How Does Ginsenoside Rh2 Mitigate Adipogenesis in Cultured Cells and Obese Mice?
Abstract
1. Introduction
2. Results
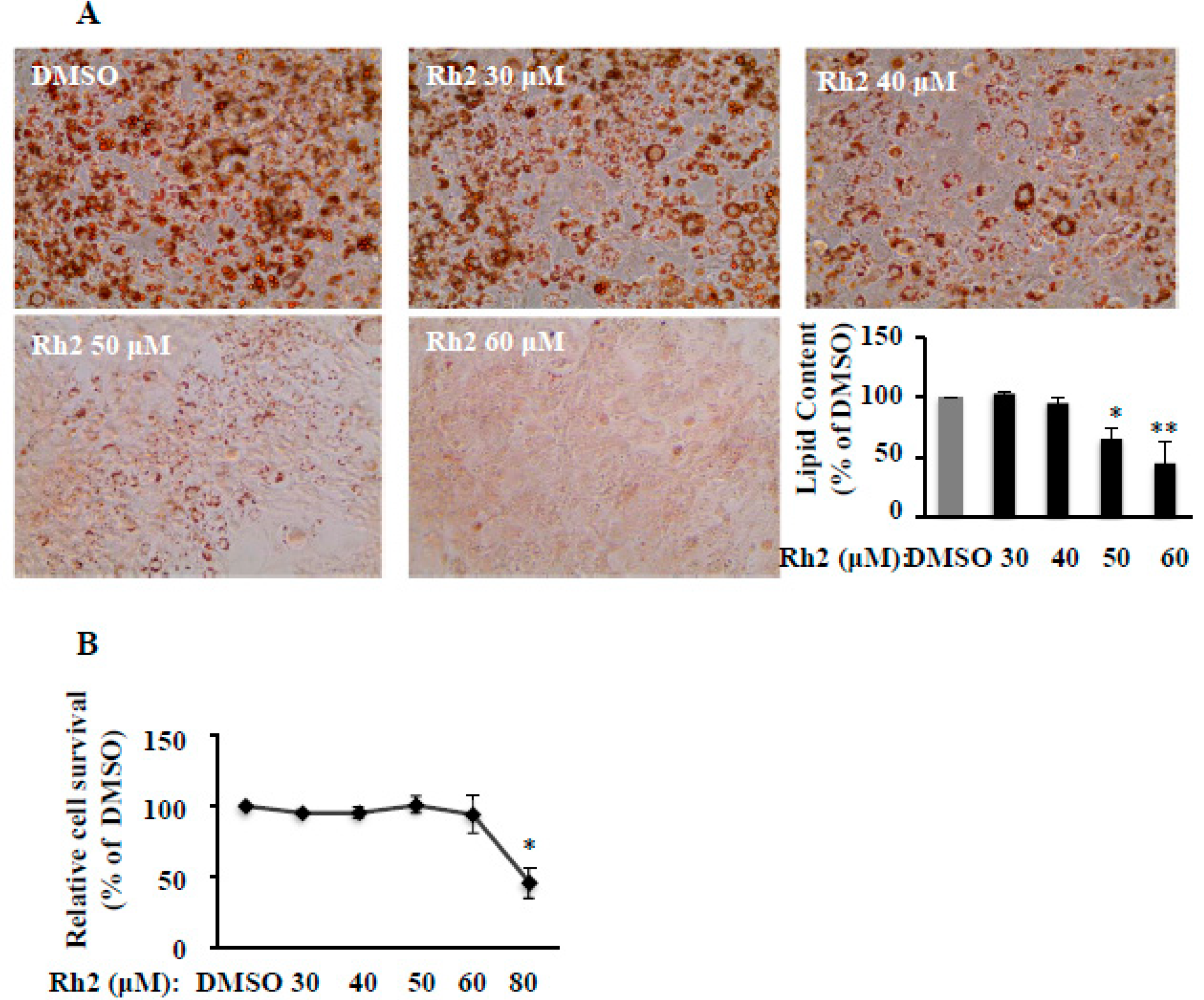
2.1. Ginsenoside Rh2 Dose-Dependently Suppresses 3T3-L1 Differentiation without Cytotoxicity
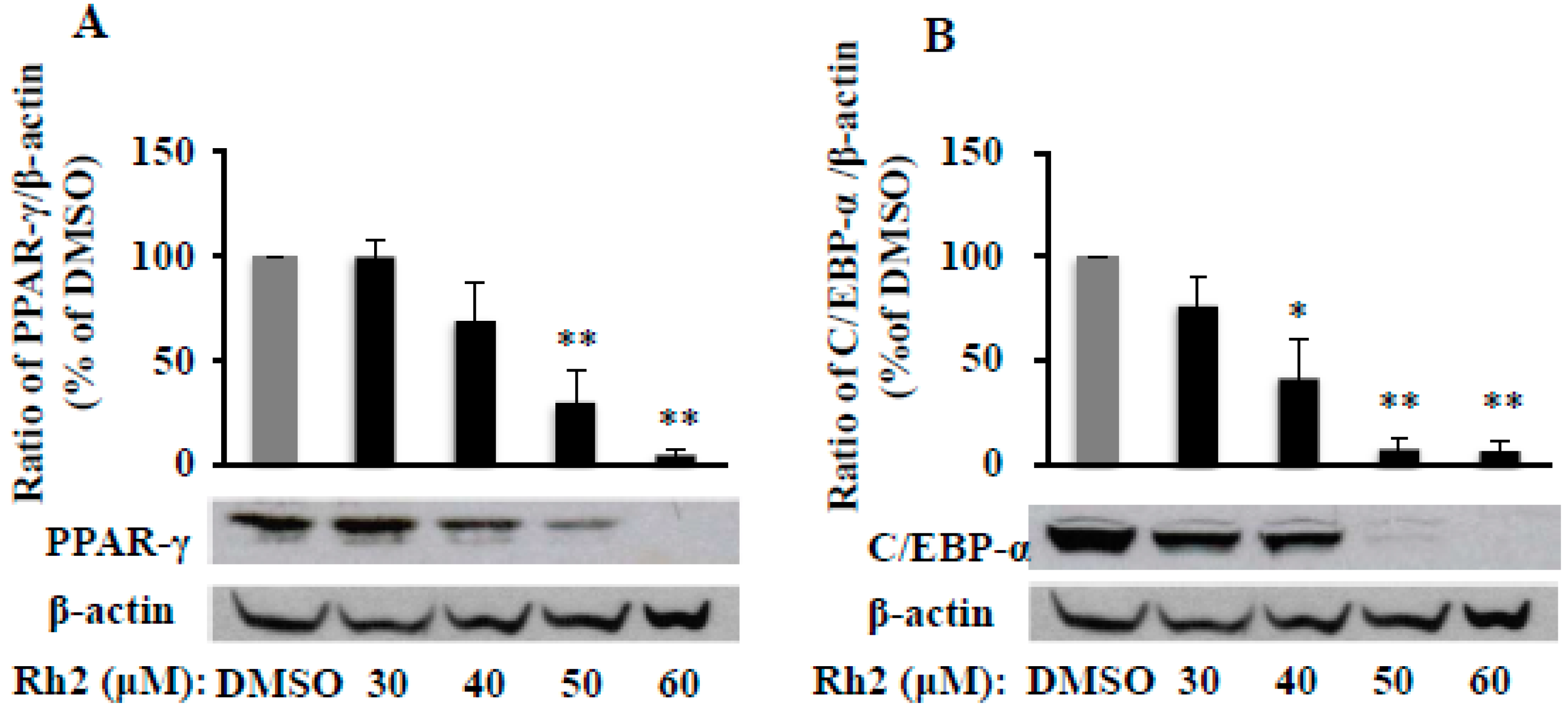
2.2. Ginsenoside Rh2 Dose-Dependently Inhibits PPAR-γ and C/EBP-α Protein Expressions in 3T3-L1 Cells
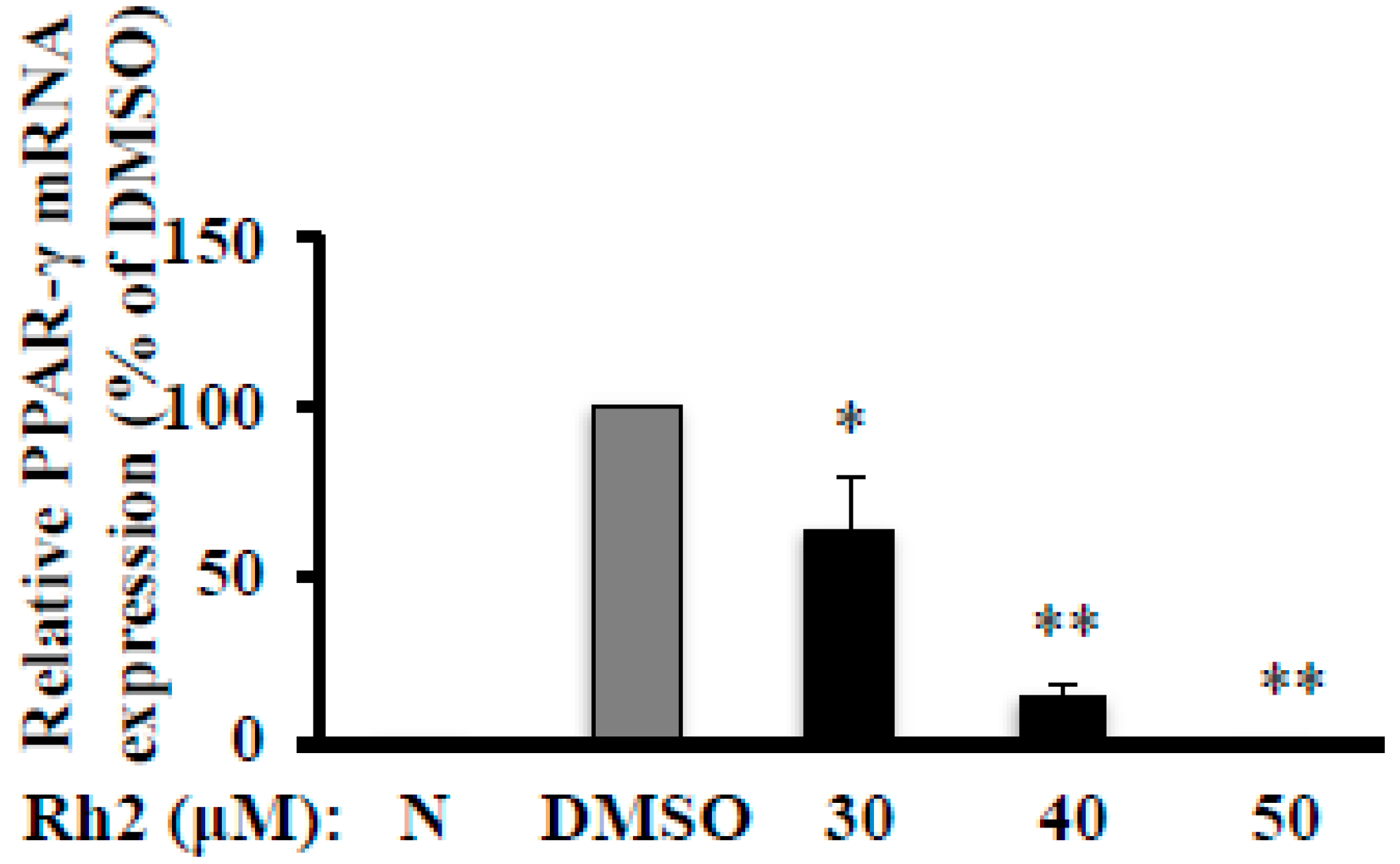
2.3. Ginsenoside Rh2 Abolishes MDI-Induced PPAR-γ mRNA Expression in 3T3-L1 Cells
2.4. Ginsenoside Rh2 Attenuates Fat Packing Proteins in 3T3-L1 Cells
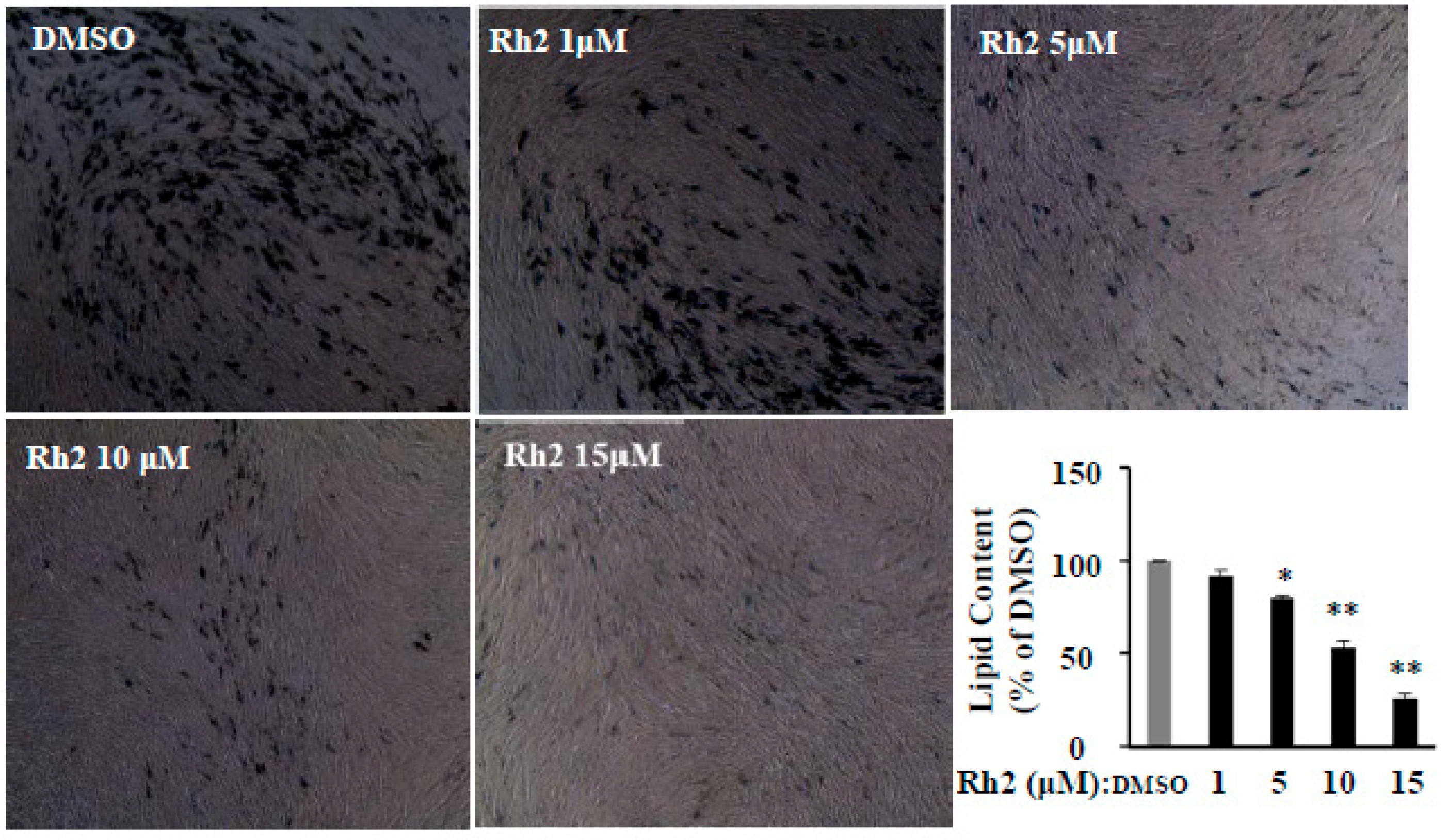
2.5. Ginsenoside Rh2 Dose-Dependently Inhibits Cell Differentiation in Human Primary Preadipocytes
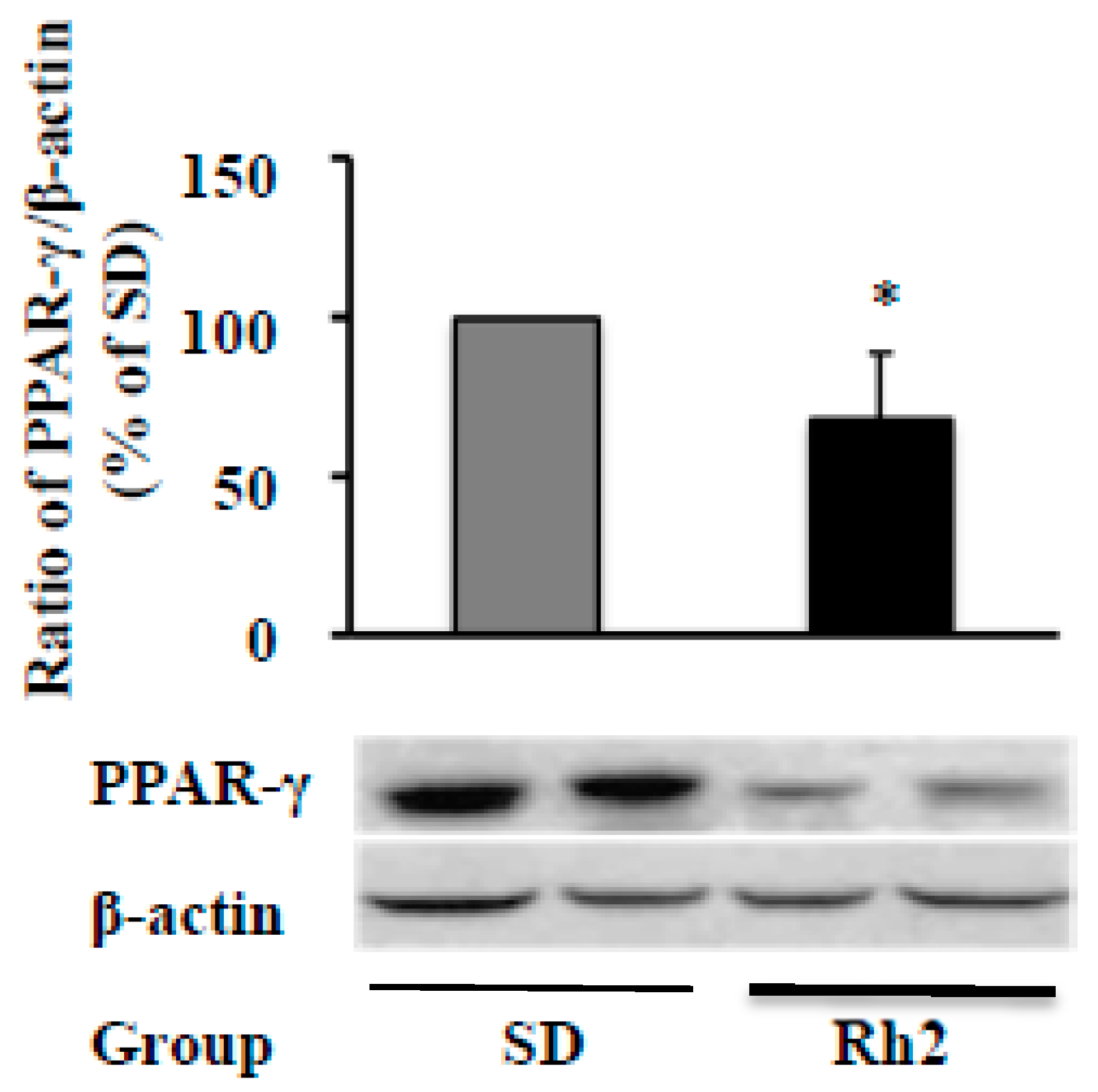
2.6. Dietary Ginsenoside Rh2 Reduces Hepatic PPAR-γ Expression in Obese Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Differentiation
4.3. Oil-Red O Staining
4.4. Cell Viability Assay
4.5. Animals
4.6. Protein Extraction
4.7. Western Blot
4.8. Total RNA Isolation and Quantitative Real-Time PCR Analysis
4.9. Hepatic Antioxidants
4.10. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| C/EBP-α | CCAAT/enhancer binding protein-alpha |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| FAS | fatty acid synthase |
| FABP4 | fatty acid binding protein 4 |
| GR | glutathione reductase |
| GST | glutathione S-transferase |
| HPPs | human primary preadipocytes |
| IBMX | 3-isobutyl-1-methylxanthine |
| MDI medium | IBMX + dexamethasone + insulin medium |
| PBS | phosphate-buffered saline |
| PCR | polymerase chain reaction |
| PPAR-γ | peroxisome proliferator-activated receptor-gamma |
| WATs | white adipose tissues |
References
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and Alternative Medicine Use Among Adults and Children: United States. 2007. Available online: https://www.cdc.gov/nchs/data/nhsr/nhsr012.pdf (accessed on 21 May 2020).
- Gui, Y.; Ryu, G.H. Effects of extrusion cooking on physicochemical properties of white and red ginseng (powder). J. Ginseng Res. 2014, 38, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Xiao-Ling, S.; Yaliu, S.; Ming-Ming, L.; Xue, F.; Xian-Sheng, M.; Li, F. Comparative Pharmacokinetics of Ginsenoside Rg3 and Ginsenoside Rh2 after Oral Administration of Ginsenoside Rg3 in Normal and Walker 256 Tumor-bearing Rats. Pharmacogn. Mag. 2016, 12, 21–24. [Google Scholar]
- Bae, E.A.; Han, M.J.; Choo, M.K.; Park, S.Y.; Kim, D.H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol. Pharm. Bull. 2002, 25, 58–63. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry 2004, 65, 337–344. [Google Scholar] [CrossRef]
- Wang, C.Z.; Aung, H.H.; Zhang, B.; Sun, S.; Li, X.L.; He, H.; Xie, J.T.; He, T.C.; Du, W.; Yuan, C.S. Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer. Res. 2008, 28, 2545–2551. [Google Scholar]
- Zhang, H.; Gong, J.; Zhang, H.; Kong, D. Induction of apoptosis and reversal of permeability glycoprotein-mediated multidrug resistance of MCF-7/ADM by ginsenoside Rh2. Int. J. Clin. Exp. Pathol. 2015, 8, 4444–4456. [Google Scholar]
- Zhang, Q.; Hong, B.; Wu, S.; Niu, T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour Biol. 2015, 36, 2377–2381. [Google Scholar] [CrossRef]
- Chen, F.; Deng, Z.Y.; Zhang, B.; Xiong, Z.X.; Zheng, S.L.; Tan, C.L.; Hu, J.N. Esterification of Ginsenoside Rh2 Enhanced Its Cellular Uptake and Antitumor Activity in Human HepG2 Cells. J. Agric. Food Chem. 2016, 64, 253–261. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, Y.; Li, C.; Qi, P.; Bao, J. Antihyperglycemic effect of ginsenoside Rh2 by inducing islet beta-cell regeneration in mice. Horm. Metab. Res. 2012, 44, 33–40. [Google Scholar]
- Chen, F.; Eckman, E.A.; Eckman, C.B. Reductions in levels of the Alzheimer’s amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006, 20, 1269–1271. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, C.H.; Kim, H.N.; Ha, T.S.; Ahn, H.Y. Ginsenoside Rg3 inhibits lipopolysaccharide-induced adhesion molecule expression in human umbilical vein endothelial cell and C57BL/6 mice. Pharmazie 2014, 69, 818–822. [Google Scholar]
- Siraj, F.M.; Kim, Y.J.; Natarajan, S.; Jung, S.K.; Yang, D.U.; Yang, D.C. Ginseng and obesity: Observations from assorted perspectives. Food Sci. Biotechnol. 2014, 23, 1007–1016. [Google Scholar] [CrossRef]
- Kim, J.H.; Hahm, D.H.; Yang, D.C.; Kim, J.H.; Lee, H.J.; Shim, I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J. Pharmacol. Sci. 2005, 97, 124–131. [Google Scholar] [CrossRef]
- Xie, J.T.; Wang, C.Z.; Ni, M.; Wu, J.A.; Mehendale, S.R.; Aung, H.H.; Foo, A.; Yuan, C.S. American ginseng berry juice intake reduces blood glucose and body weight in ob/ob mice. J. Food Sci. 2007, 72, S590–S594. [Google Scholar] [CrossRef]
- Niu, C.S.; Yeh, C.H.; Yeh, M.F.; Cheng, J.T. Increase of adipogenesis by ginsenoside (Rh2) in 3T3-L1 cell via an activation of glucocorticoid receptor. Horm. Metab. Res. 2009, 41, 271–276. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Kim, S.-H.; Lee, M.-S.; Kim, S.H.; Yang, H.-J.; Kim, M.-J.; Kim, H.-S.; Ha, J.; Kim, M.S.; Kwon, D.Y. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2007, 364, 1002–1008. [Google Scholar] [CrossRef]
- van Harmelen, V.; Skurk, T.; Rohrig, K.; Lee, Y.M.; Halbleib, M.; Aprath-Husmann, I.; Hauner, H. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 889–895. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Ahmed, B.; Liu, S.; Si, H. Antiadipogenic effects and mechanisms of combinations of genistein, epigallocatechin-3-gallate, and/or resveratrol in preadipocytes. J. Med. Food 2017, 20, 162–170. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Wang, X.; Si, H. Anti-adipogenic effects and mechanisms of ginsenoside Rg3 in pre-adipocytes and obese mice. Front. Pharmacol. 2017, 8, 113. [Google Scholar] [CrossRef]
- Fernandez-Iglesias, A.; Pajuelo, D.; Quesada, H.; Diaz, S.; Blade, C.; Arola, L.; Salvado, M.J.; Mulero, M. Grape seed proanthocyanidin extract improves the hepatic glutathione metabolism in obese Zucker rats. Mol. Nutr. Food Res. 2014, 58, 727–737. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, J.Y.; Ko, S.K. Changes in the contents of prosapogenin in Red ginseng (Panax ginseng) depending on the extracting conditions. J. Ginseng Res. 2016, 40, 86–89. [Google Scholar] [CrossRef][Green Version]
- Lee, S.A.; Jo, H.K.; Im, B.O.; Kim, S.; Whang, W.K.; Ko, S.K. Changes in the Contents of Prosapogenin in the Red Ginseng (Panax ginseng) Depending on Steaming Batches. J. Ginseng Res. 2012, 36, 102–106. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Mechanistic studies on protopanaxadiol, Rh2, and ginseng (Panax quinquefolius) extract induced cytotoxicity in intestinal Caco-2 cells. J. Biochem. Mol. Toxic. 2004, 18, 143–149. [Google Scholar] [CrossRef]
- Wang, C.Z.; Aung, H.H.; Ni, M.; Wu, J.A.; Tong, R.B.; Wicks, S.; He, T.C.; Yuan, C.S. Red American ginseng: Ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Medica 2007, 73, 669–674. [Google Scholar] [CrossRef]
- Qian, T.; Cai, Z. Biotransformation of ginsenosides Rb1, Rg3 and Rh2 in rat gastrointestinal tracts. Chin. Med. 2010, 5, 19. [Google Scholar] [CrossRef]
- Shen, H.; Leung, W.I.; Ruan, J.Q.; Li, S.L.; Lei, J.P.; Wang, Y.T.; Yan, R. Biotransformation of ginsenoside Rb1 via the gypenoside pathway by human gut bacteria. Chin. Med. 2013, 8, 22. [Google Scholar] [CrossRef]
- Hardie, D.G.; Scott, J.W.; Pan, D.A.; Hudson, E.R. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003, 546, 113–120. [Google Scholar] [CrossRef]
- Hardie, D.G. Minireview: The AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Barnes, B.; Mahlapuu, M.; Steiler, T.; Martinsson, S.; Leng, Y.; Wallberg-Henriksson, H.; Andersson, L.; Zierath, J. Role of AMP-activated protein kinase in the coordinated expression of genes controlling glucose and lipid metabolism in mouse white skeletal muscle. Diabetologia 2005, 48, 2354–2364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcia-Roves, P.M.; Osler, M.E.; Holmström, M.H.; Zierath, J.R. Gain-of-function R225Q mutation in AMP-activated protein kinase γ3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J. Biol. Chem. 2008, 283, 35724–35734. [Google Scholar] [CrossRef]
- Kajita, K.; Mune, T.; Ikeda, T.; Matsumoto, M.; Uno, Y.; Sugiyama, C.; Matsubara, K.; Morita, H.; Takemura, M.; Seishima, M.; et al. Effect of fasting on PPARgamma and AMPK activity in adipocytes. Diabetes. Res. Clin. Pract. 2008, 81, 144–149. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, Y.-H.; Park, S.K.; Kang, E.S.; Kim, H.-J.; Lee, Y.C.; Choi, C.S.; Park, S.E.; Ahn, C.W.; Cha, B.S. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metab. Clin. Exp. 2009, 58, 1170–1177. [Google Scholar] [CrossRef]
- Jeong, K.J.; Kim, G.W.; Chung, S.H. AMP-activated protein kinase: An emerging target for ginseng. J. Ginseng Res. 2014, 38, 83–88. [Google Scholar] [CrossRef]
- Yuan, H.-D.; Shin, E.-J.; Chung, S.-H. Anti-diabetic effect and mechanism of Korean red ginseng in C57BL/KsJ db/db mice. J. Ginseng Res. 2008, 32, 187–193. [Google Scholar]
- Hwang, J.T.; Lee, M.S.; Kim, H.J.; Sung, M.J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-γ signal pathways. Phytother. Res. 2009, 23, 262–266. [Google Scholar] [CrossRef]
- Park, M.W.; Ha, J.; Chung, S.H. 20 (S)-ginsenoside Rg3 enhances glucose-stimulated insulin secretion and activates AMPK. Biol. Pharm. Bull. 2008, 31, 748–751. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.-S.; Kim, C.-T.; Kim, I.-H.; Kim, Y. Ginsenoside Rg3 reduces lipid accumulation with AMP-activated protein kinase (AMPK) activation in HepG2 cells. Int. J. Mol. Sci. 2012, 13, 5729–5739. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sakamoto, K. (-)-Epigallocatechin gallate suppresses adipocyte differentiation through the MEK/ERK and PI3K/Akt pathways. Cell Biol. Int. 2011, 36, 147–153. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.; Yoon, Y. The anti-adipogenic effects of (-)epigallocatechin gallate are dependent on the WNT/beta-catenin pathway. J. Nutr. Biochem. 2013, 24, 1232–1240. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.H.; Xia, J.; Li, X.P.; Li, K.Q.; Xiong, W.; Li, J.; Chen, D.L. 20(S)-ginsenoside Rh2 inhibits the proliferation and induces the apoptosis of KG-1a cells through the Wnt/beta-catenin signaling pathway. Oncol. Rep. 2016, 36, 137–146. [Google Scholar] [CrossRef]
- Guan, N.; Huo, X.; Zhang, Z.; Zhang, S.; Luo, J.; Guo, W. Ginsenoside Rh2 inhibits metastasis of glioblastoma multiforme through Akt-regulated MMP13. Tumour Biol. 2015, 36, 6789–6795. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Cha, B.-Y.; Yamaguchi, K.; Choi, S.-S.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Effects of Korean white ginseng extracts on obesity in high-fat diet-induced obese mice. Cytotechnology 2010, 62, 367–376. [Google Scholar] [CrossRef][Green Version]
- Song, Y.B.; An, Y.R.; Kim, S.J.; Park, H.W.; Jung, J.W.; Kyung, J.S.; Hwang, S.Y.; Kim, Y.S. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J. Sci. Food Agric. 2012, 92, 388–396. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.J.; Lee, Y.H.; Lee, B.W.; Cha, B.S.; Kang, E.S.; Ahn, C.W.; Park, J.S.; Kim, H.J.; Lee, E.Y. Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague-Dawley rats. Phytother. Res. 2012, 26, 142–147. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.K.; Han, S.J.; Kim, S.H.; Hur, K.Y.; Kang, E.S.; Ahn, C.W.; Cha, B.S.; Kim, K.S.; Lee, H.C. Korean Red Ginseng Activates AMPK in Skeletal Muscle and Liver. Diabetes 2007, 56, A448. [Google Scholar]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Z.; Wang, H.; Jia, G.; Liu, G.; Zhao, H.; Nie, H. Porcine phosphotyrosine interaction domain containing 1 modulates 3T3-L1 preadipocyte proliferation and differentiation. Biologia 2013, 68, 1010–1014. [Google Scholar] [CrossRef]
- Bae, C.R.; Park, Y.K.; Cha, Y.S. Quercetin-rich onion peel extract suppresses adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J. Sci. Food Agric. 2014, 94, 2655–2660. [Google Scholar] [CrossRef]
- Issa, R.; Boeving, M.; Kinter, M.; Griffin, T.M. Effect of biomechanical stress on endogenous antioxidant networks in bovine articular cartilage. J. Orthop. Res. 2018, 36, 760–769. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds ginsenoside Rh2 are available from the authors. |

| Group | Body Weight Gain (g/mouse) | Food Intake (g/mouse/day) | Liver Weight (g) | WATs Weight (g) | Fasting Blood Glucose (mg/dL) | Liver Glutathione Reductase (nmol/min/mg protein) | Liver Glutathione S-Transferase (nmol/min/mg protein) |
|---|---|---|---|---|---|---|---|
| SD | 21.6 ± 1.85 | 3.85 ± 0.30 | 1.78 ± 0.22 | 2.71 ± 0.31 | 160 ± 16.9 | 50.2 ± 3.77 | 1171 ± 53.3 |
| Rh2 | 24.1 ± 0.82 | 3.82 ± 0.30 | 1.67 ± 0.23 | 2.69 ± 0.39 | 132 ± 11.4 * | 38.7 ± 0.91 * | 1044 ± 17.2 * |
| Ingredient | Standard Diet (g/kg diet) | Rh2 Supplemented Diet (g/kg diet) |
|---|---|---|
| Cornstarch | ||
| Casein (>85%protein) | ||
| Dextrinized cornstarch (>90% tetrasaccharides) | ||
| Sucrose | ||
| Soybean oil | ||
| Fiber | ||
| Mineral mix (AIN-93M-MX) | ||
| Vitamin mix (AIN-93-vx) | ||
| L-Cystine | 1.80 | |
| Choline bitartrate | ||
| Ter-butyhydroquinone | ||
| Ginsenoside Rh2 | 0.00 | 0.10 |
| Total | 1000 | 1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Virgous, C.; Si, H. How Does Ginsenoside Rh2 Mitigate Adipogenesis in Cultured Cells and Obese Mice? Molecules 2020, 25, 2412. https://doi.org/10.3390/molecules25102412
Zhang L, Virgous C, Si H. How Does Ginsenoside Rh2 Mitigate Adipogenesis in Cultured Cells and Obese Mice? Molecules. 2020; 25(10):2412. https://doi.org/10.3390/molecules25102412
Chicago/Turabian StyleZhang, Longyun, Carlos Virgous, and Hongwei Si. 2020. "How Does Ginsenoside Rh2 Mitigate Adipogenesis in Cultured Cells and Obese Mice?" Molecules 25, no. 10: 2412. https://doi.org/10.3390/molecules25102412
APA StyleZhang, L., Virgous, C., & Si, H. (2020). How Does Ginsenoside Rh2 Mitigate Adipogenesis in Cultured Cells and Obese Mice? Molecules, 25(10), 2412. https://doi.org/10.3390/molecules25102412




