Interaction of Reactive-Dye Chromophores and DEG on Ink-Jet Printing Performance
Abstract
1. Introduction
2. Results and Discussion
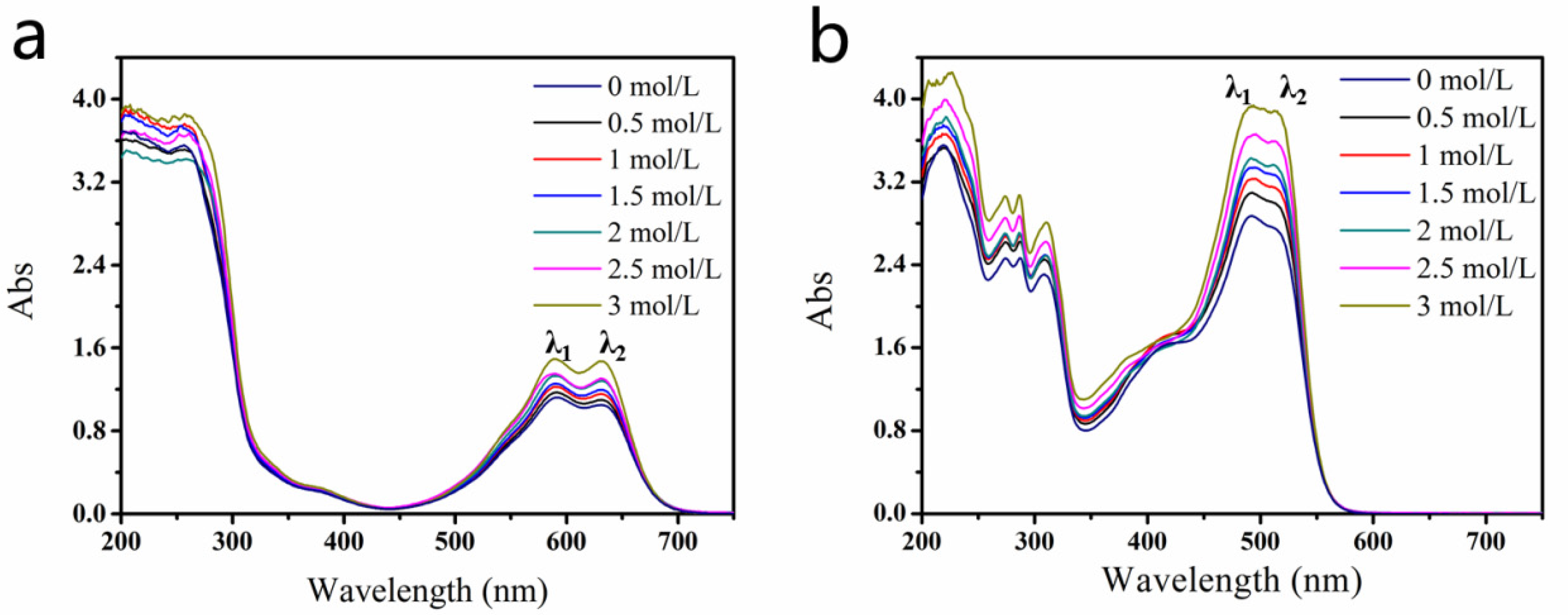
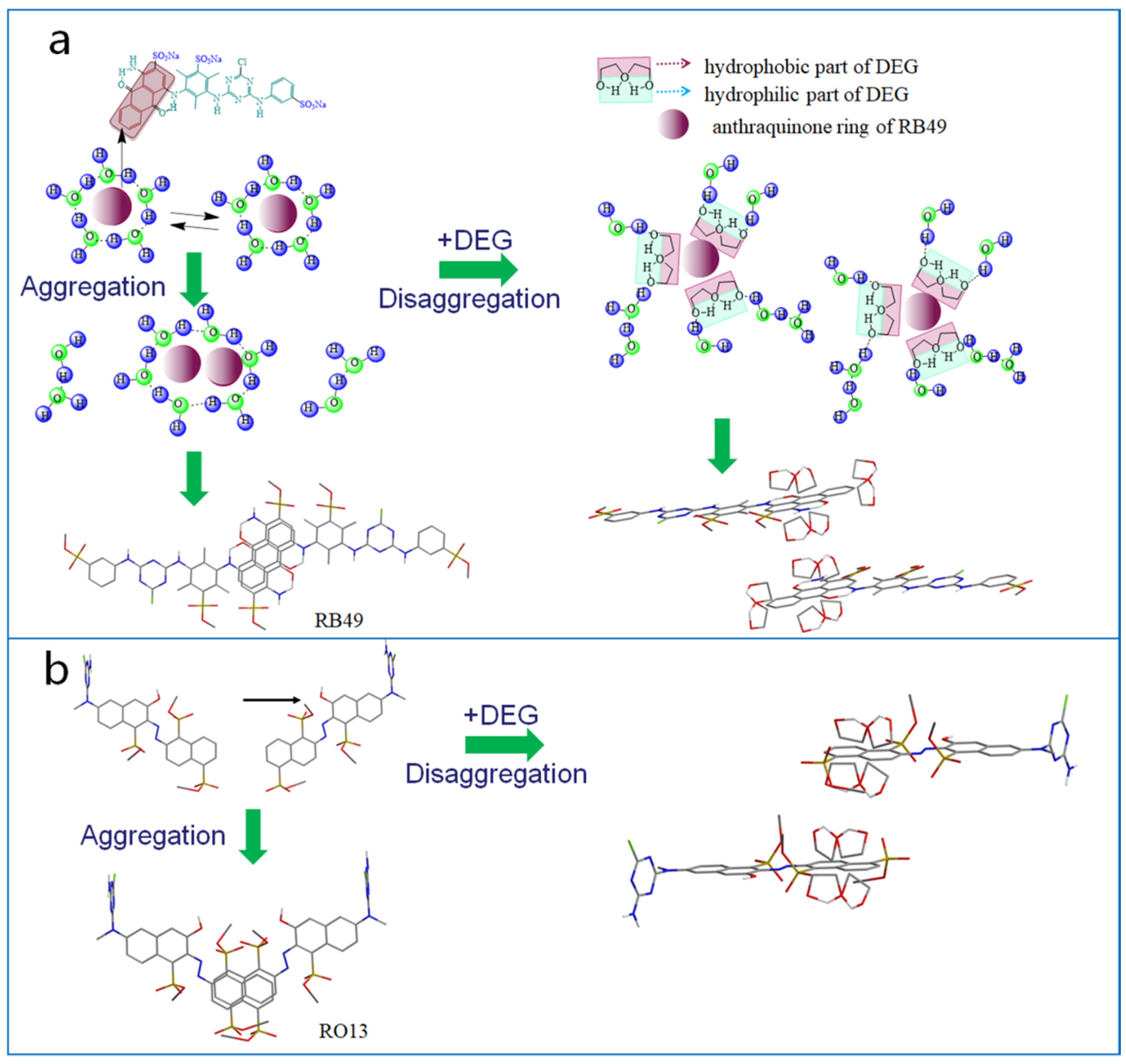
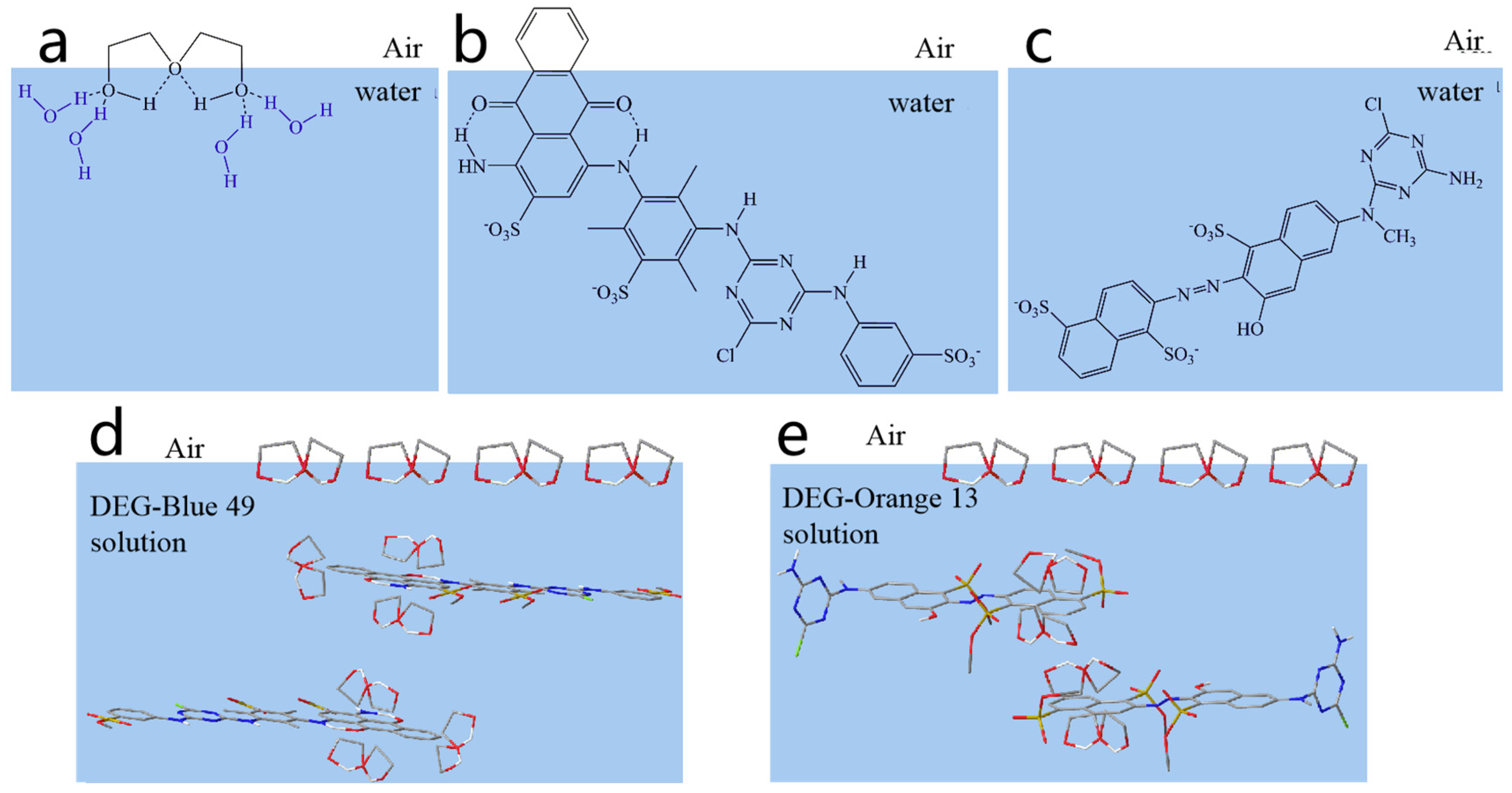
2.1. Interaction between DEG and Dyes
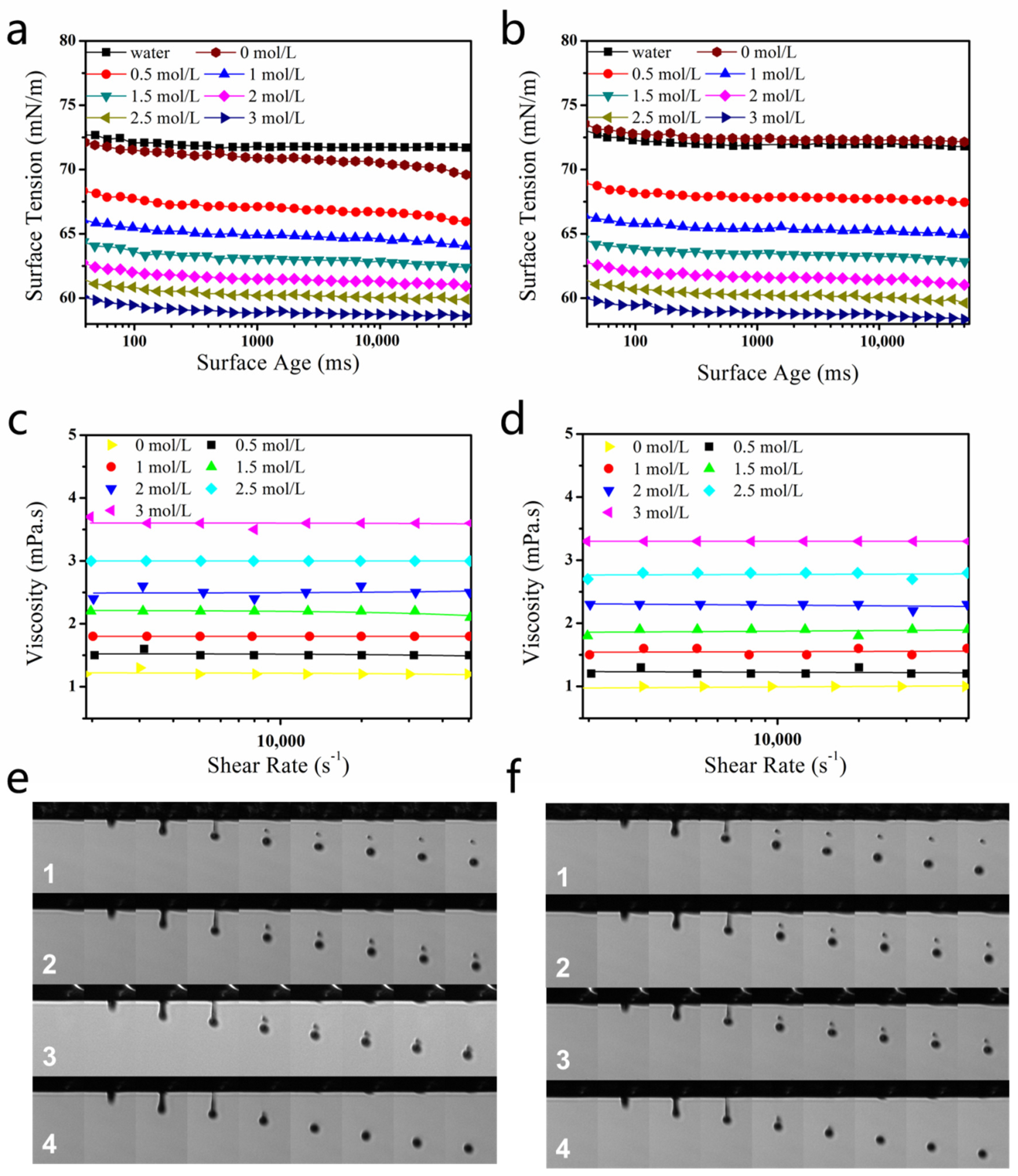
2.2. Effect of DEG on Droplet Formation
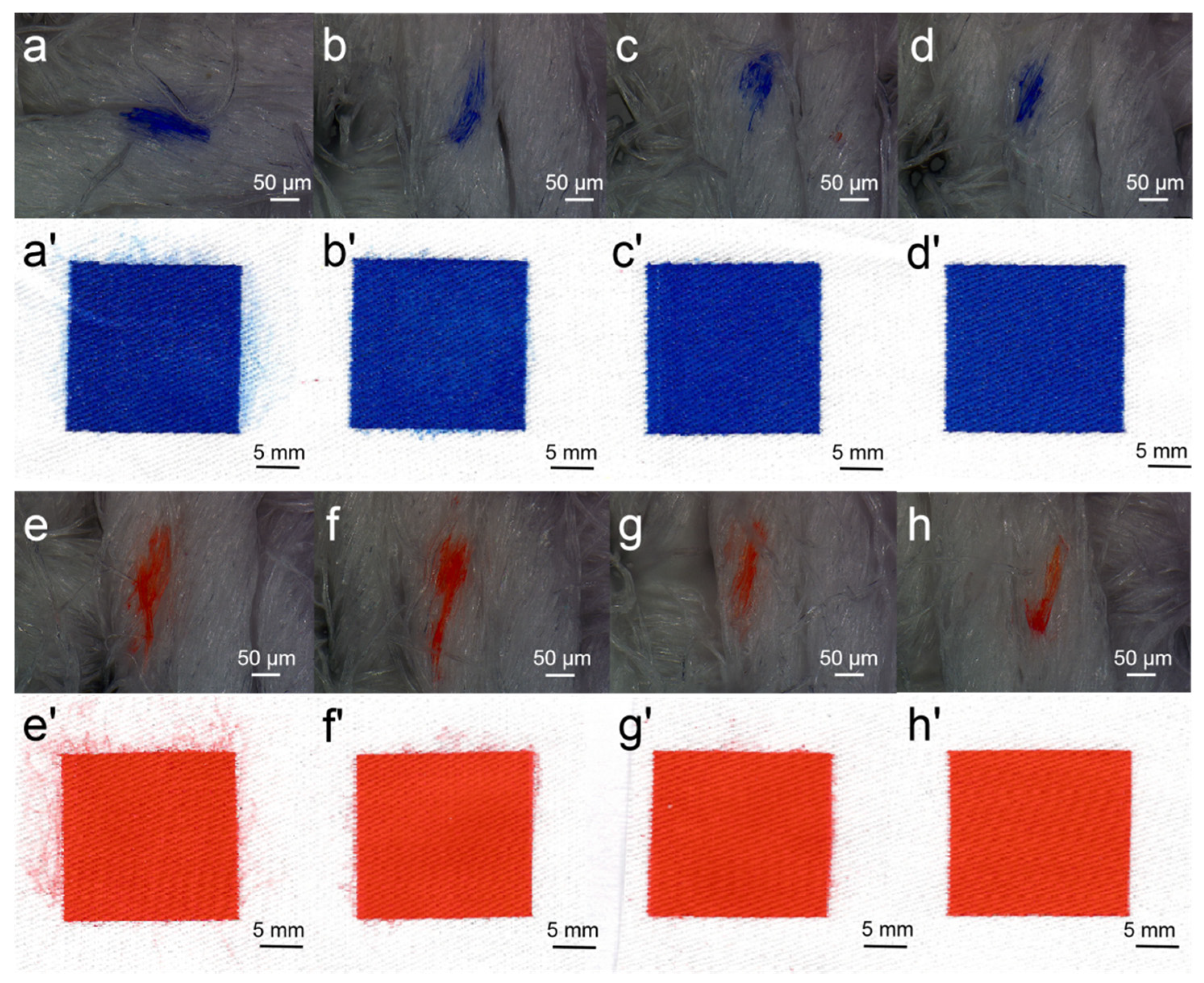
2.3. DEG Effect on Printing Performance
3. Materials and Methods
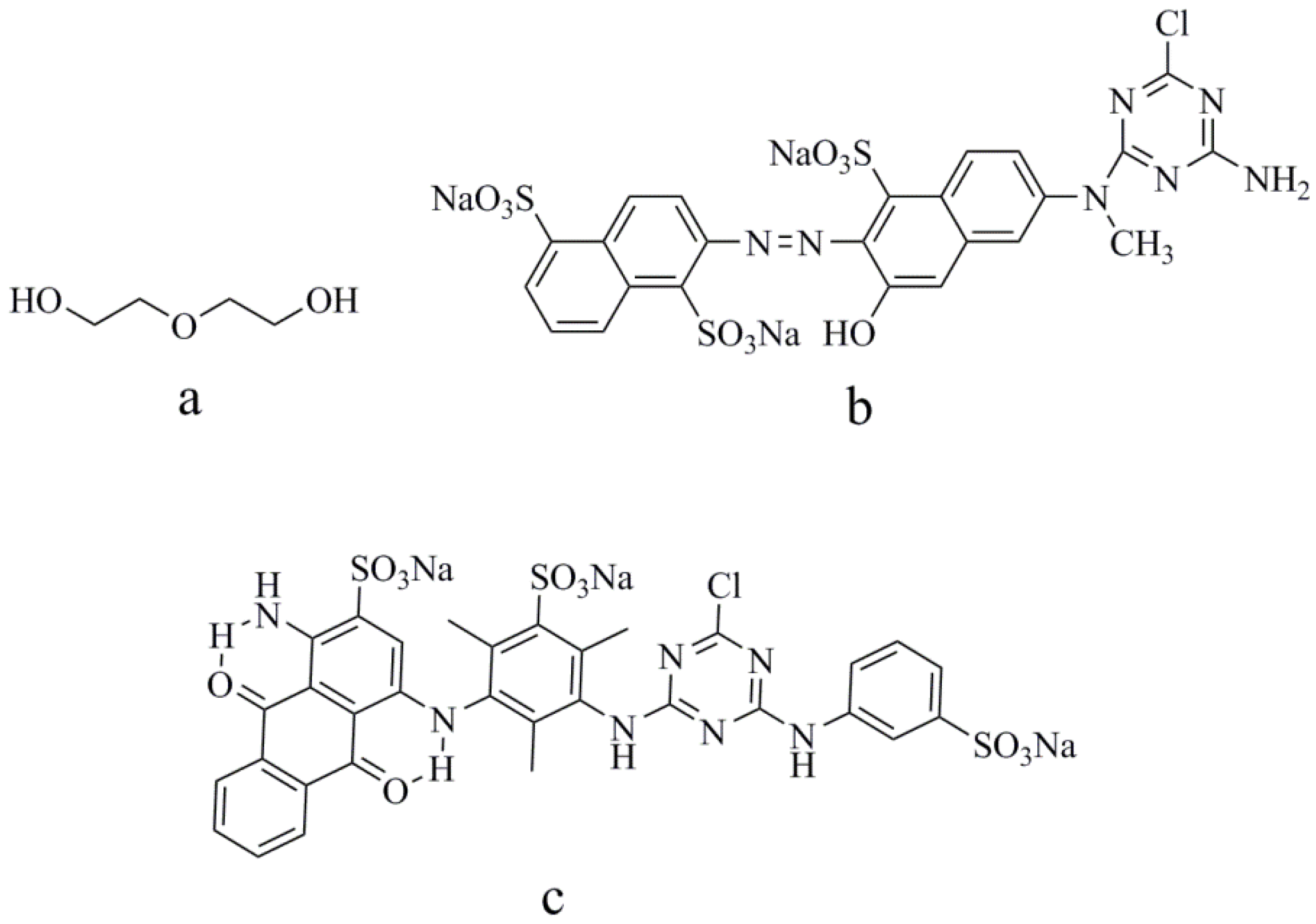
3.1. Materials
3.2. Preparation of Reactive-Dye Solution
3.3. UV–Visible Absorption Spectroscopy
3.4. Rheological-Property Test
3.5. Surface-Tension Test
3.6. Ink-Jet-Printing and Droplet-Spreading Performance on Fabric
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abe, T. Present State of Inkjet Printing Technology for Textile. Adv. Mater. Res. 2012, 441, 23–27. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Yan, K. A one-step inkjet printing technology with reactive dye ink and cationic compound ink for cotton fabrics. Carbohydr. Polym. 2018, 197, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Wang, S.; Wang, C.; Tian, A. Inkjet printing effects of pigment inks on silk fabrics surface-modified with O2 plasma. J. Appl. Polym. Sci. 2008, 107, 2949–2955. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Choi, J.W. Inkjet Printing of Carbon Nanotubes. Nanomaterials (Basel) 2013, 3, 453–468. [Google Scholar] [CrossRef]
- Carey, T.; Cacovich, S.; Divitini, G.; Ren, J.; Mansouri, A.; Kim, J.M.; Wang, C.; Ducati, C.; Sordan, R.; Torrisi, F. Fully inkjet-printed two-dimensional material field-effect heterojunctions for wearable and textile electronics. Nat. Commun. 2017, 8, 1202. [Google Scholar] [CrossRef]
- Kuang, M.; Wang, L.; Song, Y. Controllable Printing Droplets for High-Resolution Patterns. Adv. Mater. 2014, 26, 6950–6958. [Google Scholar] [CrossRef]
- Yu, J.; Seipel, S.; Nierstrasz, V.A. Digital inkjet functionalization of water-repellent textile for smart textile application. J. Mater. Sci. 2018, 53, 13216–13229. [Google Scholar] [CrossRef]
- Kawase, T.; Moriya, S.; Newsome, C.J.; Shimoda, T. Inkjet Printing of Polymeric Field-Effect Transistors and Its Applications. Jpn. J. Appl. Phys. 2005, 44, 3649. [Google Scholar] [CrossRef]
- Song, Y.; Fang, K.; Ren, Y.; Tang, Z.; Wang, R.; Chen, W.; Xie, R.; Shi, Z.; Hao, L. Inkjet Printable and Self-Curable Disperse Dyes/P(St-BA-MAA) Nanosphere Inks for Both Hydrophilic and Hydrophobic Fabrics. Polymers 2018, 10, 1402. [Google Scholar] [CrossRef]
- Song, Y.; Fang, K.; Bukhari, M.N.; Zhang, K.; Tang, Z.; Wang, R. Disperse dye/poly (styrene-methacrylic acid) nanospheres with high coloration performance for textiles. J. Clean. Prod. 2020, 263, 121538. [Google Scholar] [CrossRef]
- Ali, S.; Mughal, M.A.; Shoukat, U.; Baloch, M.A.; Kim, S.H. Cationic Starch (Q-TAC) Pre-Treatment of Cotton Fabric: Influence on dyeing with reactive dye. Carbohydr. Polym. 2015, 117, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Shamey, R.; Hussein, T. Critical Solutions in the Dyeing of Cotton Textile Materials. Text. Prog. 2005, 37, 1–84. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Xie, K.; Hou, A.; Gao, A. Novel reactive dyes with intramolecular color matching combination containing different chromophores. Dyes Pigment. 2018, 159, 576–583. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, J.D.; Wei, W.; Zhong, J.C.; Yao, J.; Ye, Y.; Yang, X.X. Effects of the Cotton Fabric Pretreatment on Application Properties of Digital Inkjet Printing with Reactive Dyes. Adv. Mater. Res. 2011, 331, 398–401. [Google Scholar] [CrossRef]
- Liu, Z.D.; Fang, K.J.; Gao, H.G.; Liu, X.M.; Zhang, J.F. Effect of cotton fabric pretreatment on drop spreading and colour performance of reactive dye inks. Coloration Technol. 2016, 132, 407–413. [Google Scholar] [CrossRef]
- Park, J.-Y.; Hirata, Y.; Hamada, K. Dye aggregation and interaction of dyes with a water-soluble polymer in ink-jet ink for textiles. Coloration Technol. 2012, 128, 184–191. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xiang, J.F.; Tang, Y.L.; Xu, G.Z.; Yan, W.P. Aggregation behaviour of two thiacarbocyanine dyes in aqueous solution. Dyes Pigment. 2008, 76, 88–93. [Google Scholar] [CrossRef]
- Tang, Z.; Fang, K.; Song, Y.; Sun, F. Jetting Performance of Polyethylene Glycol and Reactive Dye Solutions. Polymers 2019, 11, 739. [Google Scholar] [CrossRef]
- Wang, R.; Fang, K.; Ren, Y.; Song, Y.; Zhang, K.; Bukhari, M.N. Jetting performance of two lactam compounds in reactive dye solution. J. Mol. Liq. 2019, 294, 111668. [Google Scholar] [CrossRef]
- Park, J.Y.; Hirata, Y.; Hamada, K. Relationship between the dye/additive interaction and inkjet ink droplet formation. Dyes Pigment. 2012, 95, 502–511. [Google Scholar] [CrossRef]
- Takahashi, D.; Oda, H.; Izumi, T.; Hirohashi, R. Substituent effects on aggregation phenomena in aqueous solution of thiacarbocyanine dyes. Dyes Pigment. 2005, 66, 1–6. [Google Scholar] [CrossRef]
- Boruah, B.; Saikia, P.M.; Dutta, R.K. Spectrophotometric investigation of the monomer–dimer process of C.I. Basic Blue 9 in aqueous polymer–surfactant system. Dyes Pigment. 2010, 85, 16–20. [Google Scholar] [CrossRef]
- Mostafa, O.I.; Abd El-Aal, A.Y.; El Bayaa, A.A.; Sallam, H.B.; Mahmoud, A.A. Aggregation Behaviour of Some Reactive Dyes. J. Chin. Chem. Soc. 1995, 42, 507–513. [Google Scholar] [CrossRef]
- Park, J.Y.; Hirata, Y.; Hamada, K. Interactions between Dyes and Surfactants in InkJet Ink Used for Textiles. J. Oleo Sci. 2011, 60, 627–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoath, S.D.; Jung, S.; Hsiao, W.-K.; Hutchings, I.M. How PEDOT: PSS solutions produce satellite-free inkjets. Organ. Electron. 2012, 13, 3259–3262. [Google Scholar] [CrossRef]
- Chen, A.U.; Basaran, O.A. A new method for significantly reducing drop radius without reducing nozzle radius in drop-on-demand drop production. Phys. Fluids 2002, 14, L1–L4. [Google Scholar] [CrossRef]
- Du, Z.; Lin, Y.; Xing, R.; Cao, X.; Yu, X.; Han, Y. Controlling the polymer ink’s rheological properties and viscoelasticity to suppress satellite droplets. Polymer 2018, 138, 75–82. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, R.; Fang, K.; Chen, W.; Shi, Z.; Ren, Y. Effects of reactive dye structures on surface tensions and viscosities of dye solutions. J. Mol. Liq. 2019, 287, 110932. [Google Scholar] [CrossRef]
- Ghanadzadeh, A.; Zeini, A.; Kashef, A.; Moghadam, M. Concentration effect on the absorption spectra of oxazine1 and methylene blue in aqueous and alcoholic solutions. J. Mol. Liq. 2008, 138, 100–106. [Google Scholar] [CrossRef]
- Gilani, A.G.; Moghadam, M.; Hosseini, S.E.; Zakerhamidi, M.S. A comparative study on the aggregate formation of two oxazine dyes in aqueous and aqueous urea solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 100–105. [Google Scholar] [CrossRef]
- Tajalli, H.; Gilani, A.G.; Zakerhamidi, M.S.; Moghadam, M. Effects of surfactants on the molecular aggregation of rhodamine dyes in aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.G.; Poormohammadi-Ahandani, Z.; Kian, R. Additive-induced aggregate changes of two structurally similar dyes in aqueous solutions: A comparative photophysical study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, H.; Zheng, J.; Peng, H.; Cao, C.; Qi, Y.; Fang, K.; Chen, W. Systematically Exploring Molecular Aggregation and Its Impact on Surface Tension and Viscosity in High Concentration Solutions. Molecules 2020, 25, 1588. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Chen, S.; Che, B.; Xue, G. Aggregation of azo dye Orange I induced by polyethylene glycol in aqueous solution. Coll. Surf. A Physicochem. Eng. Asp. 2007, 301, 346–351. [Google Scholar] [CrossRef]
- Tull, A.G. Some Practical Observations on the Spectrophotometry of Dyes and Aggregation Effects. J. Soc. Dyers Colour. 2008, 89, 132–136. [Google Scholar] [CrossRef]
- Cui, X.; Liu, J.; Xie, L.; Huang, J.; Liu, Q.; Israelachvili, J.N.; Zeng, H. Modulation of Hydrophobic Interaction by Mediating Surface Nanoscale Structure and Chemistry, not Monotonically by Hydrophobicity. Angew. Chem. Int. Ed. 2018, 57, 11903–11908. [Google Scholar] [CrossRef]
- Graziano, G. Comment on “Water’s Structure around Hydrophobic Solutes and the Iceberg Model”. J. Phys. Chem. B 2014, 118, 2598–2599. [Google Scholar] [CrossRef]
- An, S.; Lee, M.W.; Kim, N.Y.; Lee, C.; Al-Deyab, S.S.; James, S.C.; Yoon, S.S. Effect of viscosity, electrical conductivity, and surface tension on direct-current-pulsed drop-on-demand electrohydrodynamic printing frequency. Appl. Phys. Lett. 2014, 105, 1–5. [Google Scholar] [CrossRef]
- Lee, S.-H.; Nguyen, X.H.; Ko, H.S. Study on droplet formation with surface tension for electrohydrodynamic inkjet nozzle. J. Mech. Sci. Technol. 2012, 26, 1403–1408. [Google Scholar] [CrossRef]
- Jang, D.; Kim, D.; Moon, J. Influence of Fluid Physical Properties on Ink-Jet Printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef]
- Tice, J.D.; Lyon, A.D.; Ismagilov, R.F. Effects of viscosity on droplet formation and mixing in microfluidic channels. Anal. Chim. Acta 2004, 507, 73–77. [Google Scholar] [CrossRef]
- Shin, P.; Sung, J.; Lee, M.H. Control of droplet formation for low viscosity fluid by double waveforms applied to a piezoelectric inkjet nozzle. Microelectron. Reliab. 2011, 51, 797–804. [Google Scholar] [CrossRef]
- Blokzijl, W.; Engberts, J.B.F.N. Hydrophobic Effects. Opinions and Facts. Angew. Chem. 1993, 32, 1545–1579. [Google Scholar] [CrossRef]
- Dill, K.A.; Truskett, T.M.; Vlachy, V.; Hribar-Lee, B. Modeling water, the hydrophobic effect, and ion solvation. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Rezus, Y.L.A.; Bakker, H.J. Observation of Immobilized Water Molecules around Hydrophobic Groups. Phys. Rev. Lett. 2007, 99, 148301. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.M.; Bair, S.; Casalini, R. Thermodynamic scaling of the viscosity of van der Waals, H-bonded, and ionic liquids. J. Chem. Phys. 2006, 125, 124508. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Fang, K.; Zhou, H. Interaction of Reactive-Dye Chromophores and DEG on Ink-Jet Printing Performance. Molecules 2020, 25, 2507. https://doi.org/10.3390/molecules25112507
Zhang L, Fang K, Zhou H. Interaction of Reactive-Dye Chromophores and DEG on Ink-Jet Printing Performance. Molecules. 2020; 25(11):2507. https://doi.org/10.3390/molecules25112507
Chicago/Turabian StyleZhang, Liyuan, Kuanjun Fang, and Hua Zhou. 2020. "Interaction of Reactive-Dye Chromophores and DEG on Ink-Jet Printing Performance" Molecules 25, no. 11: 2507. https://doi.org/10.3390/molecules25112507
APA StyleZhang, L., Fang, K., & Zhou, H. (2020). Interaction of Reactive-Dye Chromophores and DEG on Ink-Jet Printing Performance. Molecules, 25(11), 2507. https://doi.org/10.3390/molecules25112507



