Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Solvents and Chemicals
4.2. Reference Compounds and Standard Solutions
4.3. Material and Sample Preparation
4.4. U(H)PLC-ESI-MS Conditions
4.5. HPLC-DAD Conditions
4.6. Content Measurement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
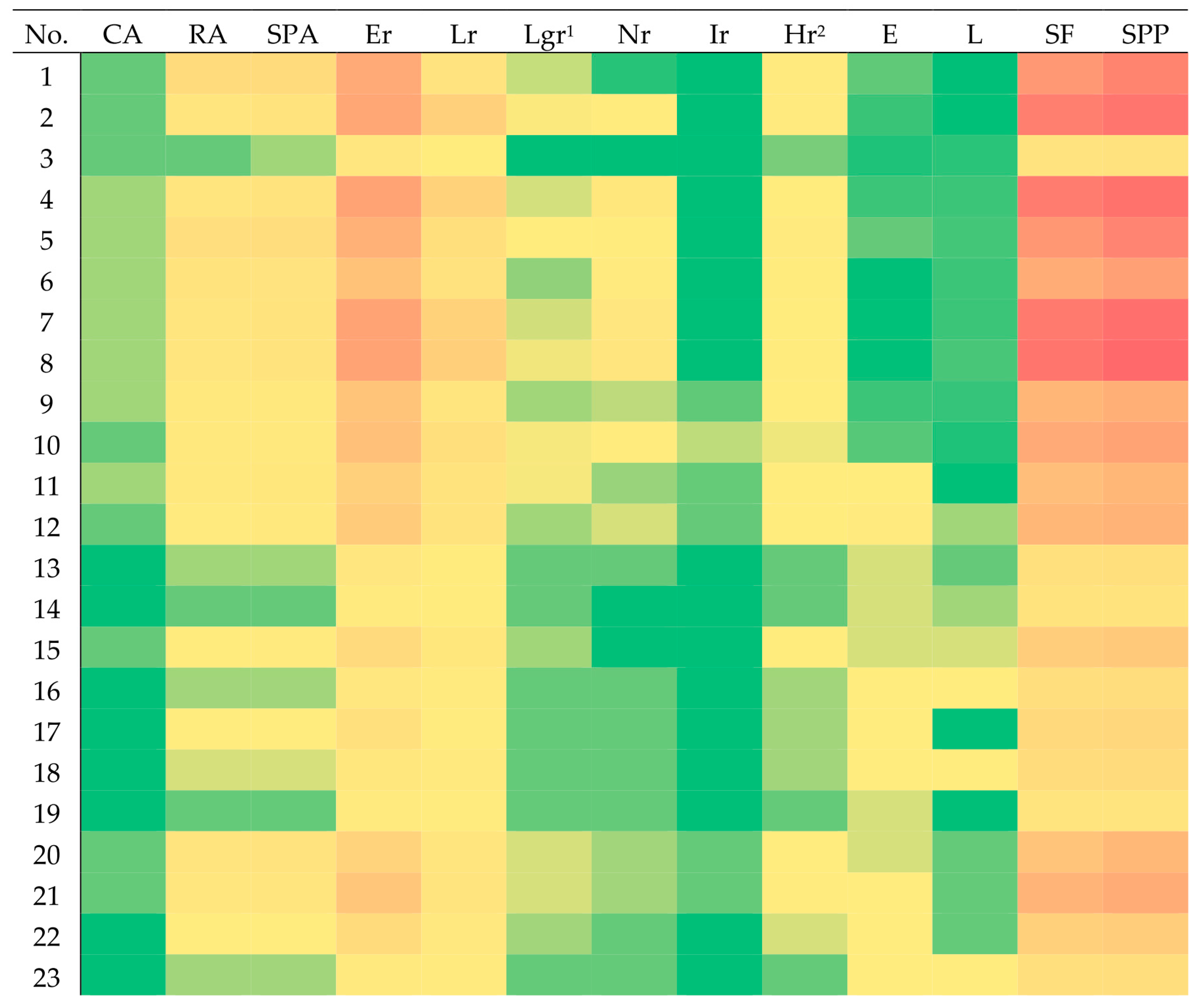
| No. | CA. | RA | SPA | Er | Lr 1 | Lgr | Nr | Ir | Hr 2 | E | L | SF | SPP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.01 a | 0.44c | 0.45c | 1.63 c | 0.27 b | 0.03 d | 0.00 | 0.00 | 0.09 b | 0.01 c | 0.00 | 2.03 c | 2.48 c |
| 2 | 0.01 a | 0.22 b | 0.23 b | 1.70 a | 0.71 a | 0.04 b | 0.08 b | 0.00 | 0.09 c | 0.01 c | 0.00 | 2.61 a | 2.84 a |
| 3 | 0.01 a | 0.01 c | 0.02 b | 0.18 a | 0.06d | 0.00 | 0.00 | 0.00 | 0.01 a | 0.00 | 0.00 | 0.26 b | 0.28 b |
| 4 | 0.02 a | 0.21 b | 0.22 b | 1.76 b | 0.66 b | 0.03 d | 0.17 c | 0.00 | 0.05 c | 0.01 b | 0.01 c | 2.68 b | 2.91 b |
| 5 | 0.02 b | 0.38 b | 0.39 b | 1.46 b | 0.36 b | 0.05d | 0.07 c | 0.00 | 0.10d | 0.01 | 0.01 a | 2.06 a | 2.45 a |
| 6 | 0.02 b | 0.24 a | 0.26 a | 1.04 a | 0.32 b | 0.02 d | 0.09 b | 0.00 | 0.07 b | 0.00 | 0.01 d | 1.54 b | 1.80 a |
| 7 | 0.02 b | 0.22 a | 0.23 a | 1.77a | 0.67 a | 0.03 b | 0.19 d | 0.00 | 0.06 a | 0.00 | 0.01 c | 2.72 a | 2.95 a |
| 8 | 0.02 b | 0.22 b | 0.24 b | 1.76 b | 0.74b | 0.04 c | 0.23d | 0.00 | 0.08 b | 0.00 | 0.01 a | 2.85b | 3.09b |
| 9 | 0.02 a | 0.13 a | 0.15 a | 0.99 b | 0.21 a | 0.02 d | 0.03 b | 0.01 c | 0.06 c | 0.01 d | 0.00 | 1.33 b | 1.48 c |
| 10 | 0.01 a | 0.13 b | 0.14 b | 1.09 a | 0.35 a | 0.04 c | 0.07 a | 0.03c | 0.04 b | 0.01 a | 0.00 | 1.62 a | 1.76 a |
| 11 | 0.02 a | 0.15 a | 0.16 a | 0.72 a | 0.24 a | 0.04 a | 0.02 b | 0.01 a | 0.05 b | 0.06 a | 0.00 | 1.14 a | 1.31 a |
| 12 | 0.01 a | 0.11 a | 0.12 d | 0.82 d | 0.25 c | 0.02 a | 0.03 a | 0.01 a | 0.04 a | 0.10d | 0.02 a | 1.29 a | 1.41 c |
| 13 | 0.00 | 0.02 a | 0.02 a | 0.19 d | 0.08 a | 0.01 a | 0.01 a | 0.00 | 0.01 a | 0.03 a | 0.01 a | 0.34 c | 0.36 c |
| 14 | 0.00 | 0.01a | 0.01a | 0.09a | 0.07 a | 0.01 a | 0.00 | 0.00 | 0.01 a | 0.03 a | 0.02 a | 0.23a | 0.24a |
| 15 | 0.01 a | 0.08 a | 0.09 d | 0.48 c | 0.17 c | 0.02 a | 0.00 | 0.00 | 0.04 a | 0.03 a | 0.03 a | 0.77 b | 0.86 b |
| 16 | 0.00 | 0.02 a | 0.02 a | 0.16 a | 0.10 c | 0.01 a | 0.01 a | 0.00 | 0.02 a | 0.04 a | 0.04 a | 0.38 c | 0.40 c |
| 17 | 0.00 | 0.04 a | 0.04 a | 0.34 c | 0.08 c | 0.01 a | 0.01 a | 0.00 | 0.02 a | 0.04 a | 0.00 | 0.50 c | 0.54 d |
| 18 | 0.00 | 0.03 a | 0.03 a | 0.18 a | 0.12 a | 0.01 a | 0.01 a | 0.00 | 0.02 a | 0.05 a | 0.04 a | 0.43 a | 0.46 a |
| 19 | 0.00 | 0.01 a | 0.01 a | 0.10 c | 0.07 c | 0.01 a | 0.01 a | 0.00 | 0.01a | 0.03 a | 0.00 | 0.23 c | 0.24 c |
| 20 | 0.01 a | 0.25 c | 0.26 c | 0.64 c | 0.22 c | 0.03 a | 0.02 a | 0.01 a | 0.04 a | 0.03 a | 0.01 a | 1.00 c | 1.26 b |
| 21 | 0.01 a | 0.20 d | 0.21 d | 0.94 c | 0.24 c | 0.03 a | 0.02 a | 0.01 a | 0.08 c | 0.04 a | 0.01 a | 1.37 d | 1.58 d |
| 22 | 0.00 | 0.06 d | 0.06 d | 0.44 c | 0.15 d | 0.02 a | 0.01 a | 0.00 | 0.03 a | 0.05 c | 0.01 a | 0.71 d | 0.77 c |
| 23 | 0.00 | 0.02 a | 0.02 d | 0.13 a | 0.09 c | 0.01 a | 0.01 a | 0.00 | 0.01 a | 0.06 a | 0.04a | 0.35 c | 0.37 b |
| MEAN | 0.01 | 0.14 | 0.15 | 0.81 | 0.27 | 0.02 | 0.05 | 0.00 | 0.04 | 0.03 | 0.01 | 1.24 | 1.39 |
| SD | 0.00 | 0.12 | 0.12 | 0.60 | 0.23 | 0.01 | 0.06 | 0.01 | 0.03 | 0.02 | 0.01 | 0.87 | 0.97 |
| MED | 0.01 | 0.13 | 0.14 | 0.72 | 0.22 | 0.02 | 0.02 | 0.00 | 0.04 | 0.03 | 0.01 | 1.14 | 1.31 |
| MAX | 0.02 | 0.44 | 0.45 | 1.77 | 0.74 | 0.05 | 0.23 | 0.03 | 0.10 | 0.10 | 0.04 | 2.85 | 3.09 |
| MIN | 0.00 | 0.01 | 0.01 | 0.09 | 0.06 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.23 | 0.24 |
References
- Sujana, P.; Sridhar, T.M.; Josthna, P.; Naidu, C.V. Antibacterial Activity and Phytochemical Analysis of Mentha piperita L. (Peppermint)—An Important Multipurpose Medicinal Plant. Am. J. Plant Sci. 2013, 4, 77–83. [Google Scholar] [CrossRef]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Guédon, D.J.; Pasquier, B.P. Analysis and Distribution of Flavonoid Glycosides and Rosmarinic Acid in 40 Mentha x piperita Clones. J. Agric. Food Chem. 1994, 42, 679–684. [Google Scholar] [CrossRef]
- Conference on Herbal Medicinal Products, European Medicines Agency. Assessment Report on Mentha x Piperita L., Folium and Aetheroleum. Available online: https://www.fitoterapia.net/archivos/201906/draft-assessment-report-mentha-x-piperita-l-folium-aetheroleum-revision-1_en.pdf?1 (accessed on 15 November 2019).
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; ISBN 928-718-505-0. [Google Scholar]
- European Medicines Agency. Menthae Piperitae Aetheroleum. Available online: https://www.ema.europa.eu/en/medicines/herbal/menthae-piperitae-aetheroleum (accessed on 15 December 2019).
- European Medicines Agency. Menthae Piperitae Folium. Available online: https://www.ema.europa.eu/en/medicines/herbal/menthae-piperitae-folium (accessed on 15 December 2019).
- United States Pharmacopeial Convention. The United States Pharmacopeia 42. National Formulary 37; United States Pharmacopeial Convention: North Bethesda, MD, USA, 2019; ISBN 193-642-485-1. [Google Scholar]
- Office for Registration of Medicinal Products, Medical Devices & Biocidal Products. Polish Pharmacopoeia XI; Polskie Towarzystwo Farmaceutyczne: Warszawa, Poland, 2017; ISBN 978-837-887-509-3. [Google Scholar]
- Areias, F.M.; Valentão, P.; Andrade, P.; Ferreres, F.; Seabra, R.M. Phenolic fingerprint of peppermint leaves. Food Chem. 2001, 73, 301–311. [Google Scholar] [CrossRef]
- Pereira, O.R.; Cardoso, S.M. Overview on Mentha and Thymus Polyphenols. Curr. Anal. Chem. 2013, 9, 382–396. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharm. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef]
- Fecka, I.; Mazur, A.; Cisowski, W. Kwas rozmarynowy, ważny składnik terapeutyczny niektórych surowców roślinnych. Postępy Fitoter. 2002, 1, 20–25. [Google Scholar]
- Plant Polyphenols. Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer Science+Business Media: New York, NY, USA, 1992; ISBN 978-1-4613-6540-2. [Google Scholar]
- Amoah, S.K.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic Acid-Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef]
- Geuenich, S.; Goffinet, C.; Venzke, S.; Nolkemper, S.; Baumann, I.; Plinkert, P.; Reichling, J.; Keppler, O.T. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology 2008, 5, 27. [Google Scholar] [CrossRef]
- Farnad, N.; Heidari, R.; Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint (Mentha piperita). J. Food Meas. Charact. 2014, 8, 113–121. [Google Scholar] [CrossRef]
- Najafian, S.; Moradi, M.; Sepehrimanesh, M. Polyphenolic contents and antioxidant activities of two medicinal plant species, Mentha piperita and Stevia rebaudiana, cultivated in Iran. Comp. Clin. Pathol. 2016, 25, 743–747. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Biskup, I.; Fecka, I. Total phenolic content and antioxidative properties of commercial tinctures obtained from some Lamiaceae plants. Nat. Prod. Commun. 2012, 7, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Riachi, L.G.; de Maria, C.A. Peppermint antioxidants revisited. Food Chem. 2015, 1, 72–81. [Google Scholar] [CrossRef]
- Nickavar, B.; Alinaghi, A.; Kamalinejad, M. Evaluation of the Antioxidant Properties of Five Mentha Species. Iran. J. Pharm. Res. 2008, 7, 203–209. [Google Scholar] [CrossRef]
- Umezu, T.; Sakata, A.; Ito, H. Ambulation-promoting effect of peppermint oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2001, 69, 383–390. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.M.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2011, 8, 322–328. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). J. Am. Med. Assoc. 2003, 289, 3095–3105. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Bakhtiarian, A.; Nikoui, V. The Antidepressant-Like Effect of the Ethanolic Extract of Mentha Piperita in Forced Swimming Test and Tail Suspension Test in Male Mice. J. Ilam Univ. 2018, 26, 34–42. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Inazu, M.; Egashira, T.; Matsumiya, T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002, 449, 261–267. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Bakhtiarian, A.; Nikoui, V. Involvement of the monoaminergic system in the antidepressant-like effect of the crude extract of Mentha piperita (Lamiaceae) in the forced swimming test in mice. Synergy 2017, 5, 21–28. [Google Scholar] [CrossRef]
- Nair, B. Final report on the safety assessment of Mentha Piperita (Peppermint) Oil, Mentha Piperita (Peppermint) Leaf Extract, Mentha Piperita (Peppermint) Leaf, and Mentha Piperita (Peppermint) Leaf Water. Int. J. Toxicol. 2001, 20, 61–73. [Google Scholar] [PubMed]
- Yang, S.T.; Wu, X.; Rui, W.; Guo, J.; Feng, Y.F. UPLC/Q-TOF-MS Analysis for Identification of Hydrophilic Phenolics and Lipophilic Diterpenoids from Radix Salviae Miltiorrhizae. Acta Chrom. 2015, 27, 711–728. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Calhelha, R.C.; Ćiric, A.; Soković, M.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C. Melissa officinalis L. decoctions as functional beverages: A bioactive approach and chemical characterization. Food Funct. 2015, 6, 2240–2248. [Google Scholar] [CrossRef]
- Ben Haj Yahia, I.; Zaouali, Y.; Ciavatta, M.L.; Ligresti, A.; Jaouadi, R.; Boussaid, M.; Cutignano, A. Polyphenolic Profiling, Quantitative Assessment and Biological Activities of Tunisian Native Mentha rotundifolia (L.) Huds. Molecules 2019, 24, 2351. [Google Scholar] [CrossRef]
- She, G.M.; Xu, C.; Liu, B.; Shi, R.B. Polyphenolic acids from mint (the aerial of Mentha haplocalyx Briq.) with DPPH radical scavenging activity. J. Food Sci. 2010, 75, 1750–3841. [Google Scholar] [CrossRef]
- Liu, A.H.; Guo, H.; Ye, M.; Lin, Y.H.; Sun, J.H.; Xu, M.; Guo, D.A. Detection, characterization and identification of phenolic acids in Danshen using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chrom. A 2007, 1, 170–182. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Janda, B.; Pecio, L.; Stochmal, A.; Oleszek, W.; Czubacka, A.; Przybys, M.; Doroszewska, T. Determination of Polyphenols in Mentha longifolia and M. piperita Field-Grown and In Vitro Plant Samples Using UPLC-TQ-MS. J. AOAC Int. 2011, 94, 43–50. [Google Scholar]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of Phenolic Composition in Lamiaceae Spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Kapp, K.; Hakala, E.; Orav, A.; Pohjala, L.; Vuorela, P.; Püssa, T.; Heikki, V.; Raal, A. Commercial peppermint (Mentha × piperita L.) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 2013, 53, 758–766. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of Water-Soluble Polyphenolic Compounds in Commercial Herbal Teas from Lamiaceae: Peppermint, Melissa, and Sage. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Haamed, E.S.; Kawashtya, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar] [CrossRef]
- Ferreres, F.; Vinholes, J.; Gil-Izquierdo, A.; Valentão, P.; Gonçalves, R.F.; Andrade, P.B. In vitrostudies ofa-glucosidase inhibitors and antiradical constituents of Glandora diffusa(Lag.) D.C. Thomas infusion. Food Chem. 2013, 136, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef]
- Dorman, H.J.; Koşar, M.; Başer, K.H.; Hiltunen, R. Phenolic profile and antioxidant evaluation of Mentha × piperita L. (peppermint) extracts. Nat. Prod. Commun. 2009, 4, 535–542. [Google Scholar] [CrossRef]
- Barchan, A.A.; Bakkali, M.; Arakrak, A.; Pagán, R.; Laglaoui, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 399–412. [Google Scholar]
- Dorman, H.J.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Caboni, P.; Saba, M.; Tocco, G.; Casu, L.; Murgia, A.; Maxia, A.; Menkissoglu-Spiroudi, U.; Ntalli, N. Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 9784–9788. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.R.; Zhang, H.M.; Ye, T.X.; Xiang, Z.J.; Yuan, Y.J.; Guo, Z.X.; Zhao, L.B. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 2008, 46, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Dahiya, P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha piperita. Int. Food Res. J. 2015, 22, 2440–2445. [Google Scholar] [CrossRef]
- The ABC Clinical Guide to Herbs; Blumenthal, M., Ed.; American Botanical Council: Austin, TX, USA, 2004; pp. 298–308. ISBN 158-890-157-2. [Google Scholar]
- Olennikov, D.; Tankhaeva, L.M. Quantitative determination of phenolic compounds in Mentha piperita leaves. Chem. Nat. Compd. 2010, 46, 22–27. [Google Scholar] [CrossRef]
- Fecka, I.; Kowalczyk, A.; Cisowski, W. Optimization of the separation of flavonoid glycosides and rosmarinic acid from Mentha piperita on HPTLC plates. J. Planar Chromatogr.–Mod. TLC 2004, 17, 22–25. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
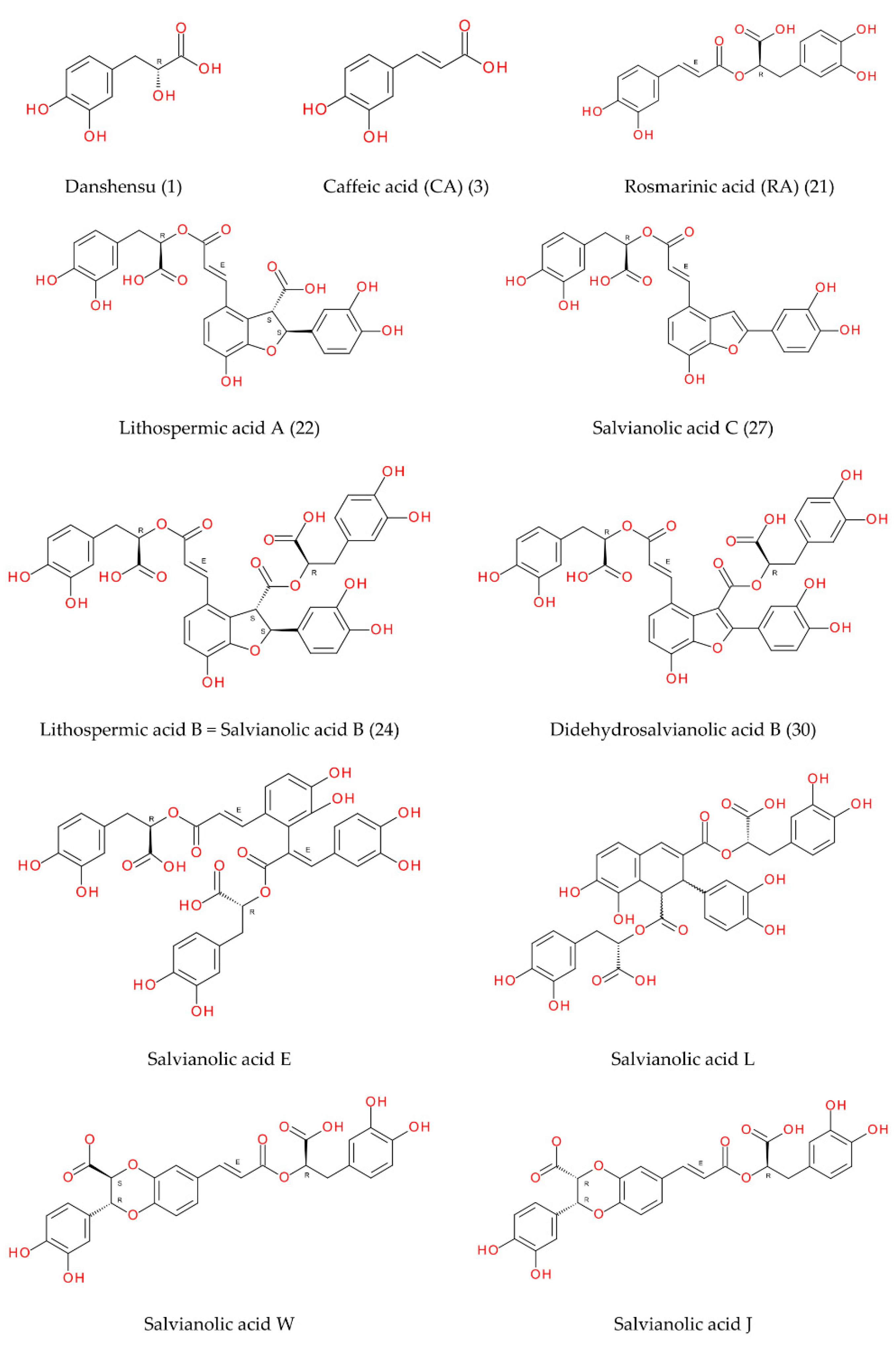
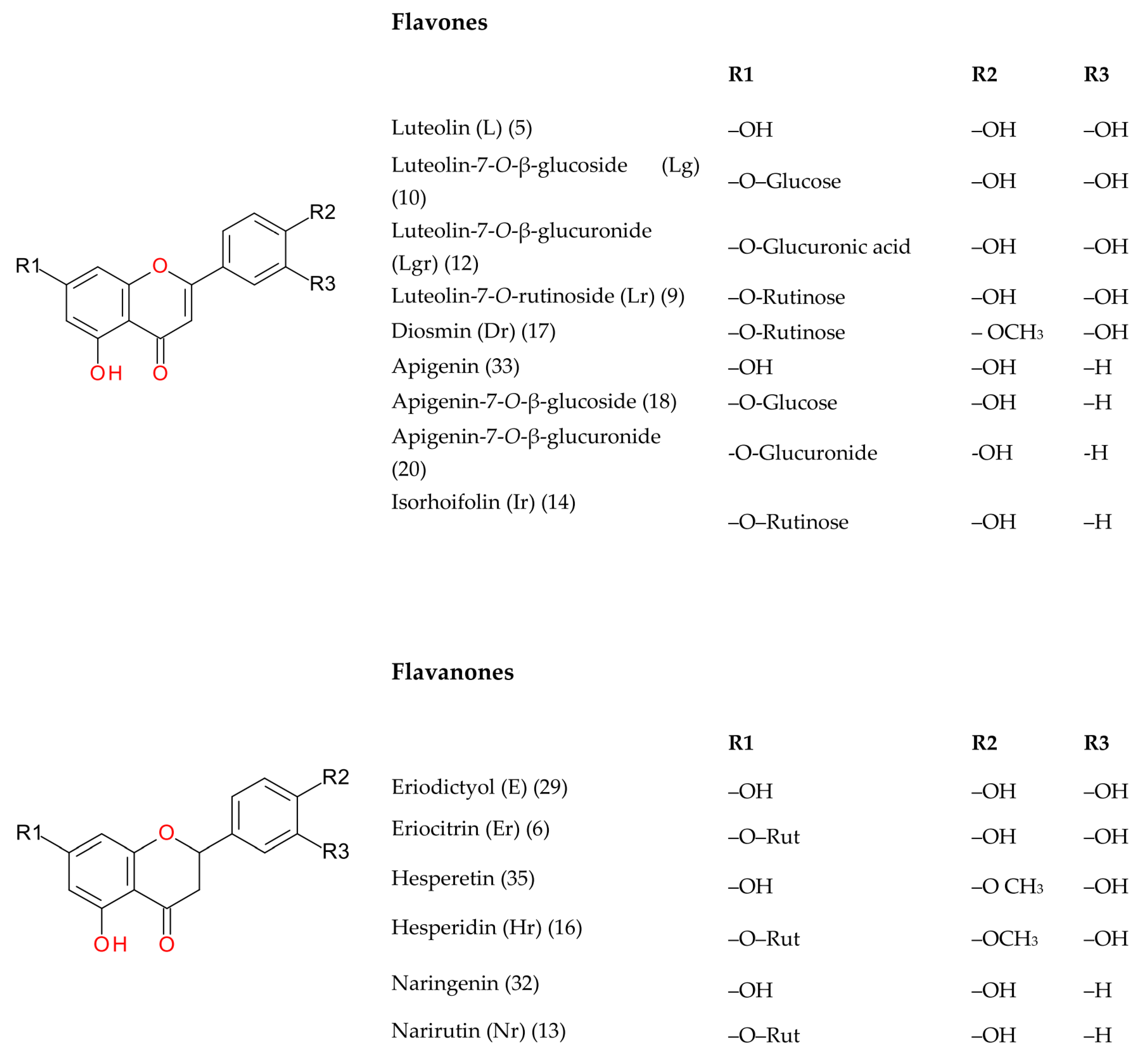
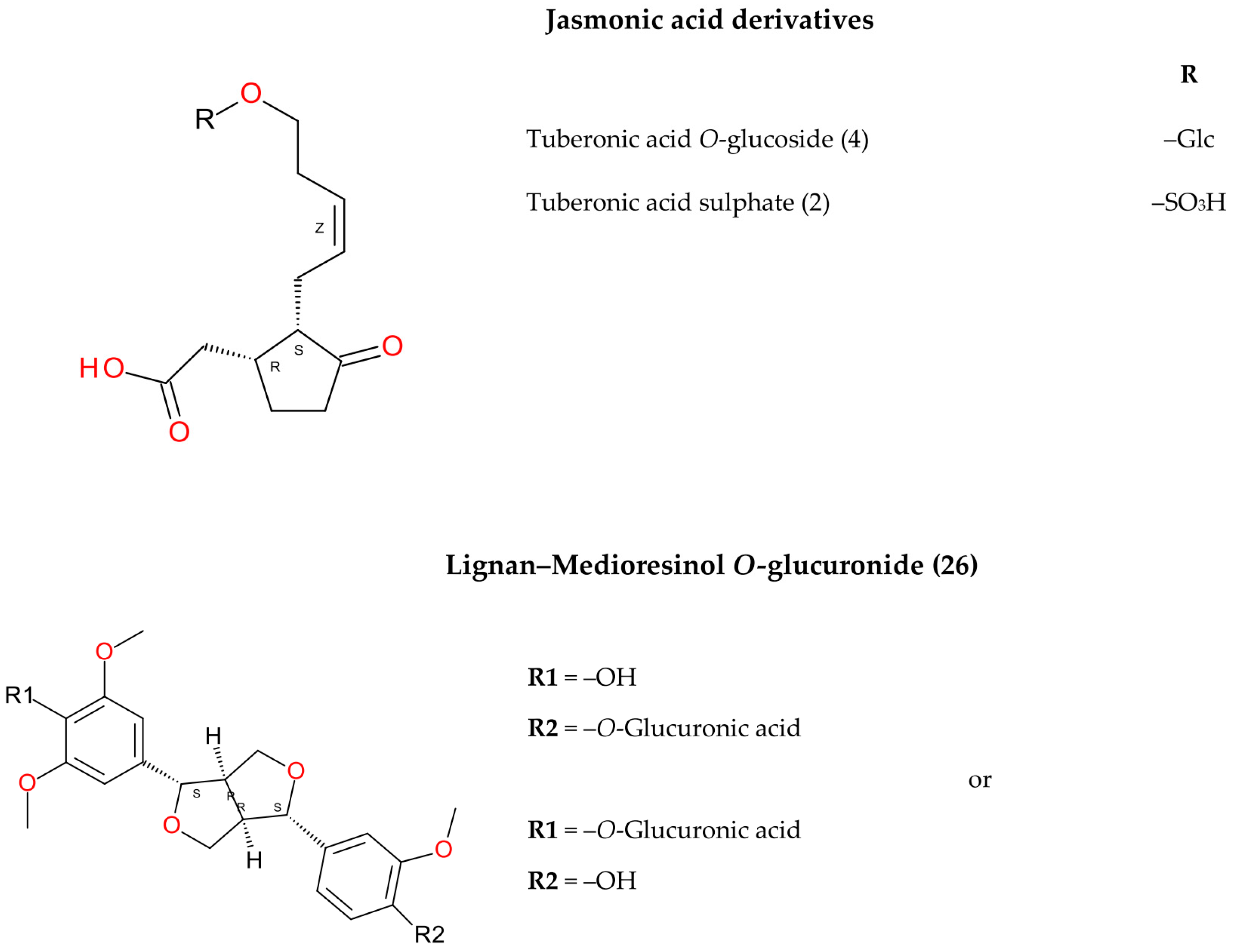
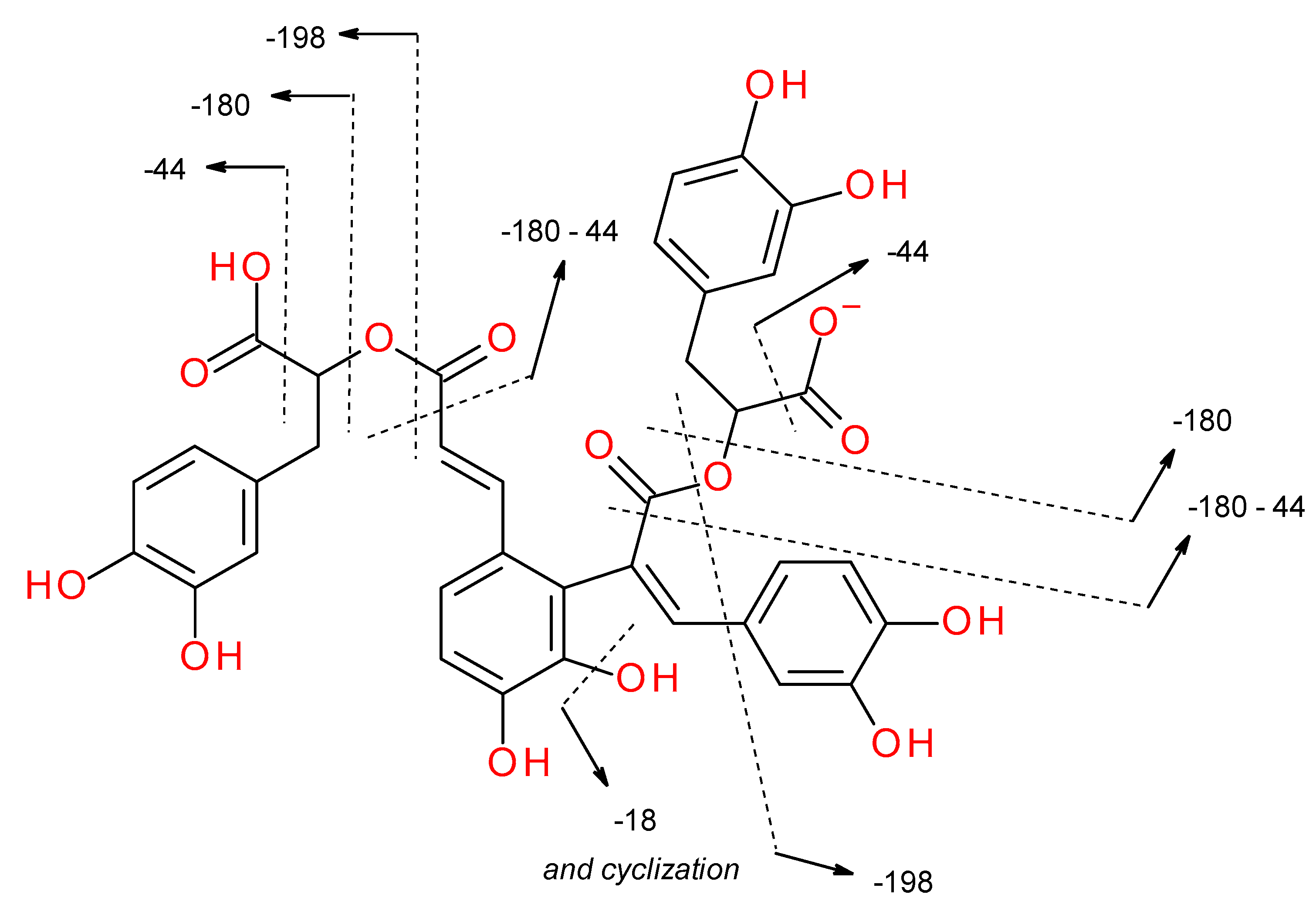
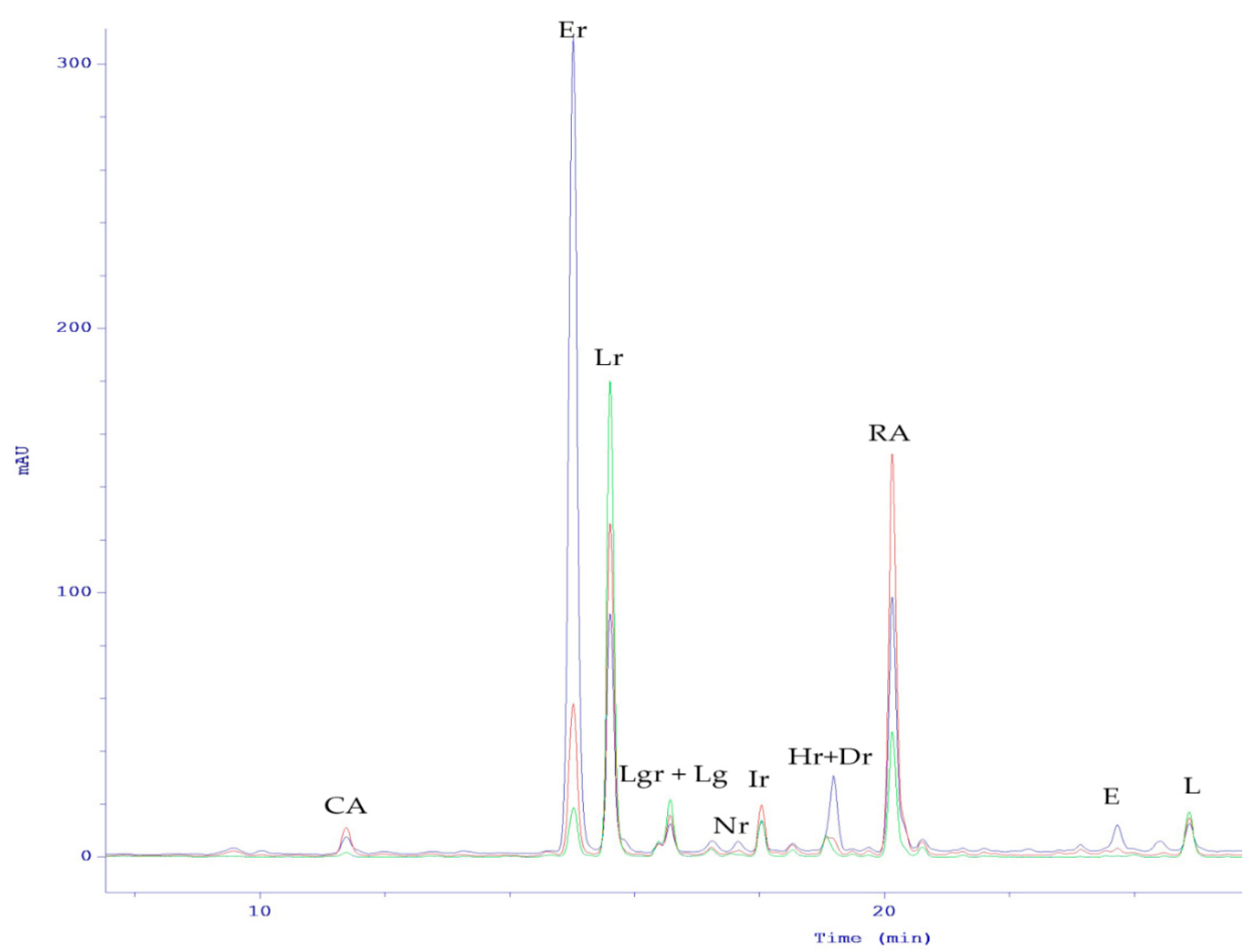
| Peak No. | tR (min) | [M-H]− (m/z, meas.) | Err. (ppm) | [M-H]− (formula) | MS/MS Fragments (m/z) | UVmax (nm) | Identification Proposal | Reference |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids—caffeetannins | ||||||||
| 1 | 1.70 | 197.0454 | 0.9 | C9H9O5 | 181, 137 | - | Salvianic acid A (danshensu) | [30] |
| 3 | 9.25 | 179.0353 | −1.7 | C9H7O4 | - | 290, 325 | Caffeic acid | s |
| 7 | 11.33 | 537.1048 | −1.8 | C27H21O12 | 295 | 285, 315, 343 | Salvianolic acid J/W or other isomer | [31,32,33] |
| 15 | 12.26 | 717.1442 | 2.6 | C36H29O16 | 475, 431, 365, 339, 321, 295 | 280, 320 | Salvianolic acid E/L or other isomer | [30,32] |
| 19 | 12.58 | 717.1440 | 3.0 | C36H29O16 | 339, 321, 295 | 285, 335 | Salvianolic acid E/L or other isomer | [30,32] |
| 21 | 12.90 | 359.0783 | −3.1 | C18H15O8 | 191, 161 | 290, 328 | Rosmarinic acid | s |
| 22 | 13.01 | 537.1040 | −0.3 | C27H21O12 | 493, 295, 185 | 254, 290, 310 | Lithospermic acid A | s |
| 24 | 13.35 | 717.1455 | 0.9 | C36H29O16 | 519, 339, 321, 295 | - | Lithospermic acid B (salvianolic acid B) | rm [34] |
| 25 | 13.53 | 717.1392 | 9.7 | C36H29O16 | - | - | Lithospermic acid B isomer | |
| 27 | 14.09 | 491.0988 | -0.8 | C26H19O10 | 311, 293 | 320 | Salvianolic acid C | [30,35] |
| 30 | 14.71 | 715.1293 | 1.6 | C36H27O16 | 337, 319, 293 | 280, 340 | Didehydrosalvianolic acid B | [36] |
| 34 | 16.15 | 387.1092 | −1.8 | C20H19O8 | 359 | - | Ethyl rosmarinate | [37] |
| Flavones | ||||||||
| 5 | 10.44 | 637.1053 | −1.0 | C27H25O18 | 285 | 265, 345 | Luteolin-O-diglucuronide | [38] |
| 9 | 11.42 | 593.1513 | −0.1 | C27H29O15 | 285 | 265, 350 | Luteolin-7-O-rutinoside | s |
| 10 | 11.71 | 447.0930 | 0.6 | C21H19O11 | 285 | 270, 345 | Luteolin-7-O-β-glucoside | s |
| 12 | 11.77 | 461.0716 | 2.0 | C21H17O12 | 285 | 270, 348 | Luteolin-7-O-β-glucuronide | s |
| 14 | 12.18 | 577.1558 | 0.8 | C27H29O14 | 269 | 268, 335 | Apigenin-7-O-rutinoside (isorhoifolin) | |
| 17 | 12.46 | 607.1668 | 0.0 | C28H30O15 | 299, 284 | 270, 340 | Diosmetin-7-O-rutinosidee (diosmin) | s |
| 18 | 12.57 | 431.0979 | 1.2 | C21H19O10 | - | 267, 335 | Apigenin-7-O-glucoside | s |
| 20 | 12.67 | 445.0762 | 3.2 | C21H17O11 | - | 267, 335 | Apigenin-7-O-glucuronide | |
| 23 | 13.08 | 461.0723 | 0.5 | C21H17O12 | - | 280, 320 | Tetrahydroxyflavone-O-glucuronide (e.g., scutellarin) | |
| 28 | 14.12 | 489.1037 | 0.2 | C23H21O12 | - | - | Luteolin-7-O-glucuronide ethyl ester | |
| 31 | 14.80 | 285.0403 | 0.5 | C15H9O6 | - | - | Luteolin | s |
| 33 | 16.10 | 269.0456 | −0.2 | C15H9O5 | - | - | Apigenin | s |
| Flavanones | ||||||||
| 6 | 11.13 | 595.1672 | −0.6 | C27H31O15 | 287 | 285 | Eriodictyol-7-O-rutinoside (eriocitrin) | s |
| 8 | 11.42 | 449.1088 | 0.2 | C21H21O11 | 287 | 285 | Eriodictyol-7-O-glucoside | [39] |
| 11 | 11.73 | 463.0862 | 4.3 | C21H19O12 | 287 | Eriodictyol-7-O-glucuronide | [38] | |
| 13 | 11.97 | 579.1709 | 1.7 | C27H31O14 | 271 | 280 | Naringenin-7-O-rutinoside (narirutin) | s |
| 16 | 12.41 | 609.1819 | 1.0 | C28H33O15 | 301, 286 | 285 | Hesperetin-7-O-rutinoside (hesperidin) | s |
| 29 | 14.41 | 287.0563 | −0.5 | C15H11O6 | - | - | Eriodictyol | s |
| 32 | 15.91 | 271.0618 | −2.1 | C15H11O5 | - | - | Naringenin | s |
| 35 | 16.35 | 301.0725 | −2.3 | C16H13O6 | - | - | Hesperetin | s |
| Jasmonic acid derivatives | ||||||||
| 2 | 9.14 | 305.0700 | 0.1 | C12H17O7S | - | 285, 320 | Tuberonic acid sulphate | [40] |
| 4 | 9.39 | 387.1661 | −0.4 | C18H27O9 | - | 280, 315 | Tuberonic acid O-glucoside | [40] |
| Lignans | ||||||||
| 26 | 13.99 | 563.2124 | 1.8 | C28H35O12 | 387 | 280, 320 | Medioresinol O-glucuronide | [40] |
| Pseudo-Molecular Ion: | Fragment Ion | Interpretation | Reference | |
|---|---|---|---|---|
| 717: | 519 [M-198 − H]− | [M-198 − H]− [M-180-18 − H]− | [34] | |
| 475 [M-242 − H]− | [M-198-44 − H]− [M-180-44-18 − H]− | [34,35] | ||
| 431 [M-286 − H]− | [M-198-44-44 − H]− [M-180-44-44-18 − H]− | |||
| 339 [M-378 − H]− | [M-198-180 − H]− [M-180-180-18 − H]− | [34] | ||
| 321 [M-396 − H]− | [M-198-198 − H]− [M-198-180-18 − H]− [M-180-180-18-18 − H]− | |||
| 295 [M-422 − H]− | [M-198-180-44 − H]− [M-180-180-44-18 − H]− | [34] | ||
| 715: | 337 [M-378 − H]− | [M-198-180 − H]− [M-180-180-18 − H]− | [36] | |
| 319 [M-396 − H]− | [M-198-198 − H]− [M-198-180-18 − H]− [M-180-180-18-18 − H]− | |||
| 293 [M-422 − H]− | [M-198-180-44 − H]− [M-180-180-44-18 − H]− | |||
| 537: | 493 [M-44 − H]− | [M-44 − H]− | [34] | |
| 295 [M-242 − H]− | [M-198-44 − H]− [M-180-44-18 − H]− | |||
| 491: | 311 [M-180 − H]− | [M-180 − H]− | [34] | |
| 293 [M-198 − H]− | [M-198 − H]− [M-180-18 − H]− | |||
| Neutral loss of: | ||||
| 198 Da → | salvianic acid A = danshensu (C9H10O5) | 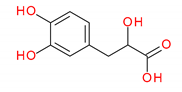 | ||
| 180 Da → | dehydroxysalvianic acid A residue = dehydroxydanshensu residue (C9H8O4) ↓ caffeic acid (C9H8O4) | 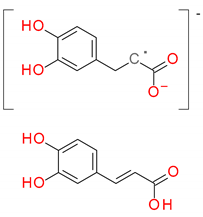 | ||
| 44 Da → | CO2 | |||
| 18 Da → | H2O | |||
| Standard | Calibration Equation | r2 | Linear Range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) | Working Wavelength |
|---|---|---|---|---|---|---|
| Flavones | ||||||
| Luteolin | y = 1224.6x − 0.1205 | 0.9990 | 10–300 | 0.245 | 0.817 | 360 |
| Luteolin-7-O-β-glucuronide | y = 615.69x − 0.9761 | 0.9996 | 10–250 | 0.487 | 1.624 | 360 |
| Luteolin-7-O-β-rutinoside | y = 457.74x − 0.9438 | 0.9998 | 10–250 | 0.655 | 2.185 | 360 |
| Isorhoifolin | y = 875.93x − 0.2331 | 0.9996 | 10–250 | 0.343 | 1.142 | 360 |
| Flavanones | ||||||
| Narirutin | y = 294.76x + 0.675 | 0.9998 | 10–250 | 1.018 | 3.393 | 280 |
| Eriodictyol | y = 330.28x + 0.2099 | 0.9998 | 10–250 | 0.908 | 3.028 | 280 |
| Eriocitrin | y = 239.18x + 0.8709 | 0.9992 | 10–300 | 1.254 | 4.189 | 280 |
| Hesperidin | y = 480.26x + 0.2943 | 0.9995 | 10–300 | 0.625 | 2.082 | 280 |
| Phenolic acids—Caffeetannins | ||||||
| Caffeic acid | y = 1824.74x − 4.64 | 0.9993 | 10–300 | 0.164 | 0.548 | 320 |
| Rosmarinic acid | y = 581.91x + 1.99 | 0.9991 | 10–300 | 0.515 | 1.718 | 320 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2020, 25, 69. https://doi.org/10.3390/molecules25010069
Bodalska A, Kowalczyk A, Włodarczyk M, Fecka I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules. 2020; 25(1):69. https://doi.org/10.3390/molecules25010069
Chicago/Turabian StyleBodalska, Agnieszka, Adam Kowalczyk, Maciej Włodarczyk, and Izabela Fecka. 2020. "Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture" Molecules 25, no. 1: 69. https://doi.org/10.3390/molecules25010069
APA StyleBodalska, A., Kowalczyk, A., Włodarczyk, M., & Fecka, I. (2020). Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules, 25(1), 69. https://doi.org/10.3390/molecules25010069





