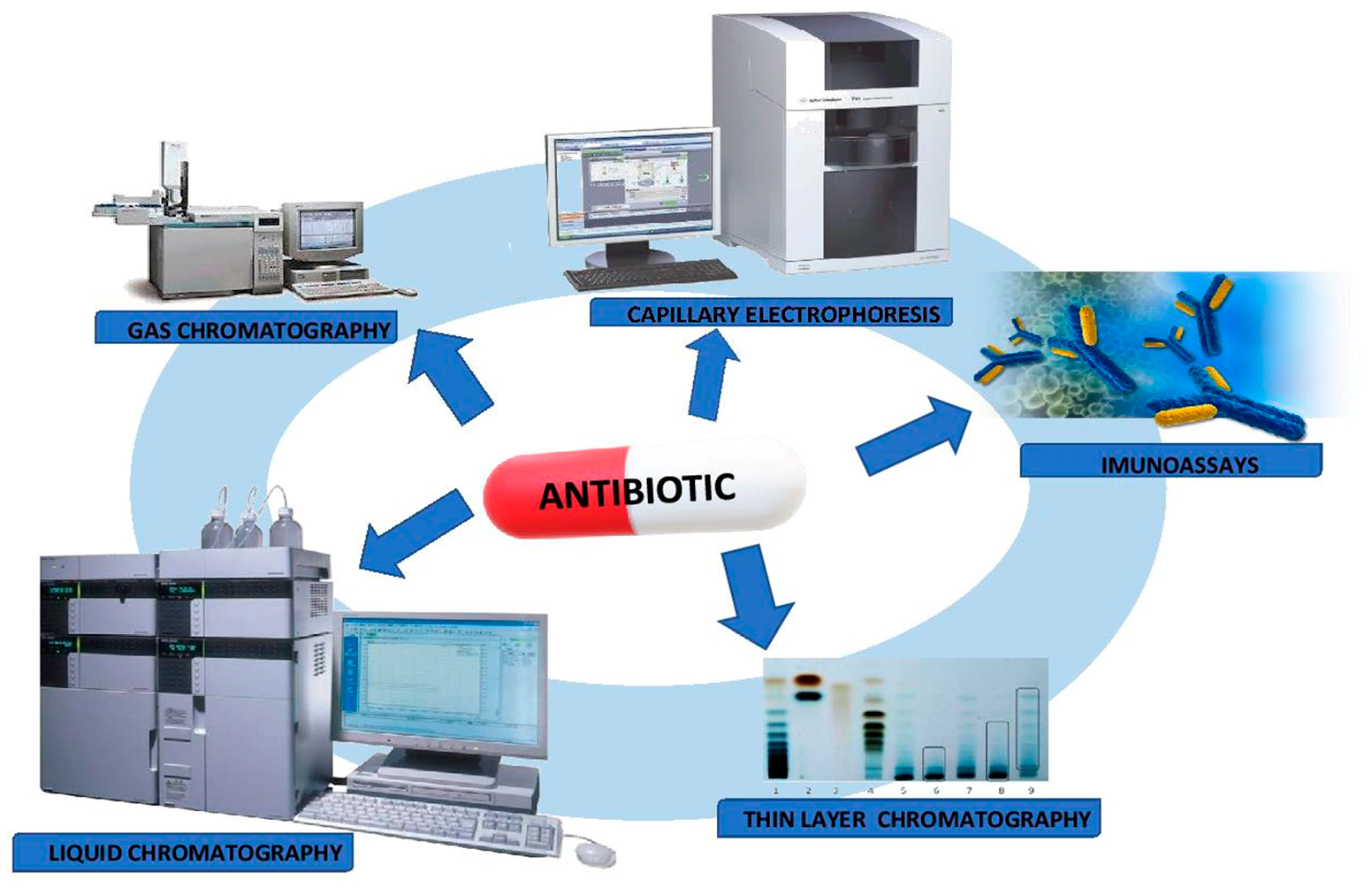
5.2. Analytical Techniques
5.2.1. Immunoassays
5.2.2. Chromatographic Techniques
Thin Layer Chromatography (TLC)
Gas Chromatography (GC)
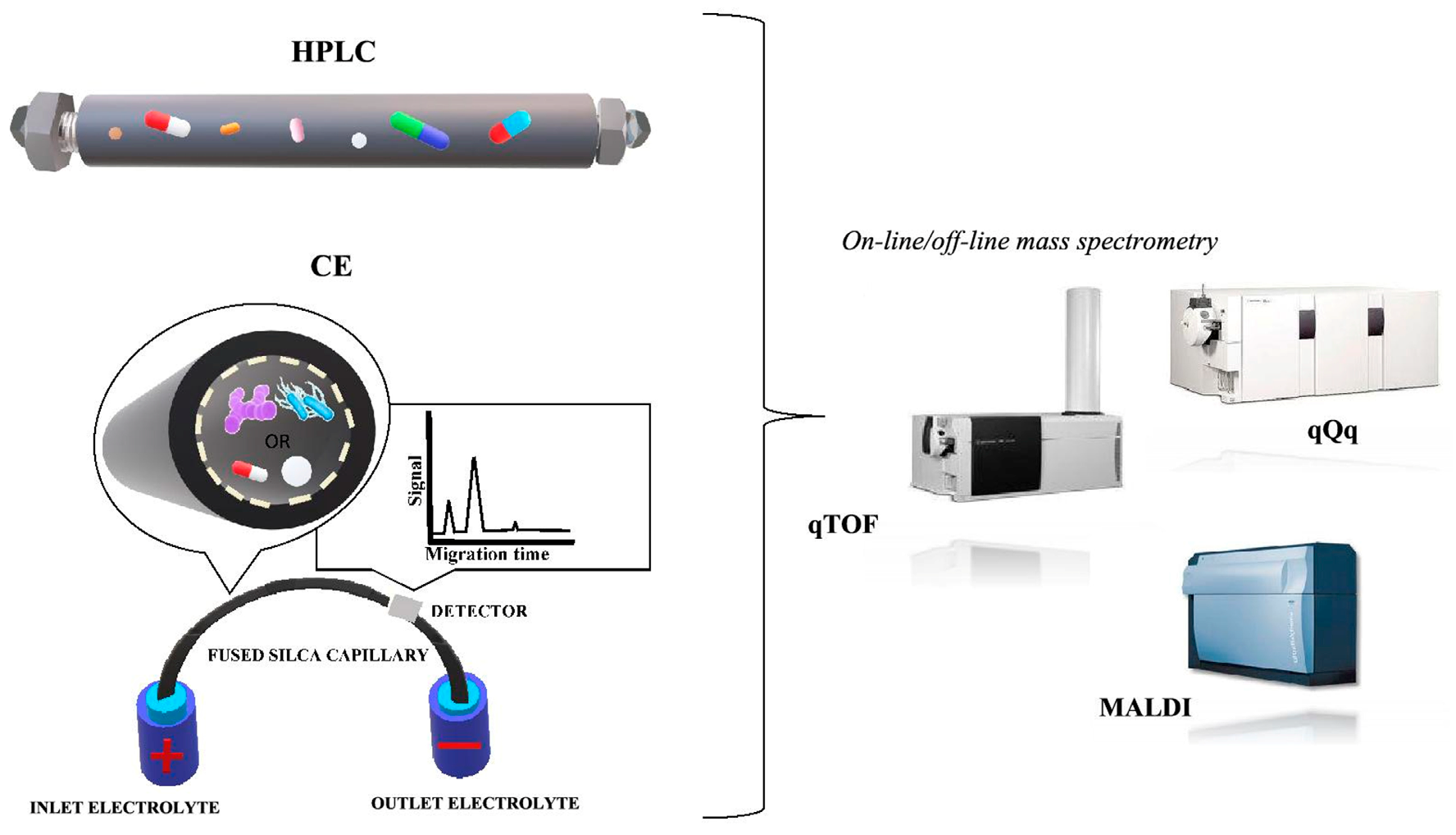
Liquid Chromatography (LC)
Electromigration Techniques
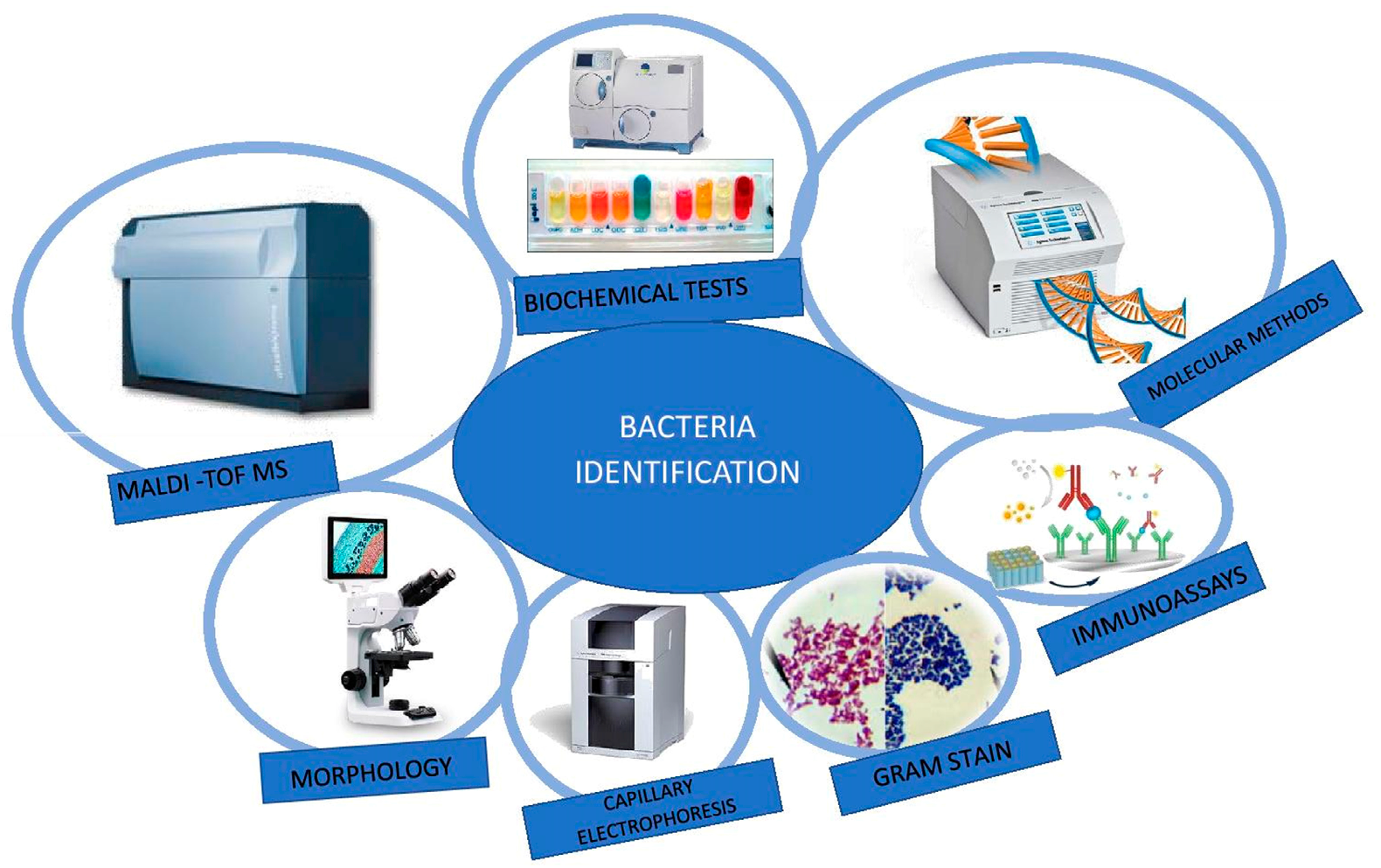
6. Different Analytical Techniques for the Determination and Identification of Microorganisms
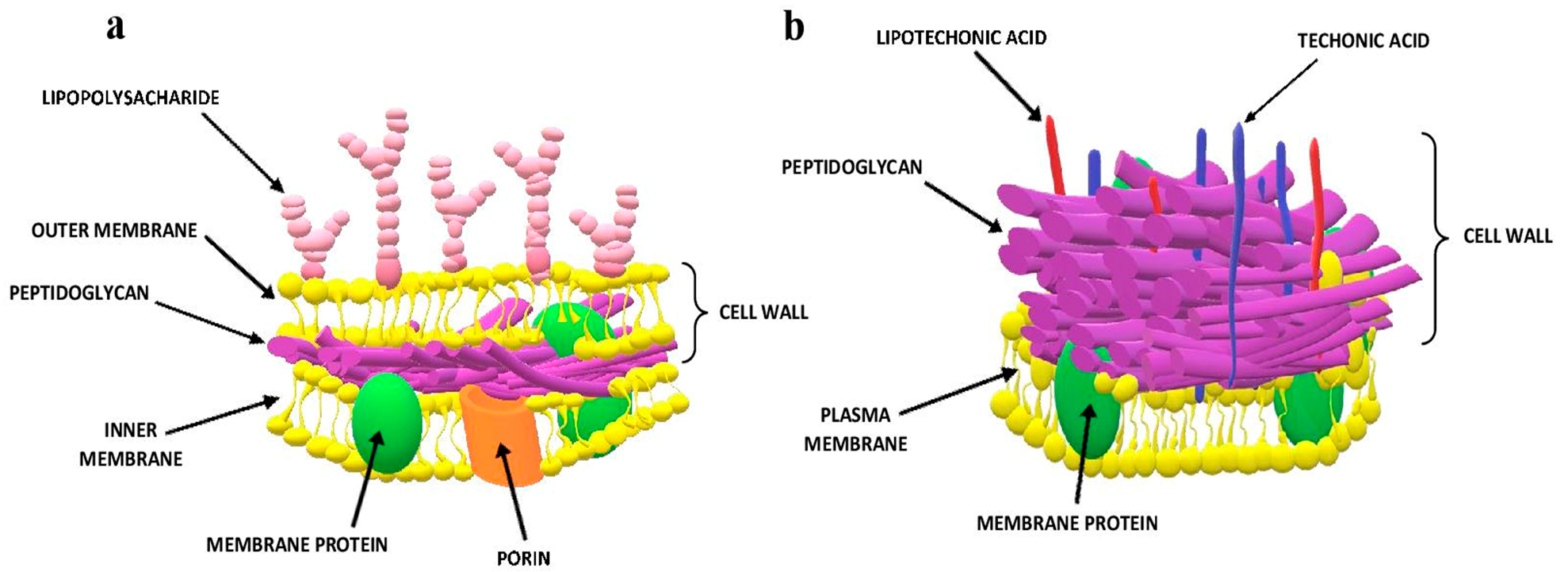
6.1. Gram Staining
6.2. Biochemical Tests
6.3. Immunoassays
6.4. Bacteriophage Typing
6.5. Fatty Acid Profile
6.6. Molecular Methods
6.6.1. DNA Hybridization
6.6.2. PCR-Based Methods
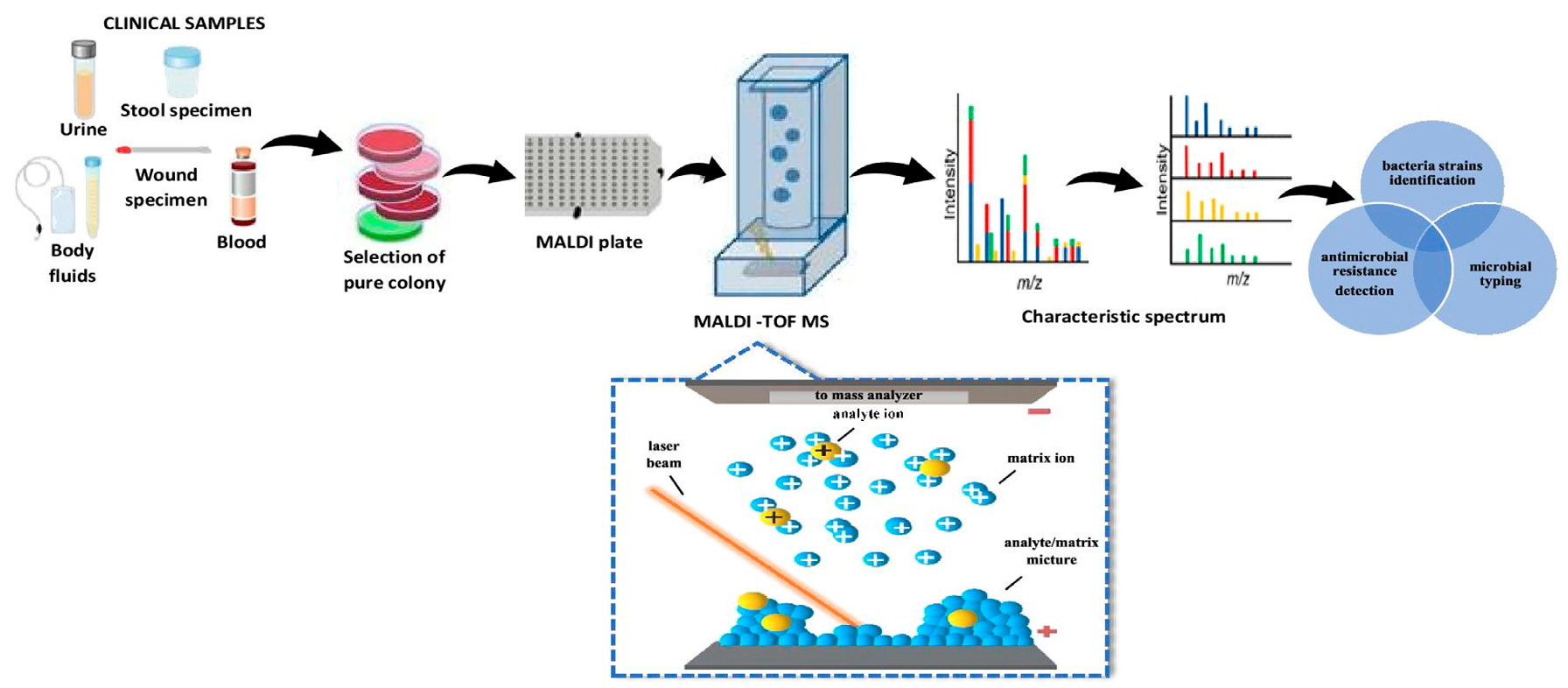
6.7. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
6.8. Capillary Electrophoresis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BGE | background electrolyte |
| C4D | non-contact conductivity detection |
| CAD | charged aerosol detector |
| CITP | capillary isotachophoresis |
| Co | cobalt |
| CZE | capillary zone electrophoresis |
| DAD | diode-array detector |
| ECD | electrochemical detection |
| ELISA | enzyme-linked immunosorbent assay |
| ELSD | evaporative light scattering detector |
| ESBL | extended-spectrum beta-lactamases |
| ESI | electrospray ionization |
| FISH | fluorescent in situ hybridization |
| FL | fluorescence detector |
| FMOC-Cl | 9-fluorenylmethyl chloroformate |
| FPIA | fluorescence polarization immunoassay |
| FTIR | Fourier-transform infrared spectroscopy |
| GC | gas chromatography |
| HILIC | hydrophilic interaction liquid chromatography |
| HPLC | high-performance liquid chromatography |
| LC | liquid chromatography |
| LIF | laser-induced fluorescence |
| LLE | liquid-liquid extraction |
| LTA | lipoteichoic acid molecules |
| MALDI | matrix-assisted laser desorption/ionization |
| MBC | minimum bactericidal concentration |
| MECK | micellar capillary electrokinetic chromatography |
| MEPS | microextraction by packed sorbent |
| MIC | minimum inhibit concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MS | mass spectrometry detector |
| NACK | capillary non-aqueous electrophoresis |
| NMR | nuclear magnetic resonance |
| PBP | penicillin binding protein |
| PCR | polymerase chain reaction |
| PDA | photodiode array detector |
| PGD | potential gradient detection |
| qQq | triple quadrupole mass spectrometer |
| Rf | retention factor |
| SPE | solid phase extraction |
| TBA | Tris-boran-EDTA |
| TDM | therapeutic drug monitoring |
| TEA | trietyloamina |
| TiN | titanium nitride |
| TiO2 | titanium dioxide |
| TLC | thin-layer liquid chromatography |
| TOF | time-of-flight |
| UHPLC | ultra-high performance liquid chromatography |
| UPLC | ultra-performance liquid chromatography |
| UV | ultraviolet |
| VAP | ventilator-associated pneumonia |
| VRE | vancomycin-resistant Enterococcus |
| WHO | World Health Organization |
References
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Trudinger, P.A.; Bubela, B. Microorganisms and the natural environment. Miner. Depos. 1967, 2, 147–157. [Google Scholar] [CrossRef]
- Patel, K.; Patel, J.; Patel, M.; Rajput, G.; Patel, H. Introduction to hyphenated techniques and their applications in pharmacy. Pharm. Methods 2010, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms-From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef]
- Feinberg, T.N. Hyphenated characterization techniques. In Handbook of Isolation and Characterization of Impurities in Pharmaceuticals; Ahuja, S., Alsante, K., Eds.; Academic Press: Cambridge, MA, USA, 2004; Volume 5, pp. 341–359. ISBN 1877-1718. [Google Scholar]
- Nelson, M.L.; Dinardo, A.; Hochberg, J.; Armelagos, G.J. Brief communication: Mass spectroscopic characterization of tetracycline in the skeletal remains of an ancient population from Sudanese Nubia 350-550 CE. Am. J. Phys. Anthropol. 2010, 143, 151–154. [Google Scholar] [CrossRef]
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881-1955): Discoverer of penicillin. Singap. Med. J. 2015, 56, 366–367. [Google Scholar] [CrossRef]
- Spížek, J.; Sigler, K.; Řezanka, T.; Demain, A. Biogenesis of antibiotics-viewing its history and glimpses of the future. Folia Microbiol. 2016, 61, 347–358. [Google Scholar] [CrossRef]
- Kon, K.; Rai, M. Antibiotic Resistance, Mechanisms and New Antimicrobial Approaches; Academic Press: London, UK, 2016. [Google Scholar]
- MacGowan, A.; Macnaughton, E. Antimicrobial therapy: Principles of use. Medicine 2017, 45, 614–621. [Google Scholar] [CrossRef]
- Walsh, C. Antibiotics That Act on Cell Wall Biosynthesis. In Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003; pp. 22–49. [Google Scholar]
- Miller, W.R.; Bayer, A.S.; Arias, C.A. Mechanism of action and resistance to daptomycin in Staphylococcus aureus and Enterococci. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Walsh, C. Antibiotics That Block Bacterial Protein Biosynthesi. In Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003; pp. 51–69. [Google Scholar]
- Walsh, C. Antibiotics That Block DNA Replication and Repair: The Quinolones. In Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003; pp. 70–77. [Google Scholar]
- Walsh, C. Other Targets of Antibacterial Drugs. In Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003; pp. 78–88. [Google Scholar]
- Ionescu, C.; Caira, M.R. Drug Metabolism: Current Concepts; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Thijssen, H.H.W. Identification of the active metabolites of the isoxazolyl-penicillins by means of mass spectrometry. J. Antibiot. 1979, 32, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Easmon, C.S.F.; Ison, C.A.; Kaye, C.M.; Timewell, R.M.; Dawsont, S.G. Pharmacokinetics of metronidazole and its principal metabolites and their activity against Gardnerella vaginalis. Br. J. Vener. Dis. 1982, 58, 246–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, F.F. Metabolism of clindamycin II: Urinary excretion products of clindamycin in rat and dog. J. Pharm. Sci. 1973, 62, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.P.; Troc, K.A.; Thompson, K.D. Activity of metronidazole and its hydroxy and acid metabolites against clinical isolates of anaerobic bacteria. Antimicrob. Agents Chemother. 1982, 22, 426–430. [Google Scholar] [CrossRef]
- Hardy, D.J.; Swanson, R.N.; Rode, R.A.; Marsh, K.; Shipkowitz, N.L.; Clement, J.J. Enhancement of the in vitro and in vivo activities of clarithromycin against Haemophilus influenzae by 14-hydroxy-clarithromycin, its major metabolite in humans. Antimicrob. Agents Chemother. 1990, 34, 1407–1413. [Google Scholar] [CrossRef]
- Piedrola, G.; Galan, I.; Leyva, A.; Maroto, M.C. Comparison of in Vitro Activity of Cefotaxime and Desacetylcefotaxime Alone and in Combination against 320 Gram-Negative Clinical Isolates. Drugs 1988, 35, 62–64. [Google Scholar] [CrossRef]
- Yanagihara, K.; Akamatsu, N.; Matsuda, J.; Kaku, N.; Katsumata, K.; Kosai, K. Susceptibility of Clostridium species isolated in Japan to fi K. Susce and its major metabolite OP-1118. J. Infect. Chemother. 2018, 24, 492–495. [Google Scholar] [CrossRef]
- Shanker, S.; Toohey, M.; Munro, R. In vitro activity of seventeen antimicrobial agents against Gardnerella vaginalis. Eur. J. Clin. Microbiol. 1982, 1, 298–300. [Google Scholar] [CrossRef]
- Adjei, M.D.; Heinze, T.M.; Deck, J.; Freeman, J.P.; Williams, A.J.; Sutherland, J.B. Transformation of the antibacterial agent norfloxacin by environmental mycobacteria. Appl. Environ. Microbiol. 2006, 72, 5790–5793. [Google Scholar] [CrossRef]
- Angehrn, P.; Hohl, P.; Then, R.L. In vitro antibacterial properties of cefetamet and in vivo activity of its orally absorbable ester derivative, cefetamet pivoxil. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 536–543. [Google Scholar] [CrossRef]
- Salmon, S.A.; Watts, J.L.; Yancey, R.J., Jr. In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance. J. Vet. Diagn. Investig. 1996, 8, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Lobanovska, M.; Pilla, G. Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar] [PubMed]
- Zhanel, G.G.; Hoban, D.J.; Schurek, K.; Karlowsky, J.A. Role of efflux mechanisms on fluoroquinolone resistance in Streptococcus pneumoniae and Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2004, 24, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.F.; Moulin, G. Antimicrobial resistance: A complex issue. Rev. Sci. Tech. 2012, 31, 23–31. [Google Scholar] [CrossRef]
- Dafale, N.A.; Semwal, U.P.; Rajput, R.K.; Singh, G.N. Selection of appropriate analytical tools to determine the potency and bioactivity of antibiotics and antibiotic resistance. J. Pharm. Anal. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics 2019, 9, 49. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; D’Antonio, D.; Catamo, G.; Ballone, E.; Piccolomini, R. Comparison of Etest, agar dilution, broth microdilution and disk diffusion methods for testing in vitro activity of levofloxacin against Staphylococcus spp. isolated from neutropenic cancer patients. Int. J. Antimicrob. Agents 2002, 19, 147–154. [Google Scholar] [CrossRef]
- Kontopidou, F.N.; Galani, I.; Panagea, T.; Antoniadou, A.; Souli, M.; Paramythiotou, E.; Koukos, G.; Karadani, I.; Armaganidis, A.; Giamarellou, H. Comparison of direct antimicrobial susceptibility testing methods for rapid analysis of bronchial secretion samples in ventilator-associated pneumonia. Int. J. Antimicrob. Agents 2011, 38, 130–134. [Google Scholar] [CrossRef]
- Gianecini, R.; Oviedo, C.; Irazu, L.; Rodríguez, M.; Galarza, P. Comparison of disk diffusion and agar dilution methods for gentamicin susceptibility testing of Neisseria gonorrhoeae. Diagn. Microbiol. Infect. Dis. 2018, 91, 299–304. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, M.H. Overview of Therapeutic Drug Monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Milone, M.C. Analytical techniques used in therapeutic drug monitoring. In Therapeutic Drug Monitoring; Dasgupta, A., Ed.; Academic Press: London, UK, 2012; pp. 49–73. [Google Scholar] [CrossRef]
- Dasgupta, A. Advances in antibiotic measurement. In Advance in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; Volume 56, pp. 75–104. [Google Scholar] [CrossRef]
- Farouk, F.; Azzazy, H.M.E.; Niessen, W.M.A. Challenges in the determination of aminoglycoside antibiotics, a review. Anal. Chim. Acta 2015, 890, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A. Limitations of immunoassays used for therapeutic drug monitoring of immunosuppressants. In Personalized Immunosuppression in Transplantation-Role of Biomarker Monitoring and Therapeutic Drug Monitoring; Oellerich, M., Dasgupta, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 29–56. [Google Scholar] [CrossRef]
- Munro, A.J.; Landon, J.; Shaw, E.J. The basis of immunoassays for antibiotics. J. Antimicrob. Chemother. 1982, 9, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Navarro, N.; Gallego-Iglesias, E.; Maquieira, Á.; Puchades, R. Immunochemical method for sulfasalazine determination in human plasma. Anal. Chim. Acta 2007, 583, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Shanin, I.A.; Shaimardanov, A.R.; Thai, N.T.D.; Eremin, S.A. Determination of fluoroquinolone antibiotic levofloxacin in urine by fluorescence polarization immunoassay. J. Anal. Chem. 2015, 70, 712–717. [Google Scholar] [CrossRef]
- Dijkstra, J.A.; Voerman, A.J.; Greijdanus, B.; Touw, D.J.; Alffenaar, J.W.C. Immunoassay Analysis of Kanamycin in Serum Using the Tobramycin Kit. Antimicrob. Agents Chemother. 2016, 60, 4646–4651. [Google Scholar] [CrossRef]
- Merola, G.; Martini, E.; Tomassetti, M.; Campanella, L. Simple and suitable immunosensor for β-lactam antibiotics analysis in real matrixes: Milk, serum, urine. J. Pharm. Biomed. Anal. 2014. [Google Scholar] [CrossRef]
- Pollap, A.; Kochana, J. Electrochemical Immunosensors for Antibiotic Detection. Biosensors 2019, 9, 61. [Google Scholar] [CrossRef]
- Council of Europe. Neomycin sulfate (Neomycin sulfas). In European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2015. [Google Scholar]
- Jain, N.; Jain, G.K.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Development and validation of an HPTLC method for determination of minocycline in human plasma. Acta Chromatogr. 2007, 19, 197–205. [Google Scholar]
- Ghoulipour, V.; Shokri, M.; Waqif-Husain, S. Determination of ampicillin and amoxicillin by high-performance thin-layer chromatography. Acta Chromatogr. 2011, 23, 483–498. [Google Scholar] [CrossRef]
- Gusev, A.I.; Proctor, A.; Hercules, D.M.; Rabinovich, Y.I. Thin-Layer Chromatography Combined with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Chem. 1995, 67, 1805–1814. [Google Scholar] [CrossRef]
- Crecelius, A.; Clench, M.R.; Richards, D.S.; Parr, V. Thin-layer chromatography-matrix-assisted laser desorption ionisation-time-of-flight mass spectrometry using particle suspension matrices. J. Chromatogr. A 2002, 958, 249–260. [Google Scholar] [CrossRef]
- Ahuja, S. Derivatization in gas chromatography. J. Pharm. Sci. 1976, 65, 63–182. [Google Scholar] [CrossRef] [PubMed]
- Kanfer, I.; Skinner, M.F.; Walker, R.B. Analysis of macrolide antibiotics. J. Chromatogr. A 1998, 812, 255–286. [Google Scholar] [CrossRef]
- Thangadurai, S. Gas chromatographic–mass spectrometric determination of azithromycin in biological fluids. J. Anal. Sci. Technol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Chiavarino, B.; Crestoni, M.E.; Di Marzio, A.; Fornarini, S. Determination of sulfonamide antibiotics by gas chromatography coupled with atomic emission detection. J. Chromatogr. B Biomed. Appl. 1998, 706, 269–277. [Google Scholar] [CrossRef]
- Główka, F.K.; Karaźniewicz-Łada, M. Determination of roxithromycin in human plasma by HPLC with fluorescence and UV absorbance detection: Application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 669–673. [Google Scholar] [CrossRef]
- Szultka-Mlynska, M.; Buszewski, B. Chromatographic behavior of selected antibiotic drugs supported by quantitative structure-retention relationships. J. Chromatogr. A 2016, 1478, 50–59. [Google Scholar] [CrossRef]
- Rambla-Alegre, M.; Martí-Centelles, R.; Esteve-Romero, J.; Carda-Broch, S. Application of a liquid chromatographic procedure for the analysis of penicillin antibiotics in biological fluids and pharmaceutical formulations using sodium dodecyl sulphate/propanol mobile phases and direct injection. J. Chromatogr. A 2011, 1218, 4972–4981. [Google Scholar] [CrossRef]
- Locatelli, M.; Ciavarella, M.T.; Paolino, D.; Celia, C.; Fiscarelli, E.; Ricciotti, G.; Pompilio, A.; di Bonaventura, G.; Grande, R.; Zengin, G.; et al. Determination of ciprofloxacin and levofloxacin in human sputum collected from cystic fibrosis patients using microextraction by packed sorbent-high performance liquid chromatography photodiode array detector. J. Chromatogr. A 2015, 1419, 58–66. [Google Scholar] [CrossRef]
- Szultka-Mlynska, M.; Pomastowski, P.; Buszewski, B. Application of solid phase microextraction followed by liquid chromatography-mass spectrometry in the determination of antibiotic drugs and their metabolites in human whole blood and tissue samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1086, 153–165. [Google Scholar] [CrossRef]
- Joseph, A.; Patel, S.; Rustum, A. Development and validation of a RP-HPLC method for the estimation of netilmicin sulfate and its related substances using charged aerosol detection. J. Chromatogr. Sci. 2010, 48, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Soliven, A.; Ahmad, I.A.H.; Tam, J.; Kadrichu, N.; Challoner, P.; Markovich, R.J.; Blasko, A. A simplified guide for charged aerosol detection of non-chromophoric compounds-Analytical method development and validation for the HPLC assay of aerosol particle size distribution for amikacin. J. Pharm. Biomed. Anal. 2017, 143, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Tzouganaki, Z.; Koupparis, M. Development and validation of an HPLC method for the determination of the macrolide antibiotic clarithromycin using evaporative light scattering detector in raw materials and pharmaceutical formulations. Mediterr. J. Chem. 2017, 6, 133–141. [Google Scholar] [CrossRef]
- Wongchang, T.; Winterberg, M.; Tarning, J.; Sriboonvorakul, N.; Muangnoicharoen, S.; Blessborn, D. Determination of ceftriaxone in human plasma using liquid chromatography-tandem mass spectrometry. Wellcome Open Res. 2019, 4, 47. [Google Scholar] [CrossRef]
- Borner, K.; Borner, E.; Lode, H. Determination of linezolid in human serum and urine by high-performance liquid chromatography. Int. J. Antimicrob. Agents 2001, 18, 253–258. [Google Scholar] [CrossRef]
- Paal, M.; Zoller, M.; Schuster, C.; Vogeser, M.; Schütze, G. Simultaneous quantification of cefepime, meropenem, ciprofloxacin, moxifloxacin, linezolid and piperacillin in human serum using an isotope-dilution HPLC-MS/MS method. J. Pharm. Biomed. Anal. 2018, 152, 102–110. [Google Scholar] [CrossRef]
- Farshchi, A.; Ghiasi, G.; Bahrami, G.A. Sensitive Liquid Chromatographic Method for the Analysis of Clarithromycin with Pre-Column Derivatization: Application to a Bioequivalence Study. Iran. J. Basic Med. Sci. 2009, 12, 25–32. [Google Scholar]
- Magréault, S.; Leroux, S.; Touati, J.; Storme, T.; Jacqz-Aigrain, E. UPLC/MS/MS assay for the simultaneous determination of seven antibiotics in human serum—Application to pediatric studies. J. Pharm. Biomed. Anal. 2019, 174, 256–262. [Google Scholar] [CrossRef]
- Kathriarachchi, U.L.; Vidhate, S.S.; Al-tannak, N.; Thomson, A.H.; Michael, J.J.; Neto, S.; Watson, D.G. Development of a LC-MS method for simultaneous determination of amoxicillin and metronidazole in human serum using hydrophilic interaction chromatography (HILIC). J. Chromatogr. B 2018, 1089, 78–83. [Google Scholar] [CrossRef]
- Ongas, M.; Standing, J.; Ogutu, B.; Waichungo, J.; Berkley, J.A.; Kipper, K. Liquid chromatography-tandem mass spectrometry for the simultaneous quantitation of ceftriaxone, metronidazole and hydroxymetronidazole in plasma from seriously ill, severely malnourished children. Wellcome Open Res. 2018, 2, 1–37. [Google Scholar] [CrossRef]
- Carlier, M.; Stove, V.; De Waele, J.J.; Verstraete, A.G. Ultrafast quantification of β-lactam antibiotics in human plasma using UPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 978–979, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; Guerra Valero, Y.C.; Roberts, D.M.; Lipman, J.; Roberts, J.A.; Wallis, S.C. Determination of Cefalothin and Cefazolin in Human Plasma, Urine and Peritoneal Dialysate by UHPLC-MS/MS: Application to a pilot pharmacokinetic study in humans. Biomed. Chromatogr. 2016, 30, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Ezzeldin, E.; El-Nahhas, T.M. New analytical method for the determination of metronidazole in human plasma: Application to bioequivalence study. Trop. J. Pharm. Res. 2012, 11, 799–805. [Google Scholar] [CrossRef][Green Version]
- Jeffery, J.; Vincent, Z.J.; Ayling, R.M.; Lewis, S.J. Development and validation of a liquid chromatography tandem mass spectrometry assay for the measurement of faecal metronidazole. Clin. Biochem. 2017, 50, 323–330. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Guo, B.; Zhang, J.; Li, Y.; Wu, X.; Fan, Y.; Chen, Y.; Cao, G.; Yu, J. Determination of the sulfate and glucuronide conjugates of levornidazole in human plasma and urine, and levornidazole and its five metabolites in human feces by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081–1082, 87–100. [Google Scholar] [CrossRef]
- Sudha, V.; Ramachandran, G.; Hemanth Kumar, A.K.; Vijayakumar, A.; Polisetty, A.K. A selective and sensitive high performance liquid chromatography assay for the determination of cycloserine in human plasma. Indian J. Tuberc. 2017, 65, 118–123. [Google Scholar] [CrossRef]
- Parker, S.L.; Lipman, J.; Roberts, J.A.; Wallis, S.C. A simple LC-MS/MS method using HILIC chromatography for the determination of fosfomycin in plasma and urine: Application to a pilot pharmacokinetic study in humans. J. Pharm. Biomed. Anal. 2015, 105, 39–45. [Google Scholar] [CrossRef][Green Version]
- Samanidou, V.F.; Evaggelopoulou, E.N.; Papadoyannis, I.N. Development of a validated HPLC method for the determination of four penicillin antibiotics in pharmaceuticals and human biological fluids. J. Sep. Sci. 2006, 29, 1550–1560. [Google Scholar] [CrossRef]
- Naicker, S.; Valero, Y.C.G.; Meija, J.L.O.; Lipman, J.; Roberts, J.A.; Wallis, S.C.; Parker, S.L. A UHPLC–MS/MS method for the simultaneous determination of piperacillin and tazobactam in plasma (total and unbound), urine and renal replacement therapy effluent. J. Pharm. Biomed. Anal. 2017, 148, 324–333. [Google Scholar] [CrossRef]
- Carlier, M.; Stove, V.; Roberts, J.A.; Van De Velde, E.; De Waele, J.J.; Verstraete, A.G. Quantification of seven β-lactam antibiotics and two β-lactamase inhibitors in human plasma using a validated UPLC-MS/MS method. Int. J. Antimicrob. Agents 2012, 40, 416–422. [Google Scholar] [CrossRef]
- Legrand, T.; Vodovar, D.; Tournier, N.; Khoudour, N. Simultaneous Determination of Eight-Lactam Antibiotics, Performance Liquid Chromatography with Ultraviolet Detection. Antimicrob. Agents Chemother. 2016, 60, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Brunner, L.J.; Dipiro, J.T. Capillary electrophoresis for therapeutic drug monitoring. Electrophoresis 2005, 19, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Mallampati, S.; Pauwels, J.; Hoogmartens, J.; Van Schepdael, A. CE in impurity profiling of drugs. Sep. Sci. Technol. 2008, 9, 259–315. [Google Scholar] [CrossRef]
- El Deeb, S.; Wätzig, H.; Abd El-Hady, D.; Sänger-van de Griend, C.; Scriba, G.K.E. Recent advances in capillary electrophoretic migration techniques for pharmaceutical analysis (2013–2015). Electrophoresis 2016, 37, 1591–1608. [Google Scholar] [CrossRef] [PubMed]
- Greño, M.; Castro-Puyana, M.; García, M.Á.; Marina, M.L. Analysis of antibiotics by CE and CEC and their use as chiral selectors: An update. Electrophoresis 2018, 39, 235–259. [Google Scholar] [CrossRef]
- Paul, P.; Van Laeken, C.; Sänger-van de Griend, C.; Adams, E.; Van Schepdael, A. CE-C4D method development and validation for the assay of ciprofloxacin. J. Pharm. Biomed. Anal. 2016, 129, 1–8. [Google Scholar] [CrossRef]
- Sánchez-Hernández, L.; Marina, M.L. Potential of Vancomycin for the Enantiomeric Resolution of FMOC-AMINO Acids by Capillary Electrophoresis-Ion-Trap-Mass Spectrometry. Electrophoresis 2014, 35, 1244–1250. [Google Scholar] [CrossRef]
- Solangi, A.R.; Memon, S.Q.; Khuhawar, M.Y.; Bhanger, M.I. Quantitative analysis of eight cephalosporin antibiotics in pharmaceutical products and urine by capillary zone electrophoresis. Acta Chromatogr. 2007, 19, 81–96. [Google Scholar]
- Hernández-Mesa, M.; D’Orazio, G.; Rocco, A.; García-Campaña, A.M.; Blanco, C.C.; Fanali, S. Capillary electrochromatography-mass spectrometry for the determination of 5-nitroimidazole antibiotics in urine samples. Electrophoresis 2015, 36, 2606–2615. [Google Scholar] [CrossRef]
- Tůma, P.; Jaček, M.; Fejfarová, V.; Polák, J. Electrophoretic stacking for sensitive determination of antibiotic ceftazidime in human blood and microdialysates from diabetic foot. Anal. Chim. Acta 2016, 942, 139–145. [Google Scholar] [CrossRef]
- Andrási, M.; Gáspár, A.; Klekner, Á. Analysis of cephalosporins in bronchial secretions by capillary electrophoresis after simple pretreatment. J. Chromatogr. B 2007, 846, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Berzas Nevado, J.J.; Castañeda Peñalvo, G.; Guzman Bernardo, F.J. Micellar electrokinetic chromatography method for the determination of sulfamethoxazole, trimethoprim and their main metabolites in human serum. J. Sep. Sci. 2005, 28, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, A.; Kardos, S.; Andrási, M.; Klekner, Á. Capillary electrophoresis for the direct determination of cephalosporins in clinical samples. Chromatographia 2006, 56, S109–S114. [Google Scholar] [CrossRef]
- Kitahashi, T.; Furuta, I. Determination of vancomycin in human serum by micellar electrokinetic capillary chromatography with direct sample injection. Clin. Chim. Acta 2001, 312, 221–225. [Google Scholar] [CrossRef]
- Griese, N.; Blaschke, G.; Boos, J.; Hempel, G. Determination of free and liposome-associated daunorubicin and daunorubicinol in plasma by capillary electrophoresis. J. Chromatogr. A 2002, 979, 379–388. [Google Scholar] [CrossRef]
- Mrestani, Y.; Neubert, R.H.H.; Härtl, A.; Wohlrab, J. Determination of cephalosporins in urine and bile by capillary zone electrophoresis. Anal. Chim. Acta 1997, 349, 207–213. [Google Scholar] [CrossRef]
- Ferdig, M.; Kaleta, A.; Thanh Vo, T.D.; Buchberger, W. Improved capillary electrophoretic separation of nine (fluoro)quinolones with fluorescence detection for biological and environmental samples. J. Chromatogr. A 2004, 1047, 305–311. [Google Scholar] [CrossRef]
- Kłodzińska, E.; Jaworski, M.; Kupczyk, W.; Jackowski, M.; Buszewski, B. A study of interactions between bacteria and antibiotics by capillary electrophoresis. Electrophoresis 2012, 33, 3095–3100. [Google Scholar] [CrossRef]
- Buszewski, B.; Rogowska, A.; Pomastowski, P.; Złoch, M.; Railean-Plugaru, V. Identification of microorganisms by modern analytical techniques. J. AOAC Int. 2017, 100, 1607–1623. [Google Scholar] [CrossRef]
- Raghavendra, P.; Pullaiah, T. Pathogen Identification Using Novel Sequencing Methods. In Advances in Cell and Molecular Diagnostics; Academic Press: London, UK, 2018; pp. 161–199. [Google Scholar] [CrossRef]
- Bailón-Salas, A.M.; Medrano-Roldán, H.; Valle-Cervantes, S.; Ordaz-Díaz, L.A.; Urtiz-Estrada, N.; Rojas-Contreras, J.A. Review of molecular techniques for the identification of bacterial communities in biological effluent treatment facilities at pulp and paper mills. BioResources 2017, 12, 4384–4409. [Google Scholar] [CrossRef]
- Depelteau, J.S.; Brenzinger, S.; Briegel, A. Bacterial and Archaeal Cell Structure. Ref. Modul. Life Sci. 2018, 9, 414–426. [Google Scholar] [CrossRef]
- Frank, K.M. Microbiology in Clinical Pathology. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; McManus, L.M., Mitchell, R.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3237–3268. [Google Scholar] [CrossRef]
- Becerra, S.C.; Roy, D.C.; Sanchez, C.J.; Christy, R.J.; Burmeister, D.M. An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue. BMC Res. Notes 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Bishop, B.; Geffen, Y.; Plaut, A.; Kassis, O.; Bitterman, R.; Paul, M.; Neuberger, A. The use of matrix-assisted laser desorption/ionization time of-flight mass spectrometry for rapid bacterial identification in patients with smear-positive bacterial meningitis. Clin. Microbiol. Infect. 2018, 24, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Nonhoff, C.; Rottiers, S.; Struelens, M.J. Evaluation of the Vitek 2 system for identification and antimicrobial susceptibility testing of Staphylococcus spp. Clin. Microbiol. Infect. 2005, 11, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Marr, I.; Sarmento, N.; Obrien, M.; Lee, K.; Gusmao, C.; de Castro, G.; Janson, S.; Tong, S.Y.C.; Baird, R.; Francis, J.R. Antimicrobial resistance in urine and skin isolates in Timor-Leste. J. Glob. Antimicrob. Resist. 2018, 13, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Kierzkowska, M.; Majewska, A.; Kuthan, R.T.; Sawicka-Grzelak, A.; Młynarczyk, G. A comparison of Api 20A vs MALDI-TOF MS for routine identification of clinically significant anaerobic bacterial strains to the species level. J. Microbiol. Methods 2013, 92, 209–212. [Google Scholar] [CrossRef]
- Hogan, C.A.; Watz, N.; Budvytiene, I.; Banaei, N. Rapid antimicrobial susceptibility testing by VITEK®2 directly from blood cultures in patients with Gram-negative rod bacteremia. Diagn. Microbiol. Infect. Dis. 2019, 94, 6–11. [Google Scholar] [CrossRef]
- Chung, J.W.; Jeon, H.S.; Sung, H.; Kim, M.N. Evaluation of MicroScan and Phoenix system for rapid identification and susceptibility testing using direct inoculation from positive BACTEC blood culture bottles. Korean J. Lab. Med. 2009, 29, 25–34. [Google Scholar] [CrossRef]
- Sellenriek, P.; Holmes, J.; Ferrett, R.; Drury, R.; Storch, G.A. Comparison of MicroScan Walk-Away, Phonix and VITEK-TWO Microbiology Systems Used in the Identification and Susceptibility Testing of Bacteria. In Proceedings of the 105th General Meeting ASM, Atlanta, GA, USA, 9 June 2005. [Google Scholar]
- Idelevich, E.A.; Sparbier, K.; Kostrzewa, M.; Becker, K. Rapid detection of antibiotic resistance by MALDI-TOF mass spectrometry using a novel direct-on-target microdroplet growth assay. Clin. Microbiol. Infect. 2018, 24, 738–743. [Google Scholar] [CrossRef]
- Perry, J.D.; Freydière, A.M. The application of chromogenic media in clinical microbiology. J. Appl. Microbiol. 2007, 103, 2046–2055. [Google Scholar] [CrossRef]
- Akter, M.L.; Haque, R.; Salam, M.A. Comparative evaluation of chromogenic agar medium and conventional culture system for isolation and presumptive identification of uropathogens. Pak. J. Med. Sci. 2014, 30, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Glupczynski, Y.; Berhin, C.; Bauraing, C.; Bogaerts, P. Evaluation of a new selective chromogenic agar medium for detection of extended-spectrum β-lactamase-producing Enterobacteriaceae. J. Clin. Microbiol. 2007, 45, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Krämer, P.M. Immunochemical Methods. Rapid Chem. Biol. Tech. Water Monit. 2009, 1, 157–173. [Google Scholar] [CrossRef]
- Verma, J.; Saxena, S.; Babu, S.G. Analyzing Microbes. In Anlyzing Microbes; Arora, D.K., Das, S., Sukuman, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 169–186. [Google Scholar] [CrossRef]
- Richter, Ł.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Paczesny, J.; Hołyst, R. Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov. Today 2018, 23, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Wang, L.; Li, Y.; Shi, F.; Wang, X. Differentiation of bacteria using fatty acid profiles from gas chromatography-tandem mass spectrometry. J. Sci. Food Agric. 2010, 90, 1380–1383. [Google Scholar] [CrossRef]
- Cody, R.B.; McAlpin, C.R.; Cox, C.R.; Jensen, K.R.; Voorhees, K.J. Identification of bacteria by fatty acid profiling with direct analysis in real time mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 2007–2012. [Google Scholar] [CrossRef]
- Mothershed, E.A.; Whitney, A.M. Nucleic acid-based methods for the detection of bacterial pathogens: Present and future considerations for the clinical laboratory. Clin. Chim. Acta 2006, 363, 206–220. [Google Scholar] [CrossRef]
- Falkow, S. The Use of DNA Hybridization for the Identification of Pathogenic Bacteria. In Rapid Methods and Automation in Microbiology and Immunology; Habermehl, K.O., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 30–33. [Google Scholar] [CrossRef]
- McLoughlin, K.S. Microarrays for pathogen detection and analysis. Brief. Funct. Genom. 2011, 10, 342–353. [Google Scholar] [CrossRef]
- Jin, D.Z.; Wen, S.Y.; Chen, S.H.; Lin, F.; Wang, S.Q. Detection and identification of intestinal pathogens in clinical specimens using DNA microarrays. Mol. Cell. Probes 2006, 20, 337–347. [Google Scholar] [CrossRef]
- Mullis, K.B. The unusual origin of the polymerase chain reaction. Sci. Am. 1990, 262, 56–65. [Google Scholar] [CrossRef]
- Wolk, D.M.; Kaleta, E.J.; Wysocki, V.H. PCR-electrospray ionization mass spectrometry: The potential to change infectious disease diagnostics in clinical and public health laboratories. J. Mol. Diagn. 2012, 14, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Fdhila, K.; Slama, R.B.; Mahdouani, K.; Hentati, H.; Najjari, F.; Bakhrouf, A.; Chaieb, K. Molecular detection of bacteria associated to dental caries in 4-12-year-old Tunisian children. Microb. Pathog. 2014, 71, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Pechorsky, A.; Nitzan, Y.; Lazarovitch, T. Identification of pathogenic bacteria in blood cultures: Comparison between conventional and PCR methods. J. Microbiol. Methods 2009, 78, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Parta, M.; Goebel, M.M.; Matloobi, M.C.; Stager, C.D.M.; Musher, D.M. Identification of Methicillin-Resistant or Methicillin-Susceptible Staphylococcus aureus in Blood Cultures and Wound Swabs by GeneXper. J. Clin. Microbiol. 2009, 47, 1609–1610. [Google Scholar] [CrossRef]
- Olsen, J.E.; Aabo, S.; Hill, W.E.; Notermans, S.; Wernars, K.; Granum, P.E.; Popovic, T.; Rasmussen, H.N.; Olsvik, O. Probes and polymerase chain reaction for detection of food-borne bacterial pathogens. Int. J. Food Microbiol. 1995, 28, 1–78. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Koduru, J.R.; Park, T.J.; Wu, H.F.; Lin, Y.C. Progress of electrospray ionization and rapid evaporative ionization mass spectrometric techniques for the broad-range identification of microorganisms. Analyst 2019, 144, 1073–1103. [Google Scholar] [CrossRef]
- Brinkman, C.L.; Vergidis, P.; Uhl, J.R.; Pritt, B.S.; Cockerill, F.R.; Steckelberg, J.M.; Baddour, L.M.; Maleszewski, J.J.; Edwards, W.D.; Sampath, R.; et al. PCR-electrospray ionization mass spectrometry for direct detection of pathogens and antimicrobial resistance from heart valves in patients with infective endocarditis. J. Clin. Microbiol. 2013, 51, 2040–2046. [Google Scholar] [CrossRef]
- Buszewski, B.; Szumski, M.; Kłodzińska, E.; Dahm, H. Separation of bacteria by capillary electrophoresis. J. Sep. Sci. 2003, 26, 1045–1049. [Google Scholar] [CrossRef]
- Jackowski, M.; Szeliga, J.; Kłodzińska, E.; Buszewski, B. Application of capillary zone electrophoresis (CZE) to the determination of pathogenic bacteria for medical diagnosis. Anal. Bioanal. Chem. 2008, 391, 2153–2160. [Google Scholar] [CrossRef]
- Buszewski, B.; Kłodzińska, E. Determination of pathogenic bacteria by CZE with surface-modified capillaries. Electrophoresis 2008, 29, 4177–4184. [Google Scholar] [CrossRef]
- Szeliga, J.; Klodzinska, E.; Jackowski, M.; Buszewski, B. The clinical use of a fast screening test based on technology of capillary zone electrophoresis (CZE) for identification of Escherichia coli infection in biological material. Med. Sci. Monit. 2011, 17. [Google Scholar] [CrossRef]
- Klodzińska, E.; Kupczyk, W.; Jackowski, M.; Buszewski, B. Capillary electrophoresis in the diagnosis of surgical site infections. Electrophoresis 2013, 34, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Sing, A. Leptospira spp. Strain identification by MALDI TOF MS is an equivalent tool to 16S rRNA gene sequencing and multi locus sequence typing Leptospira spp. strain identification by MALDI TOF MS is an equivalent tool to 16S rRNA gene sequencing and multi loc. BMC Microbiol. 2016, 12, 1–14. [Google Scholar] [CrossRef]
- Cendejasbueno, E.; Kolecka, A.; Alastrueyizquierdo, A.; Theelen, B.; Groenewald, M.; Kostrzewa, M.; Cuencaestrella, M.; Gomezlopez, A.; Boekhout, T. Reclassification of the Candida haemulonii complex as Candida haemulonii (C. haemulonii group I), C. duobushaemulonii sp. nov. (C. haemulonii group II), and C. haemulonii var. vulnera var. nov.: Three multiresistant human pathogenic yeasts. J. Clin. Microbiol. 2012, 50, 3641–3651. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.P.; Reid, M.; Hadfield, S.J.; Johnston, S.; Mikhail, J.; Harris, L.G.; Jenkinson, H.F.; Berry, N.; Lewis, A.M.; Elbouri, K.; et al. Identification of clinical isolates of α-hemolytic streptococci by 16S rRNA gene sequencing, matrix-assisted laser desorption ionization-time of flight mass spectrometry using MALDI biotyper, and conventional phenotypic methods: A comparison. J. Clin. Microbiol. 2012, 50, 4087–4090. [Google Scholar] [CrossRef][Green Version]
- Krásný, L.; Hynek, R.; Hochel, I. Identification of bacteria using mass spectrometry techniques. Int. J. Mass. Spectrom. 2013, 353, 67–79. [Google Scholar] [CrossRef]
- Pomastowski, P.; Buszewski, B. Complementarity of matrix-and nanostructure-assisted laser desorption/ionization approaches. Nanomaterials 2019, 9, 260. [Google Scholar] [CrossRef]
- Van Belkum, A.; Welker, M.; Pincus, D.; Charrier, J.P.; Girard, V. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry in clinical microbiology: What are the current issues? Ann. Lab. Med. 2017, 37, 475–483. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.; Qiao, L. Direct MALDI-TOF MS Identification of Bacterial Mixtures. Anal. Chem. 2018, 90, 10400–10408. [Google Scholar] [CrossRef]
- Karger, A.; Stock, R.; Ziller, M.; Elschner, M.C.; Bettin, B.; Melzer, F.; Maier, T.; Kostrzewa, M.; Scholz, H.C.; Neubauer, H.; et al. Rapid identification of Burkholderia mallei and Burkholderia pseudomallei by intact cell Matrix-assisted Laser Desorption/Ionisation mass spectrometric typing. BMC Microbiol. 2012, 12, 1–15. [Google Scholar] [CrossRef]
- Saha, R.; Farrance, C.E.; Verghese, B.; Hong, S.; Donofrio, R.S. Klebsiella michiganensis sp. nov., a new bacterium isolated from a tooth brush holder. Curr. Microbiol. 2013, 66, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Mkrtchyan, H.V.; Russell, C.A.; Wang, N.; Cutler, R.R. Could Public Restrooms Be an Environment for Bacterial Resistomes? PLoS ONE 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhang, G.; Fan, Y.Y.; Yang, X.; Sui, W.J.; Lu, X.X. Direct identification of bacteria causing urinary tract infections by combining matrix-assisted laser desorption ionization-time of flight mass spectrometry with UF-1000i urine flow cytometry. J. Microbiol. Methods 2013, 92, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Karamonova, L.; Junkova, P.; Mihalova, D.; Javůrkova, B.; Fukal, L.; Rauch, P.; Blažkova, M. The potential of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for the identification of biogroups of Cronobacter sakazakii. Rapid Commun. Mass Spectrom. 2013, 27, 409–418. [Google Scholar] [CrossRef]
- Mailhac, A.; Durand, H.; Boisset, S.; Maubon, D.; Berger, F.; Maurin, M.; Chiquet, C.; Bidart, M. MALDI-TOF mass spectrometry for rapid diagnosis of postoperative endophthalmitis. J. Proteom. 2017, 152, 150–152. [Google Scholar] [CrossRef]
- Haiko, J.; Savolainen, L.E.; Hilla, R.; Pätäri-Sampo, A. Identification of urinary tract pathogens after 3-hours urine culture by MALDI-TOF mass spectrometry. J. Microbiol. Methods 2016, 129, 81–84. [Google Scholar] [CrossRef]
- Hou, T.Y.; Chiang-Ni, C.; Tengs, S.H. Current status of MALDI-TOF mass spectrometry in clinical microbiology. J. Food Drug Anal. 2019, 27, 404–414. [Google Scholar] [CrossRef]
- Vrioni, G.; Tsiamis, C.; Oikonomidis, G.; Theodoridou, K.; Kapsimali, V.; Tsakris, A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: Current achievements and future perspectives. Ann. Transl. Med. 2018, 6, 240. [Google Scholar] [CrossRef]
- Barberino, M.G.; Silva, M.D.; Arraes, A.C.P.; Correia, L.C.; Mendes, A.V. Direct identification from positive blood broth culture by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS). Braz. J. Infect. Dis. 2017, 21, 339–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ma, Q.; Song, Y.; Zhang, Q.; Wang, X.; Chen, F. Identification of Lactobacillus from the saliva of adult patients with caries using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Spanu, T.; De Carolis, E.; Fiori, B.; Sanguinetti, M.; D’Inzeo, T.; Fadda, G.; Posteraro, B. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to rpoB gene sequencing for species identification of bloodstream infection staphylococcal isolates. Clin. Microbiol. Infect. 2011, 17, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Genc, G.E.; Demir, M.; Yaman, G.; Kayar, B.; Koksal, F.; Satana, D. Evaluation of MALDI-TOF MS for identification of nontuberculous mycobacteria isolated from clinical specimens in mycobacteria growth indicator tube medium. New Microbiol. 2018, 41, 214–219. [Google Scholar] [PubMed]
- Ivanovna Shilnikova, I. Species Identification of Clinical Veillonella Isolates by MALDI-TOF Mass Spectrometry and Evaluation of Their Antimicrobial Susceptibility. Am. J. Biomed. Life Sci. 2017, 5, 82–87. [Google Scholar] [CrossRef]
- Panda, A.; Kurapati, S.; Samantaray, J.C.; Srinivasan, A.; Khalil, S. MALDI-TOF mass spectrometry proteomic based identification of clinical bacterial isolates. Indian J. Med. Res. 2014, 140, 770–777. [Google Scholar]
- Akyar, I.; Can, S. Rapid identification of Aeromonas species in stool samples with chromogenic media and matrix-assisted laser desorption ionization-time of flight mass spectrometry: An institutional experience. Turk. J. Med. Sci. 2013, 43, 388–392. [Google Scholar] [CrossRef]
- Ferreira, L.; Sánchez-Juanes, F.; Muñoz-Bellido, J.L.; González-Buitrago, J.M. Rapid method for direct identification of bacteria in urine and blood culture samples by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: Intact cell vs. extraction method. Clin. Microbiol. Infect. 2011, 17, 1007–1012. [Google Scholar] [CrossRef][Green Version]
- Liderot, K.; Ratcliffe, P.; Lüthje, P.; Thidholm, E.; Özenci, V. Microbiological diagnosis of Eggerthella lenta blood culture isolates in a Swedish tertiary hospital: Rapid identification and antimicrobial susceptibility profile. Anaerobe 2016, 38, 21–24. [Google Scholar] [CrossRef]
- Buszewski, B.; Kłodzińska, E. Rapid microbiological diagnostics in medicine using electromigration techniques. TrAC Trends Anal. Chem. 2016, 78, 95–108. [Google Scholar] [CrossRef]
- Hu, A.; Chen, C.T.; Tsai, P.J.; Ho, Y.P. Using capillary electrophoresis-selective tandem mass spectrometry to identify pathogens in clinical samples. Anal. Chem. 2006, 78, 5124–5133. [Google Scholar] [CrossRef]
- Hjerten, S.; Elenbring, K.; Kilar, F.; Liao, J.; Chen, A.J.C.; Siebert, C.J.; Zhu, M. Carrier-free zone electrophoresis, displacement electrophoresis and isoelectric focusing in a high-performance electrophoresis apparatus. J. Chromatogr. A 1987, 403, 47–61. [Google Scholar] [CrossRef]
- Ebersole, R.C.; McCormick, R.M. Separation and isolation of viable bacteria by capillary zone electrophoresis. Biotechnology 1993, 11, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Torimura, M.; Ito, S.; Kano, K.; Ikeda, T.; Esaka, Y.; Ueda, T. Surface characterization and on-line activity measurements of microorganisms by capillary zone electrophoresis. J. Chromatogr. B Biomed. Appl. 1999, 721, 31–37. [Google Scholar] [CrossRef]
- Pfetsch, A.; Welsch, T. Determination of the electrophoretic mobility of bacteria and their separation by capillary zone electrophoresis. Fresenius J. Anal. Chem. 1997, 359, 198–201. [Google Scholar] [CrossRef]
- Glynn, J.R.; Belongia, B.M.; Arnold, R.G.; Ogden, K.L.; Baygents, J.C. Capillary electrophoresis measurements of electrophoretic mobility for colloidal particles of biological interest. Appl. Environ. Microbiol. 1998, 64, 2572–2577. [Google Scholar] [CrossRef]
- Schneiderheinze, J.M.; Armstrong, D.W.; Schulte, G.; Westenberg, D.J. High efficiency separation of microbial aggregates using capillary electrophoresis. FEMS Microbiol. Lett. 2000, 189, 39–44. [Google Scholar] [CrossRef][Green Version]
- Armstrong, D.W.; Girod, M.; He, L.; Rodriguez, M.A.; Wei, W.; Zheng, J.; Yeung, E.S. Mechanistic aspects in the generation of apparent ultrahigh efficiencies for colloidal (microbial) electrokinetic separations. Anal. Chem. 2002, 74, 5523–5530. [Google Scholar] [CrossRef]
- Zheng, J.; Yeung, E.S. Mechanism of microbial aggregation during capillary electrophoresis. Anal. Chem. 2003, 75, 818–824. [Google Scholar] [CrossRef]
- Kłodzińska, E.; Szumski, M.; Hrynkiewicz, K.; Dziubakiewicz, E.; Jackowski, M.; Buszewski, B. Differentiation of Staphylococcus aureus strains by CE, zeta potential and coagulase gene polymorphism. Electrophoresis 2009, 30, 3086–3091. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Kłodzińska, E.; Dahm, H.; Szeliga, J.; Jackowski, M.; Buszewski, B. Combination of capillary electrophoresis, PCR and physiological assays in differentiation of clinical strains of Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 286, 1–8. [Google Scholar] [CrossRef]
| Antibiotic (Antibiotics Group) | Metabolite | Activity of the Metabolite Compared to Initial Compound | MIC (µg/mL) | Ref. | |
|---|---|---|---|---|---|
| A | M | ||||
| Metronidazole (nitroimidazole) | 1-(2-hydroxyethyl)-2-hydroxymethyl-5-nitroimidazole | <Bacteroides spp. <Clostridium perfringens <Clostridium spp. =Peptococcus spp. | 0.5 0.5 0.5 0.25 | 1.0 1.0 2.0 0.25 | [21] |
| Metronidazole (nitroimidazole) | 2-methyl-5-nitroimidazole-1-acetic acid | <Bacteroides spp. <Clostridium perfringens <Clostridium spp. <Peptococcus spp. | 0.5 0.5 0.5 0.25 | 16.0 32.0 16.0 16.0 | [21] |
| Clarithromycin (macrolide) | 14-hydroxyclarithromycin | >Haemophilus influenzae | 2.4 | 1.2 | [22] |
| Cefotaxime (β-lactam) | desacetylcefotaxime | =Pseudomonas aeruginosa <Escherichia coli >Proteus mirabilis <Shigella spp. <Klebsiella pneumoniae | >128 0.25 0.5 0.125 0.25 | >128 0.5 0.25 1.0 0.5 | [23] |
| Fidaxcomicin (macrolide) | OP-1118 | <Clostridium perfringens <Clostridium difficile | 0.008 0.12 | 0.25 4.0 | [24] |
| Tinidazole (nitroimidazole) | hydroxytinidzole | >Gardnerella vaginalis | 32 | 2 | [25] |
| Metronidazole (nitroimidazole) | hydroxymetronidazole | >Gardnerella vaginalis | 32 | 4 | [25] |
| norfloxacin (quinolone) | N-nitrosonorfloxacin | <Enterococcus faecalis <Escherichia coli <Staphylococcus aureus <Mycobacterium gilvum <Pseudomonas aeruginosa | 3.01 0.05 1.6 6.2 1.6 | 7.5 1.9 3.8 12.5 7.5 | [26] |
| norfloxacin (quinolone) | N-acetylnorfloxacin | <Enterococcus faecalis <Escherichia coli <Staphylococcus aureus <Mycobacterium gilvum <Pseudomonas aeruginosa | 3.01 0.05 1.6 6.2 1.6 | ≥50 ≥50 ≥50 ≥50 ≥50 | [26] |
| Cefetamet (β-lactam) | cefetamet pivoxil | =Escherichia coli =Streptococcus pyogenes | 0.5 0.06 | 0.5 0.06 | [27] |
| Ceftiofur (β-lactam) | desfuroylceftiofur | <Salmonella spp. <Actinobacillus pleuropneumoniae | 1.0 0.0078 | 2.0 0.015 | [28] |
| Antibiotic/Metabolite | Matrix | Sample Preparation | Detection | Conditions | LOD/LOQ (units) | Ref. |
|---|---|---|---|---|---|---|
| ceftriaxone metronidazole hydroxymetronidazole | human plasma | protein precipitation (ACN) | HPLC-MS/MS m/z Q1→Q3 555.1→ 396.0 172.2→128.2 188.0→125.9 | Column: Polaris 5 C18-A (150 mm × 3.0 mm i.d., 3.0 µm) Mobile phase: 10mM ammonium formate (pH 2.5)/acetonitrile (0.1% FA) gradient elution 300 µL/min, 30 °C, 5 µL | -/0.4–300 µg/mL (ceftriaxone) -/0.05–50 µg/ mL (metronidazole) -/0.02–30 µg/mL (hydroxymetronidazole) | [71] |
| ceftriaxone | human plasma | protein precipitation (MeOH) | LC-MS/MS m/z Q1→Q3 555.0→396.1 | Column: Agilent Zorbax Eclipse Plus C18 (100 mm × 2.1 mm i.d., 3.5 μm) Mobile phase: 10mM ammonium formate/acetonitrile (2% FA) (87.5:12.5 v/v) methanol/acetonitrile (75:25 v/v) 20 mM ammonium bicarbonate gradient elution 0.4 mL/min, 40 °C, 2 µL | -/1.01–200 µg/mL | [65] |
| amoxicillin ampicillin cloxacillin dicloxacillin | urine | Filtration (0.45 µm) | LC-UV 210 nm | Column: Zorbax C18 (150 mm × 4.6 mm i.d., 5.0 µm) Mobile phase: 0.11M SDS/6% propanol/0.01M NaH2PO4 buffer (pH 3.0) mL/min, 25 °C, 20 µL | 1.5–15/50 ng/mL | [58] |
| amoxicillin meropenem ceftazidime cefuroxime piperacillin | human plasma | protein precipitation (ACN) | UPLC-MS/MS m/z Q1→Q3 366.1→114.0 384.2→141.2 547.1→468.0 442.2→364.1 518.2→143.1 | Column: Waters Acquity UPLC BEH C18 (100 mm × 2.1 mm i.d., 1.7 µm) Mobile phase: 2 mM ammonium acetate/water (0.1% FA) 2 mM ammonium acetate/methanol (0.1% FA) gradient elution 0.4 mL/min, 50 °C, 40 µL | -/1.0–100 mg/L (amoxicillin, cefuroxime) -/0.5–80 mg/L (meropenem, ceftazidime) -/1.0–150 mg/L (piperacillin) | [72] |
| cefazolin cefalothin | human plasma urine peritoneal dialysate | protein precipitation (ACN) filtration (0.45 µm) direct injection | UHPLC-MS/MS m/z Q1→Q3 455.1→323.1 419.1→315.0 | Column: Phenomenex Kinetex C8 (50 mm × 2.1 i.d., 1.7 μm) Mobile phase: 0.1% formic acid 0.1% formic acid/methanol gradient elution 50 °C, 0.2 µL | 0.04–0.05/1 µg/mL (plasma) 0.46–4.6/0.1–0.2 µg/mL (urine) 0.01–0.03 /0.2 µg/mL (peritoneal dialysate) | [73] |
| clarithromycin | human serum | LLE (DCM) derivatization (FMOC-Cl) | HPLC-FD 265 nm (Ex) 315 nm (Em) | Column: Shimpack CLC-ODS (150 mm × 4.6 mm i.d., 5 µm) Mobile phase: 0.05 M phosphate buffer/TEA/methanol 2.0 mL/min., 58 °C, 20 µL | 0.01/0.025 µg/mL | [68] |
| metronidazole | human plasma | LLE protein precipitation (ACN) | HPLC-UV 320 nm | Column: Eclipse XDB-phenyl (250 mm × 4.6 mm i.d., 5 µm) Mobile phase: 0.05 M sodium acetate/acetonitrile/glacial acetic acid (75:25:1 v/v/v) (pH 4.0) 50 µL | -/0.05–30 µg/mL | [74] |
| metronidazole | human feces | LLE (MeOH) | LC-MS/MS m/z Q1→Q3 172.2→128.0 | Column: Waters Acquity UPLC BEH C18 (50 mm × 2.1 mm i.d., 1.7 µm) Mobile phase: 2 mM ammonium acetate/water (0.1% FA) 2 mM ammonium acetate/water (0.1% FA) gradient elution 0.4 mL/min, 55 °C | 5/66 ng/mL | [75] |
| levornidazole hydroxylation metabolite N-dealkylation metabolite oxidative dechlorination metabolite | human feces | LLE protein precipitation (MeOH) | HPLC-MS/MS m/z Q1→Q3 220.0→128.0 236.0→171.0 202.0→128.0 299.9→128.1 | Column: Atlantis T3 columns (150 mm × 2.1 mm i.d., 5.0 µm) Mobile phase: acetonitrile-methanol/water (0.5% FA) gradient elution 0.4 mL/min, 30 °C | -/0.005–2.0 µg/mL | [76] |
| cefepime meropenem ciprofloxacin moxifloxacin linezolid piperacillin | human serum | protein precipitation (methanol -methyl-tert-butyl ether (90:10, v/v) | HPLC-MS/MS m/z Q1→Q3 481.0→167.0 384.1→114.0 332.0→231.0 402.0→261.0 338.0→235.0 518.0→143.0 | Column: Fortis C8 (100 mm × 2.1 mm i.d., 3 µm) Mobile phase: 10 mM ammonium formiate/water (0.1% FA) methanol gradient elution 0.5 mL/min, 30 °C, 15 µL | -/0.25–200 mg/L (cefepime) -/0.25–120 mg/L (meropenem, -/0.05–10 mg/L (ciprofloxacin) -/0.125–10 mg/L (moxifloxacin) -/0.125–50 mg/L (linezolid) -/0.5–400 mg/L (piperacillin) | [67] |
| cycloserine | human plasma | SPE (ACN) | HPLC-PDA 240 nm | Column: Allantis T3 (150 mm × 4.6 mm id, 3 µm) Mobile phase:10Mm phosphate buffer/acetonitrile (95:5 v/v) 0.4 mL/min, 30 °C, 50 µL | 0.3/1.2 µg/mL | [77] |
| linezolid | human serum urine | dilution (acetate buffer, pH 3.5) | HPLC-UV 250 nm | Column: Nucleosil-100 5C18 (125 mm × 4 mm id, 5 µm) Mobile phase: Acetonitrile/sodium acetate buffer/water (180:100:720, v/v), (pH 3.7) 1.3 mL/min, 25 °C, 50 µL | 0.07/0.14 mg/L (serum) 2.4/4.7 mg/L (urine) | [66] |
| fosfomycin | human plasma urine | protein precipitation (ACN) filtration (0.22 µm) | LC-MS/MS m/z Q1→Q2 137.1→78.9 | Column: Merck SeQuant zic-HILIC (50 mm × 2.1 mm i.d., 5 µm) Mobile phase: 2 mM ammonium acetate/acetonitrile (15:85 v/v) 0.3 mL/min, 24 °C, 0.1µl (plasma), 0.5 µL (urine) | 0.01/1.02 µg/mL (plasma) 0.01/0.1 mg/mL (urine) | [78] |
| amoxicillin oxacillin cloxacillin dicloxacillin | plasma whole blood urine | protein precipitation (ACN) SPE (MeOH) | HPLC-PDA 240 nm | Column: Inertsil ODS-3 (250 mm × 4.0 mm i.d., 5 µm) Mobile phase: acetonitrile (0.1% TFA) 1.0 mL/min, 1.3 mL/min, 25 °C, 20 µL | 3.3–6.6/10–20 ng/mL (plasma) 6.6/20 ng/mL (whole blood, urine) | [79] |
| amoxicillin cefotaxime ciprofloxacin clindamycin metronidazole amoxycilloic acid 4-hydroxyphenylglycyl amoxicillin desacetyl cefotaxime 3-desacetyl cefotaxime lactone ciprofloxacin N-oxide N-demethylclindamycin clindamycin sulfoxide hydroxymetronidazole | whole blood surgical wound | SPME (MeOH) | HPLC-QqQ-MS m/z Q1→Q3 366.0→114.0 456.0→396.0 332.0→314.0 425.0→162.0 172.0→128.0 384.0→189.0 515.0→263.0 414.0→354.0 396.0→336.0 348.0→328.0 411.0→148.0 441.0→178.0 188.0→144.0 | Column: Phenomex GRACE C18 (50 mm × 2.0 mm i.d., 4 µm) Mobile phase: acetonitrile/water (0.1% FA) gradient elution 0.4 mL/min, 25 °C, 5 µL | 0.031/0.093 µg/mL (amoxicillin) 0.033/0.098 µg/mL (amoxycilloic acid) 0.037/0.112 µg/mL (4-hydroxyphenylglycyl amoxicillin) 0.039/0.118 µg/mL (cefotaxime) 0.041/0.123 µg/mL (3-desacetyl cefotaxime lactone) 0.044/0.131 µg/mL (desacetyl cefotaxime) 0.028/0.085 µg/mL (ciprofloxacin) 0.032/0.096 µg/mL (ciprofloxacin N-oxide) 0.033/0.098 µg/mL (clindamycin) 0.039/0.117 µg/mL (N-demethylclindamycin) 0.042/0.126 µg/mL (clindamycin sulfoxide) 0.043/0.129 µg/mL (metronidazole) 0.045/0.135 µg/mL (hydroxymetronidazole) | [61] |
| piperacillin tazobactam | plasma urine | ultrafiltration filtration (0.45 µm) | UHPLC-MS/MS m/z Q1→Q3 518.0→143.0 229.0→138.0 | Column: C18 Shimadzu Shim-pack XR-ODS III (50 × 2.0 mm i.d, 1.6 μm) Mobile phase: acetonitrile (0.1% FA)/water (0.1% FA) gradient elution 1 µL | 0.01/0.5 µg/mL (piperacillin) 0.01/5 µg/mL (tazobactam) | [80] |
| amoxicillin ampicillin piperacillin meropenem cefuroxime ceftazidime cefazolin | human plasma | protein precipitation (ACN) | UPLC-MS/MS m/z Q1→Q3 366.16→113.94 350.16→106.00 518.26→359.09 384.18→141.03 423.09→207.00 547.22→468.10 455.16→323.00 | Column: ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm i.d. 1.6 μm) Mobile phase: acetonitrile (0.1% FA)/water (0.1% FA) gradient elution 0.4 mL/min, 50 °C, 10 µL | -/0.5–1.5 mg/L | [81] |
| amoxicillin cefazolin cefepime cefotaxime ceftazidime cloxacillin oxacillin piperacillin | human plasma | protein precipitation (ACN) | UHPLC-UV 230 nm 260 nm | Column: Hypersil Gold PFP column (100 mm × 2.1 mm i.d. 1.9 μm) Mobile phase: 10 mM phosphoric/acetonitrile gradient elution 500 µL/min, 40 °C,10 µL | -/2–100 mg/L | [82] |
| Antibiotic/Metabolite | Matrix | Sample Peparation | Detection | Capillary Parameters | LOD/LOQ (units) | Ref. |
|---|---|---|---|---|---|---|
| cefazolin cefamandol cefuroxim ceftazidim ceftriaxon cefepim | serum cerebrospinal fluid sputum | lyophilization direct injection | CZE-PD 270 nm | 25 mM borate buffer (pH 9.1), 50 mM SDS Ltot = 48 cm, Leff = 40 cm, i.d. = 50 µm 20 kV, 25 °C, 2 s | 0.42–0.84/ µg/mL | [92] |
| sulfamethoxazole N4-acetylsulfamethoxazole trimethoprim trimethoprim 1-oxide trimethoprim 3-oxide | human serum | protein precipitation (ACN) | MEKC-DAD 260 nm 206 nm | 20 mM borate buffer (pH 9.3), 25 mM SDS + 5% ACN Ltot = 60.2 cm, Leff = 50 cm, i.d. = 75 µm 30 kV, 20 °C, 5 s | 0.04–0.06/0.13–0.24 mg/L | [93] |
| ceftazidime cefotaxime cefuroxime ceftriaxone | wound drainage cerebrospinal fluid serum urine | filtration (0.45 µm) | CZE-UV 270 nm | 25 mM borate, buffer (pH 9.2) Ltot = 48.5 cm, Leff = 40 cm, i.d. = 50 µm 25 kV, 25 °C, 0.2 s | 0.21–0.48/ µg/mL | [94] |
| ceftazidime | human blood | protein precipitation (ACN) | CE-DAD 200 nm 260 nm | 50 mM chloroacetic acid, 20% v/v methanol, 0.5% v/v INST (pH 2.32) Ltot = 31.5 cm, Leff = 23 cm, i.d. = 25 µm 30 kV, 25 °C, 30 s | 0.42/ µg/mL | [91] |
| vancomycin | human serum | direct injection | MEKC-PDA 210 nm | 25 mM borate buffer (pH 10.0), 100 mM SDS Ltot = 67 cm, Leff = 50 cm, i.d. = 75 µm 25 kV, 25 °C, 4 s | 1 µg/mL 1 µg/mL | [95] |
| daunorubicin | human plasma | SPE (MeOH) | CE-LIF 520 nm | 100 mM sodium dihydrogenphosphate (pH 5.0) Leff = 40 cm, i.d. = 50 µm 10 kV, 25 °C, 10 s | -/1 µg/L | [96] |
| cephalexi cefadroxil cefaclor ceftazidim cefsulodin cefotaxim cefamandol cefuroxim cefodizim | urine | filtration (0.2 µm) | CZE-DAD 210 nm | 50 mM citrate buffer (pH 6) Ltot = 48.5 cm, i.d. = 50 µm 30 kV, 25 °C, 9 s | 2.5–5/ µg/mL | [97] |
| cefadroxil cefixime cefuroxime sodium ceftriaxone sodium ceftizoxime cefaclor cefradine cefotoxime | urine | filtration (0.42 µm) | CE-UV 214 nm | 50 mM sodium tetraborate buffer (pH 9.0) Ltot = 57 cm, Leff = 50 cm, i.d. = 75 µm 30 kV, 25 °C, 4 s | 0.5–5/-µg mL | [89] |
| moxifloxacin lomefloxacin norfloxacin ciprofloxacin ofloxacin enrofloxacin oxolinic acid flumequine | human blood | protein precipitation (MeOH) | CE-FD 240–400 nm | 50 mM phosphoric acid (pH 7.55), 40% acetonitrile Ltot = 70 cm, Leff = 55 cm, i.d. = 75 µm 50 mbar, 25 °C, 8 s | 0.5–15/1.5–45 µg/L | [98] |
| gentamicin | smear of the wound | direct injection | CZE-DAD | TBE buffer, 0.0125% PEO (pH 8.53) Ltot = 33.5 cm, Leff = 25 cm, i.d. = 75 µm 20 kV, 25 °C, 10 s | -/- | [99] |
| Bacteria Speices | Clinical Samples | Matrix Solution | Sampling Technique | Identification System | Degree of Compliance Identification (%) | Ref. |
|---|---|---|---|---|---|---|
| Eschericha coli Klebsiella pneumoniae Klebsiella oxytoca Citrobacter spp. Enterobacter spp. Pseudomonas aeruginosa Proteus mirabilis | urine | HCCA | direct application | MALDI VITEK® MS | 86 100 67 100 75 100 100 | [152] |
| Eschericha coli Klebsiella pneumoniae Klebsiella oxytoca Enterococcus faecium Enterococcus faecalis Pseudomonas aeruginosa Proteus mirabilis | urine | HCCA | protein extraction | MALDI BioTyper | 95 93 100 82 90 86 98 | [149] |
| Staphylococcus epidermidis Klebsiella pneumoniae Eschericha coli Staphylococcus haemolyticus | blood | HCCA | direct application | MALDI VITEK® MS | 65 97 93 80 | [155] |
| Lactobacillus fermentum Lactobacillus salivarius Lactobacillus rhamnosus Lactobacillus plantarum | saliva | HCCA | protein extraction | MALDI BioTyper | 80 36 75 100 | [156] |
| Staphylococcus epidermidis Staphylococcus hominis Staphylococcus haemolyticus | blood | HCCA | protein extraction | MALDI BioTyper | 99 100 100 | [157] |
| Mycobacterium abscessus Mycobacterium fortuitum Mycobacterium avium | sputum pus peritoneal fluid urine | HCCA | protein extraction | MALDI BioTyper | 97 | [158] |
| Veillonella spp. | abdomen fluid pleural fluid bile surgical wounds pus operating material blood | HCCA | direct application | MALDI BioTyper | 100 | [159] |
| Escherichia coli Streptoccocus aureus Staphylococcus epidermidis | blood urine pus swab cerebrospinal fluid respiratory tract wound specimens | HCCA | protein extraction | MALDI BioTyper | 100 100 100 | [160] |
| Aeromonas spp. | feces | HCCA | direct application | MALDI BioTyper | 100 | [161] |
| Streptoccocus spp. | vitreous samples | HCCA | protein extraction | MALDI BioTyper | 96 | [149] |
| Escherichia coli | urine blood | HCCA | direct application protein extraction | MALDI BioTyper | 94 | [162] |
| Eggerthella lenta | blood | - | direct application | MALDI BioTyper MALDI VITEK® MS | 94 100 | [163] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauter, K.; Szultka-Młyńska, M.; Buszewski, B. Determination and Identification of Antibiotic Drugs and Bacterial Strains in Biological Samples. Molecules 2020, 25, 2556. https://doi.org/10.3390/molecules25112556
Pauter K, Szultka-Młyńska M, Buszewski B. Determination and Identification of Antibiotic Drugs and Bacterial Strains in Biological Samples. Molecules. 2020; 25(11):2556. https://doi.org/10.3390/molecules25112556
Chicago/Turabian StylePauter, Katarzyna, Małgorzata Szultka-Młyńska, and Bogusław Buszewski. 2020. "Determination and Identification of Antibiotic Drugs and Bacterial Strains in Biological Samples" Molecules 25, no. 11: 2556. https://doi.org/10.3390/molecules25112556
APA StylePauter, K., Szultka-Młyńska, M., & Buszewski, B. (2020). Determination and Identification of Antibiotic Drugs and Bacterial Strains in Biological Samples. Molecules, 25(11), 2556. https://doi.org/10.3390/molecules25112556








