Griseaketides A–D, New Aromatic Polyketides from the Pathogenic Fungus Magnaporthe grisea
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Microbial Material
4.3. Cultivation, Extraction and Isolation
4.4. Nematicidal Activity
Author Contributions
Funding
Conflicts of Interest
References
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.J.; Xiao, J.H. Secondary metabolites from higher fungi: Discovery, bioactivity, and bioproduction. Adv. Biochem. Eng. Biotechnol. 2009, 113, 79–150. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, T.J.; Dunkle, L.D.; Ciuffetti, L.M. Host-selective toxins and avirulence determinants: What’s in a name? Annu. Rev. Phytopathol. 2002, 40, 251–285. [Google Scholar] [CrossRef] [PubMed]
- Dufour, N.; Rao, R.P. Secondary metabolites and other small molecules as intercellular pathogenic signals. FEMS Microbiol. Lett. 2011, 314, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Yang, D.S.; Li, G.H.; Pu, X.J.; Mo, M.H.; Zhao, P.J. Antibacterial diketopiperazines from an endophytic fungus Bionectria sp. Y1085. J. Antibiot. 2019, 72, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Sekido, S.; Yamaguchi, I.; Kondo, H.; Suzuki, Y.; Neto, G.C.; Sakurai, A.; Yaegashi, H. Structures of two novel pyriculol-related compounds and identification of naturally produced epipyriculol from Pyricularia oryzae. Agric. Biol. Chem. 1991, 55, 2785–2791. [Google Scholar] [CrossRef]
- Sviridov, S.I.; Ermolinskii, B.S. Secondary metabolites of Pyricularia oryzae I. o-nitrophenol derivatives. Chem. Nat. Compd. 1990, 26, 691–696. [Google Scholar] [CrossRef]
- Iwasaki, S.; Muro, H.; Nozoe, S.; Okuda, S.; Sato, Z. Isolation of 3,4-dihydro-3,4,8-trihydroxy-1(2H)-naphthalenone and tenuazonic acid from Pyricularia oryzae cavara. Tetrahedron Lett. 1972, 13, 13–16. [Google Scholar] [CrossRef]
- Scharf, D.H.; Heinekamp, T.; Brakhage, A.A. Human and plant fungal pathogens: The role of secondary metabolites. PLoS Pathog. 2014, 10, e1003859. [Google Scholar] [CrossRef]
- Mobius, N.; Hertweck, C. Fungal phytotoxins as mediators of virulence. Curr. Opin. Plant Biol. 2009, 12, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.N.; Chowdhury, R.; Hasan, C.M.; Rashid, M.A. Steroids and polyketides from Uvaria hamiltonii stem bark. Acta Pharm. 2004, 54, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Myobatake, Y.; Takemoto, K.; Kamisuki, S.; Inoue, N.; Takasaki, A.; Takeuchi, T.; Mizushina, Y.; Sugawara, F. Cytotoxic alkylated hydroquinone, phenol, and cyclohexenone derivatives from Aspergillus violaceofuscus Gasperini. J. Nat. Prod. 2014, 77, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Peng, W.B.; Li, Z.L.; Wang, X.; Wei, J.G.; He, Q.X.; Yang, Y.P.; Liu, K.C.; Li, X.L. Chemical constituents from Euphorbia stracheyi and their biological activities. Fitoterapia 2014, 97, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Jang, Y.W.; Kim, Y.S.; Yu, S.H.; Lee, K.J.; Park, S.M.; Oh, B.T.; Chae, J.C.; Yun, B.S. Xylarinols A and B, two new 2-benzoxepin derivatives from the fruiting bodies of Xylaria polymorpha. J. Antibiot. 2009, 62, 163–165. [Google Scholar] [CrossRef]
- Leyva, A.; Blum, F.E.; Ley, S.V. A new synthesis of (–)-epipyriculol: A phytotoxic metabolite. Tetrahedron 2008, 64, 4711–4717. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugiyama, T.; Watanabe, M.; Murayama, T.; Yamashita, K. Syntheses of all four stereoisomers of pyriculol. Agric. Biol. Chem. 1987, 51, 2161–2166. [Google Scholar] [CrossRef]
- Sasaki, A.; Tanaka, K.; Sato, Y.; Kuwahara, S.; Kiyota, H. First synthesis and absolute configuration of (–)-pyriculariol, a phytotoxin isolated from rice blast fungus, Magnaporthe grisea. Use of microwave irradiation to control Stille coupling reaction products. Tetrahedron Lett. 2009, 50, 4637–4638. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Gorecki, M.; Mandoli, A.; Di Bari, L.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Clement, S.; Evidente, A. Pyriculins A and B, two monosubstituted hex-4-ene-2,3-diols and other phytotoxic metabolites produced by Pyricularia grisea isolated from buffelgrass (Cenchrus ciliaris). Chirality 2017, 29, 726–736. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kiyota, H.; Ueda, R.; Kuwahara, S. Synthesis to determine the absolute configuration of (–)-pyricuol, a phytotoxin isolated from rice blast disease fungus Magnaporthe grisea. Tetrahedron Lett. 2005, 46, 7107–7109. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakamura, Y.; Sasaki, A.; Ueda, R.; Suzuki, Y.; Kuwahara, S.; Kiyota, H. Synthesis and plant growth inhibitory activity of both enantiomers of pyricuol, a phytotoxin isolated from rice blast disease fungus Magnaporthe grisea. Tetrahedron 2009, 65, 6115–6122. [Google Scholar] [CrossRef]
- Martín-matute, B.; Nevado, C.; Cárdenas, D.J.; Echavarren, A.M. Intramolecular reactions of alkynes with furans and electron rich arenes catalyzed by PtCl2: the role of platinum carbenes as intermediates. J. Am. Chem. Soc. 2003, 125, 5757–5766. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Kuwahara, Y. 7-Hydroxyphthalide: A new natural salicylaldehyde analog from Oulenzia sp. (Astigmata: Winterschmitiidae). Biosci. Biotechnol. Biochem. 2001, 65, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Gulder, T.A.M.; Hong, H.; Correa, J.; Egereva, E.; Wiese, J.; Imhoff, J.F.; Gross, H. Isolation, structure elucidation and total synthesis of lajollamide A from the marine fungus Asteromyces cruciatus. Mar. Drugs 2012, 10, 2912–2935. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.J.; Wang, C.H.; Zeng, Q.; Guan, B.; Zhang, W.D.; Jin, H.Z. Chemical constituents of the stems of Celastrus rugosus. Arch. Pharm. Res. 2013, 36, 1291–1301. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Malonne, H.; Duez, R.; Vanhaelen-Fastre, R.; Vanhaelen, M.; Fontaine, J. Cytotoxic constituents from Plumbago zeylanica. Fitoterapia 2004, 75, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Zhang, H.J.; Yao, P.; Niu, X.M.; Xiang, W.; Sun, H.D. Non-taxane compounds from the bark of Taxus yunnanensis. J. Asian Nat. Prod. Res. 2002, 4, 147–154. [Google Scholar] [CrossRef]
- Kim, J.C.; Min, J.Y.; Kim, H.T.; Cho, K.Y.; Yu, S.H. Pyricuol, a new phytotoxin from Magnaporthe grisea. Biosci. Biotech. Bioch. 1998, 62, 173–174. [Google Scholar] [CrossRef]
- Jacob, S.; Grotsch, T.; Foster, A.J.; Schuffler, A.; Rieger, P.H.; Sandjo, L.P.; Liermann, J.C.; Opatz, T.; Thines, E. Unravelling the biosynthesis of pyriculol in the rice blast fungus Magnaporthe oryzae. Microbiology 2017, 163, 541–553. [Google Scholar] [CrossRef]
- Caboni, P.; Aissani, N.; Cabras, T.; Falqui, A.; Marotta, R.; Liori, B.; Ntalli, N.; Sarais, G.; Sasanelli, N.; Tocco, G. Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 1794–1803. [Google Scholar] [CrossRef]
- Sajid, M.; Azim, M.K. Characterization of the nematicidal activity of natural honey. J. Agric. Food Chem. 2012, 60, 7428–7434. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.S.; Li, G.H.; Zhao, P.J.; Zheng, X.; Luo, S.L.; Li, L.; Niu, X.M.; Zhang, K.Q. Nematicidal activity of Trichoderma spp. and isolation of an active compound. World J. Microbiol. Biotechnol. 2010, 26, 2297–2302. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
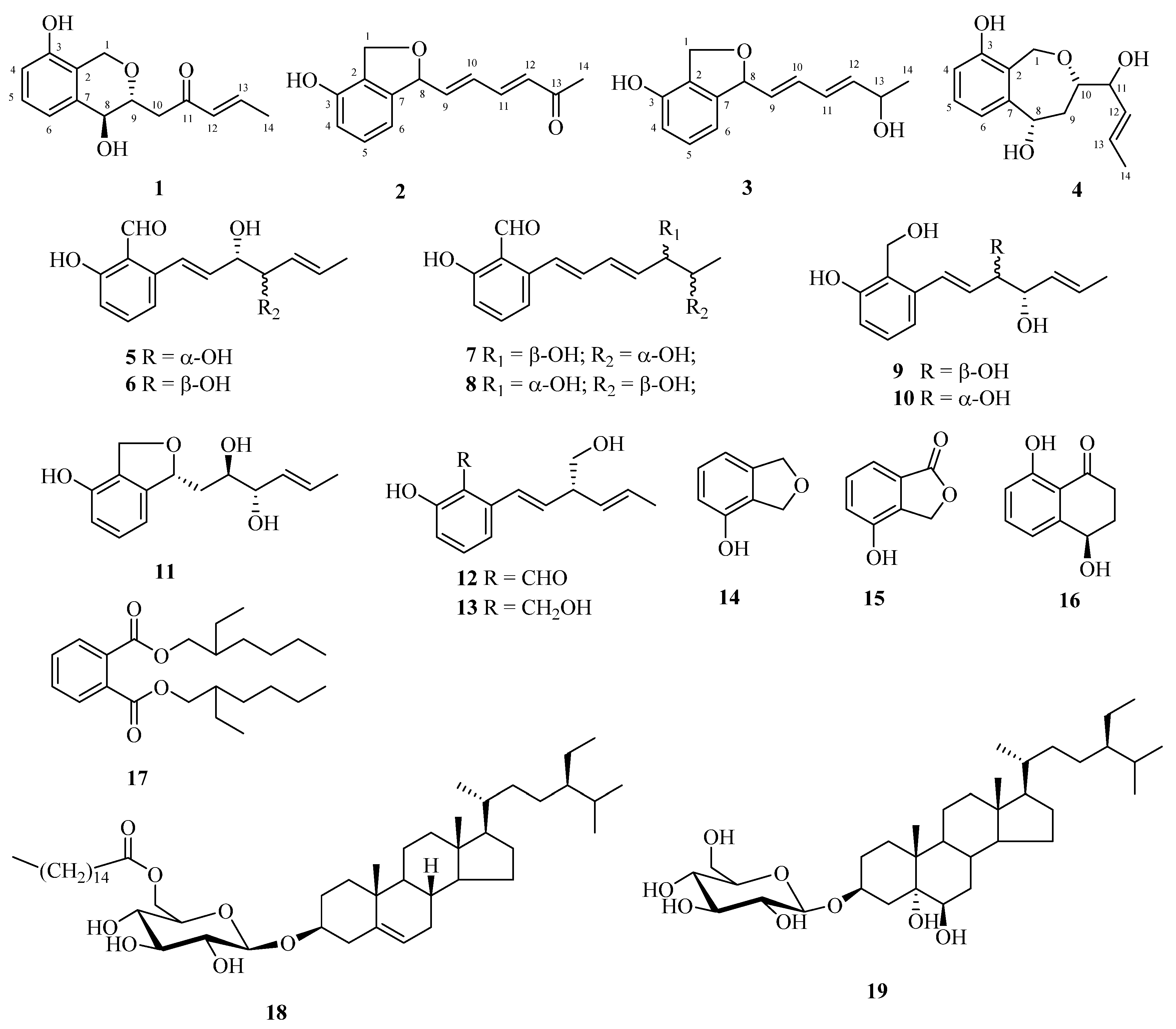
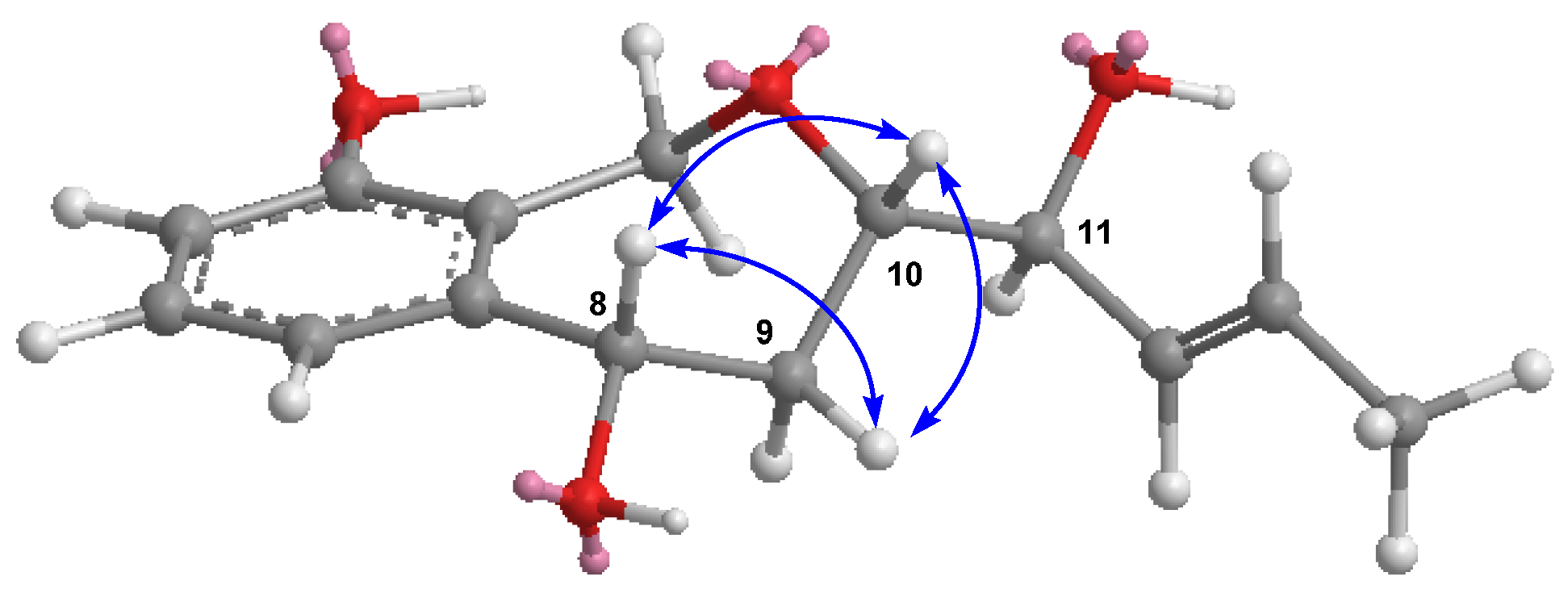
 ) and 1H-1H COSY (
) and 1H-1H COSY ( ) correlations of compounds 1–4.
) correlations of compounds 1–4.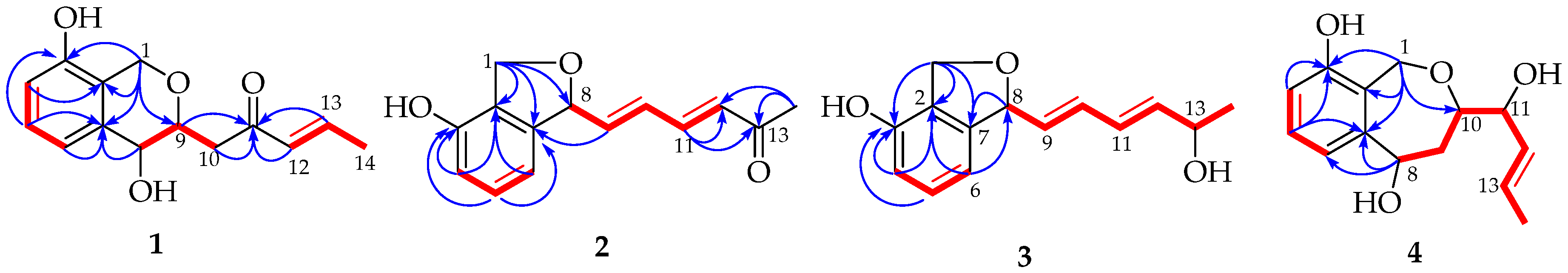
| No. | 1 (in CDCl3) | 2 (in CDCl3) | ||||
|---|---|---|---|---|---|---|
| δH | δC | HMBC | δH | δC | HMBC | |
| 1 | 4.85, d (15.8) | 64.3, t | 2, 3, 7, 9 | 5.23, dd (11.9, 2.1) | 71.2, t | 2, 7, 8 |
| 4.70, d (15.8) | 2, 3, 7, 9 | 5.15, d (11.9) | 2, 7, 8 | |||
| 2 | - | 121.9, s | - | - | 125.2, s | - |
| 3 | - | 150.8, s | - | - | 150.3, s | - |
| 4 | 6.65, d (8.7) | 113.7, d | 6 | 6.71, d (8.0) | 114.5, d | 2, 3, 6 |
| 5 | 7.11, t (7.5, overlap) | 127.7, d | 3, 4, 7 | 7.18, t (7.7) | 129.4, d | 3, 7 |
| 6 | 7.12, d (7.5, overlap) | 118.4, d | 2, 4, 7 | 6.73, d (7.5) | 113.9, d | 2, 4, 8 |
| 7 | - | 138.3, s | - | - | 142.4, s | - |
| 8 | 4.49, d (7.5) | 69.2, d | 7, 9, 10 | 5.73, brd (6.0) | 84.4, d | 9, 10 |
| 9 | 3.93, m | 75.4, d | 1, 11 | 6.25, dd (15.1, 6.8) | 141.8, d | 7, 8 |
| 10 | 2.98, dd (16.2, 4.7) | 43.2, t | 8, 9, 11 | 6.53, dd (15.1, 11.0) | 129.0, d | 8, 11, 12 |
| 3.03, dd (16.2, 7.1) | - | |||||
| 11 | - | 199.2, s | - | 7.15, dd (15.7, 11.0) | 142.4, d | 9, 10, 13 |
| 12 | 6.24, dd (15.8, 1.4) | 132.2, d | 11, 14 | 6.21, d (15.7) | 131.4, d | 10, 14 |
| 13 | 6.99, dq (15.8, 6.8) | 144.6, d | 11, 14 | - | 198.8, s | - |
| 14 | 1.94, dd (6.8, 1.4) | 18.4, q | 12, 13 | 2.28, s | 27.3, q | 12, 13 |
| No. | 3 (in CD3OD) | 4 (in CD3OD) | ||||
|---|---|---|---|---|---|---|
| δH | δC | HMBC | δH | δC | HMBC | |
| 1 | 5.09, dd (12.0, 2.6) | 71.7, t | 2, 7, 8 | 4.91, d (15.6) | 65.8, t | 2, 3, 7, 10 |
| 4.98, brd (12.0) | 2, 7, 8 | 4.50, d (15.6) | 2, 3, 7 | |||
| 2 | - | 126.0, s | - | - | 123.1, s | - |
| 3 | - | 153.0, s | - | - | 154.0, s | - |
| 4 | 6.67, d (7.7) | 115.1, d | 1, 2, 3, 6 | 6.68, d (7.9) | 114.6, d | 3, 6 |
| 5 | 7.10, t (7.7) | 130.3, d | 3, 4, 7 | 7.08, t (7.9) | 128.4, d | 3, 6, 7 |
| 6 | 6.58, d (7.7) | 113.8, d | 2, 4, 8 | 6.85, d (7.5) | 122.0, d | 2, 4, 8 |
| 7 | - | 144.1, s | - | - | 138.2, s | - |
| 8 | 5.57, d (7.7) | 86.6, d | 7, 9 | 4.28, brs | 67.5, d | 2, 6, 7 |
| 9 | 5.71, dd (15.1, 7.7) | 133.5, d | 8, 11 | 2.06, m; 1.80, m | 39.6, t | 10, 11, 12 |
| 10 | 6.38, dd (15.1, 10.6) | 132.8, d | 8, 11, 12 | 3.63, brt (6.8) | 76.9, d | 8, 9 |
| 11 | 6.24, dd (15.2, 10.6) | 129.3, d | 10, 13 | 4.30, t (6.8) | 71.2, d | 13 |
| 12 | 5.81, dd (15.2, 6.2) | 139.9, d | 10, 13 | 5.52, dd (14.6, 6.8) | 135.3, d | 11, 14 |
| 13 | 4.29, dq (6.2, 6.4) | 68.7, d | 11, 14 | 5.73, dq (14.6, 6.1) | 127.7, d | 11, 14 |
| 14 | 1.24, d (6.4) | 23.5, q | 12, 13 | 1.71, d (6.1) | 17.9, q | 12, 13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-H.; Yang, D.-S.; Lei, H.-M.; Li, C.-Y.; Li, G.-H.; Zhao, P.-J. Griseaketides A–D, New Aromatic Polyketides from the Pathogenic Fungus Magnaporthe grisea. Molecules 2020, 25, 72. https://doi.org/10.3390/molecules25010072
Yang Y-H, Yang D-S, Lei H-M, Li C-Y, Li G-H, Zhao P-J. Griseaketides A–D, New Aromatic Polyketides from the Pathogenic Fungus Magnaporthe grisea. Molecules. 2020; 25(1):72. https://doi.org/10.3390/molecules25010072
Chicago/Turabian StyleYang, Yin-He, Da-Song Yang, Hong-Mei Lei, Cheng-Yun Li, Guo-Hong Li, and Pei-Ji Zhao. 2020. "Griseaketides A–D, New Aromatic Polyketides from the Pathogenic Fungus Magnaporthe grisea" Molecules 25, no. 1: 72. https://doi.org/10.3390/molecules25010072
APA StyleYang, Y.-H., Yang, D.-S., Lei, H.-M., Li, C.-Y., Li, G.-H., & Zhao, P.-J. (2020). Griseaketides A–D, New Aromatic Polyketides from the Pathogenic Fungus Magnaporthe grisea. Molecules, 25(1), 72. https://doi.org/10.3390/molecules25010072





