Toxicity and Antitumor Activity of a Thiophene–Acridine Hybrid
Abstract
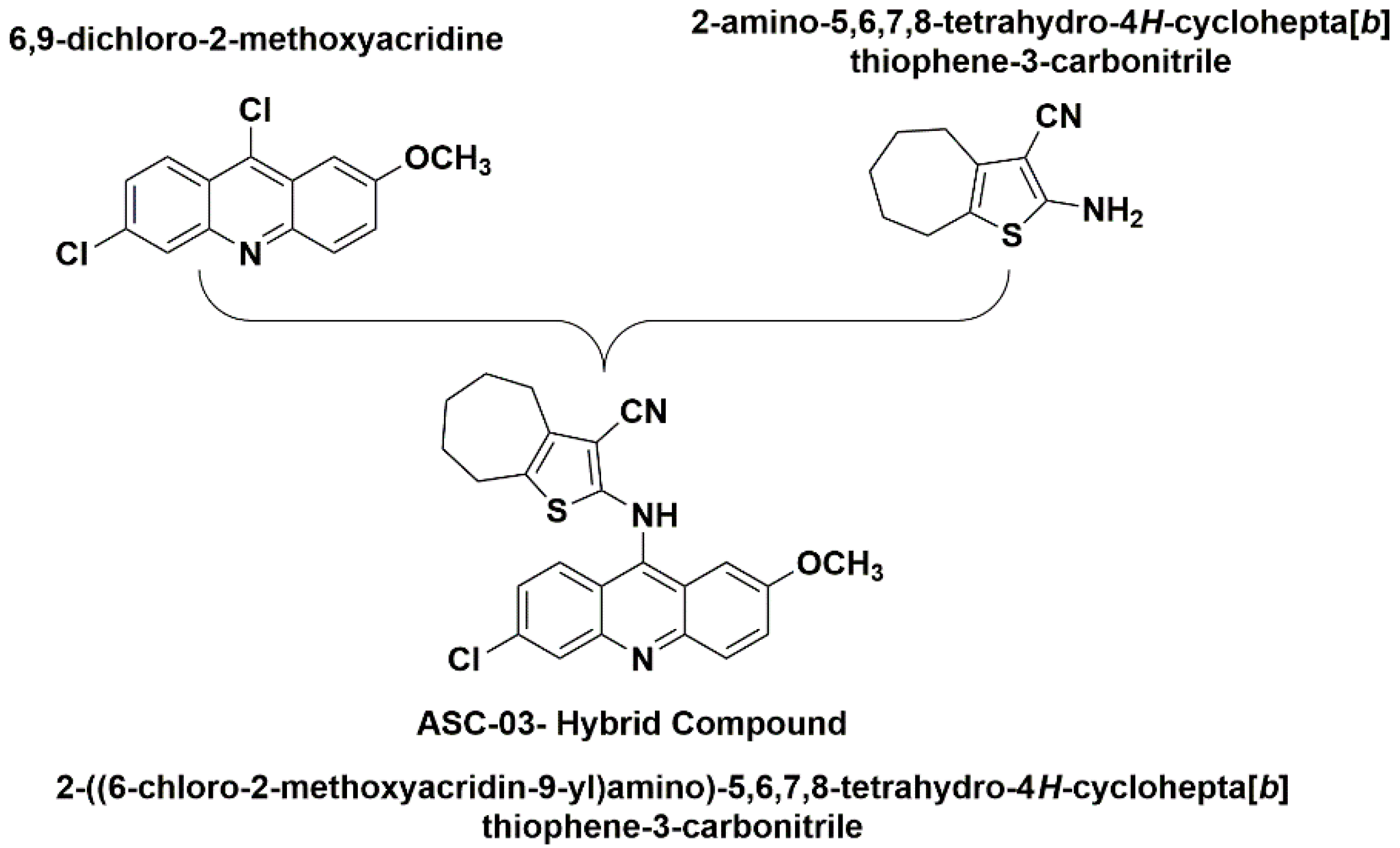
1. Introduction
2. Results
2.1. Cytotoxicity Assay
2.2. Toxicity in Zebrafish Embryos
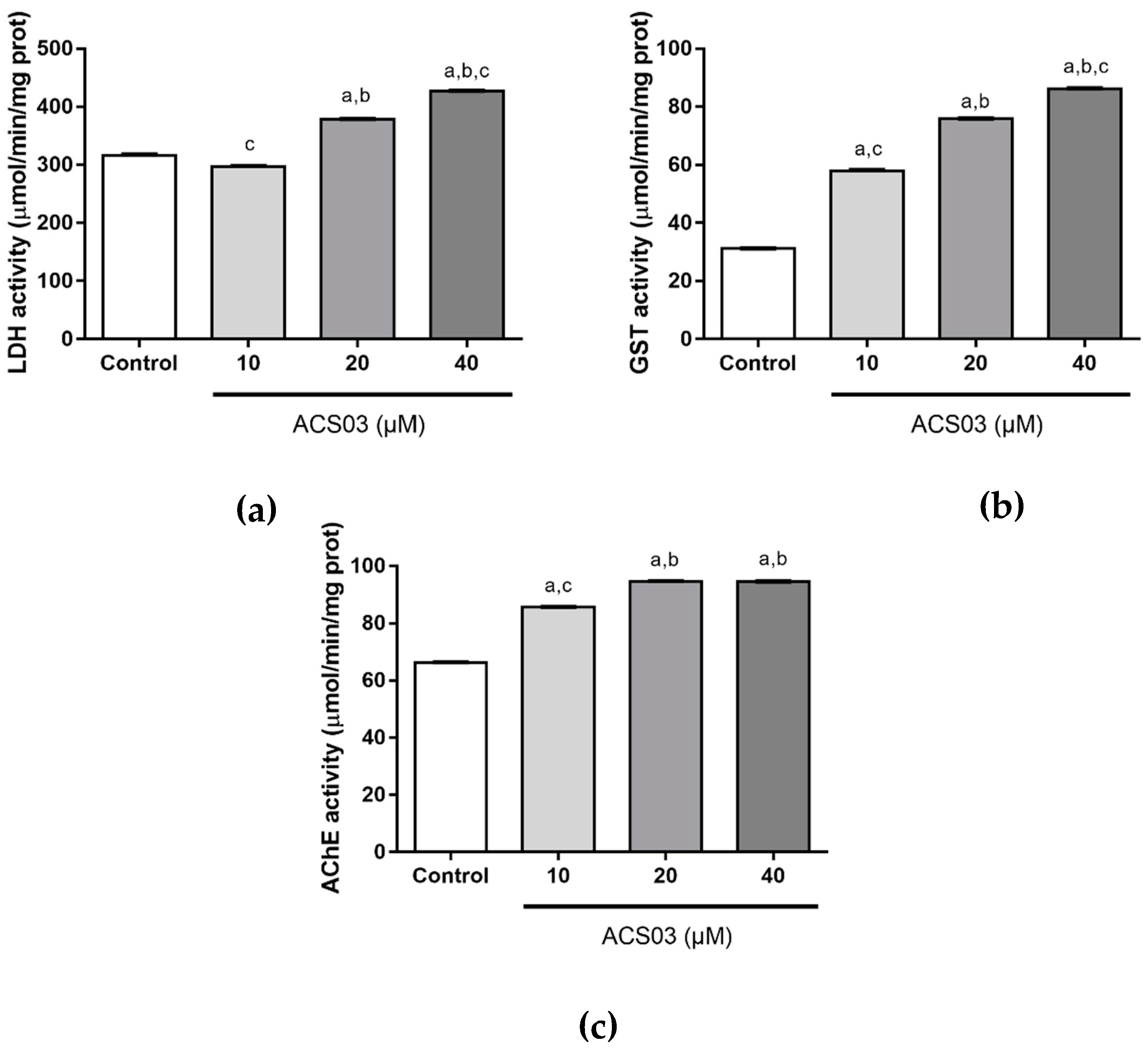
2.3. Biochemical Biomarkers in Zebrafish Embryos
2.4. Acute Preclinical Toxicity
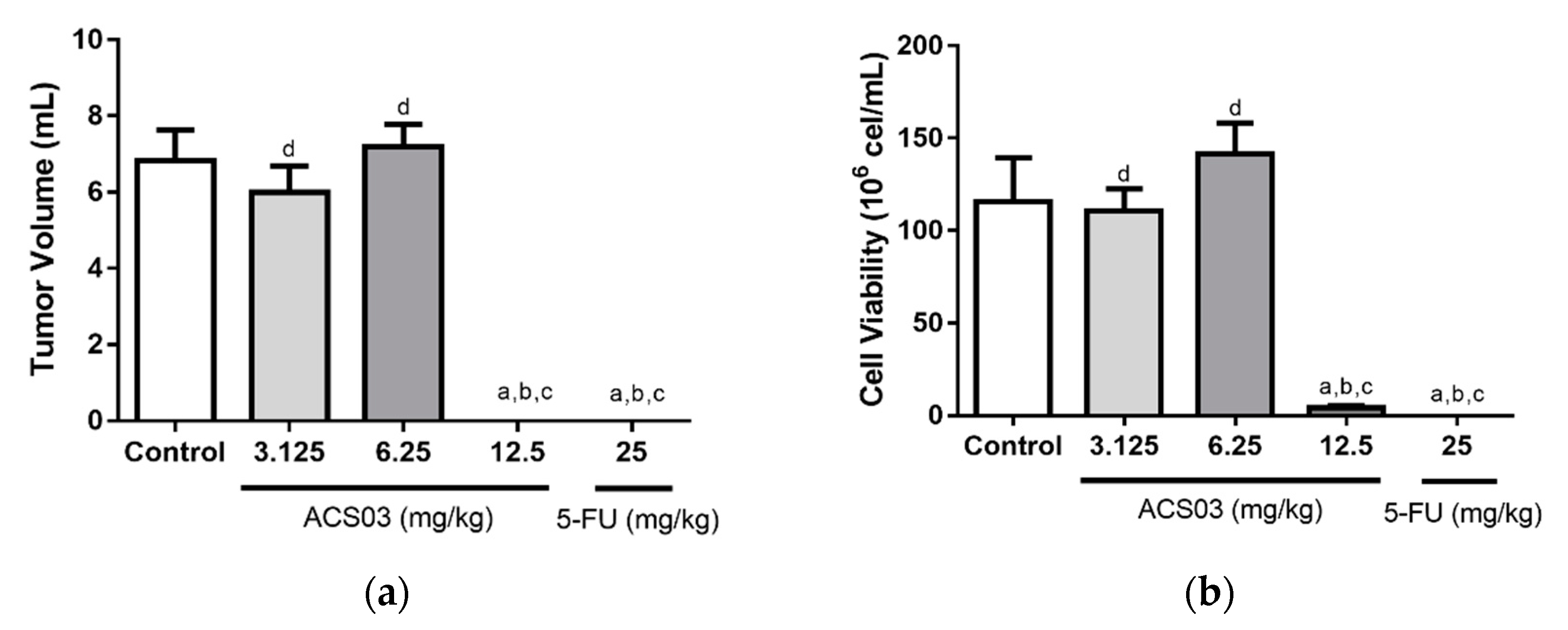
2.5. In Vivo Antitumor Activity
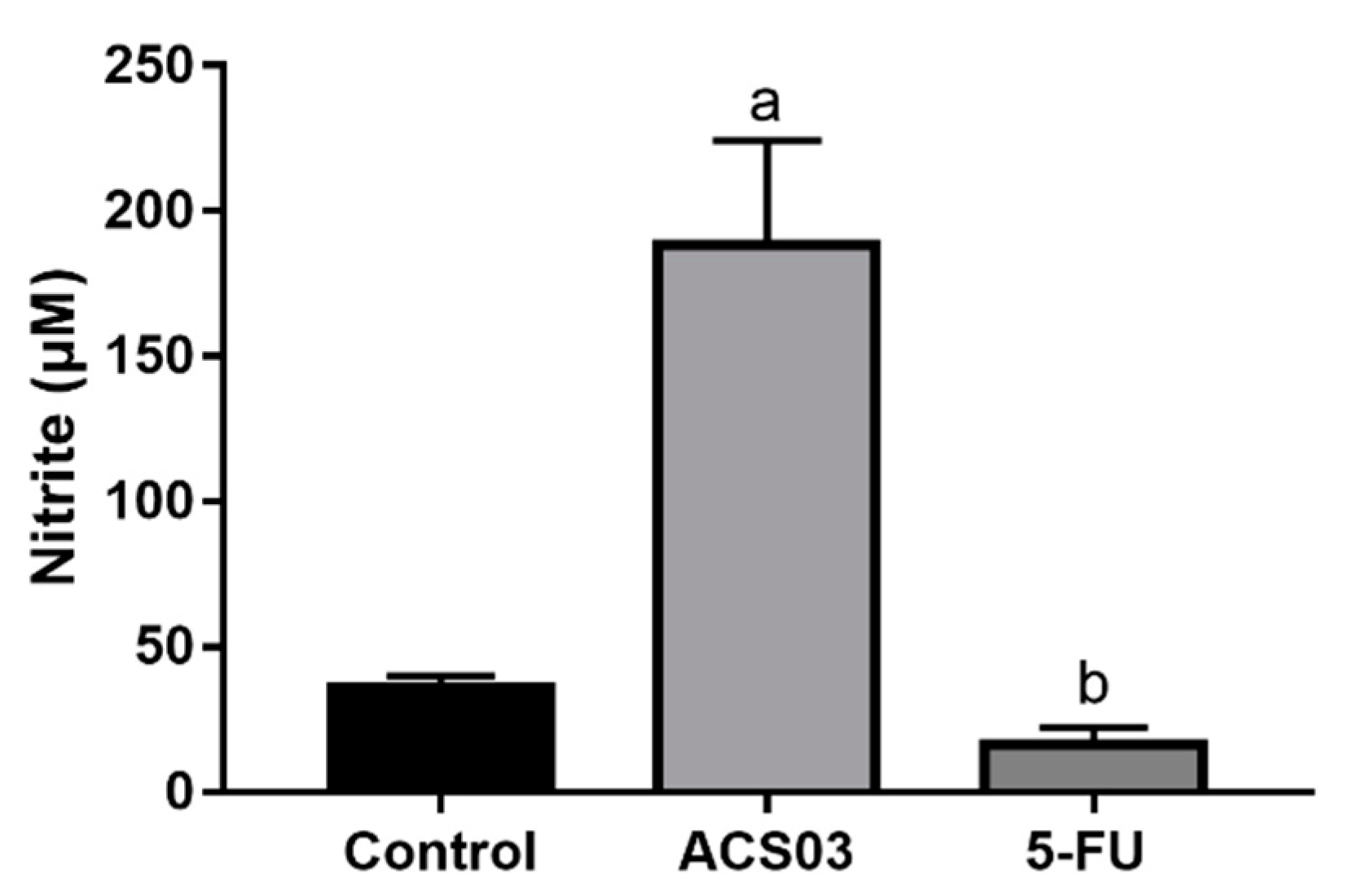
2.6. Quantification of Nitrite Levels
3. Discussion
4. Materials and Methods
4.1. Treatment
4.2. Cell Lines
4.3. Peripheral Blood Mononuclear Cells (PBMC)
4.4. Zebrafish Embryos
4.5. Animals
4.6. In Vitro Assay
Cell Viability Assay
4.7. In Vivo Assays
4.7.1. Acute Toxicity Test Using Zebrafish Embryos
4.7.2. Enzyme Testing Using Zebrafish Larvae
4.7.3. Assessment of Acute Preclinical Toxicity in Mice
4.7.4. Evaluation of In Vivo Antitumor Activity in the Ehrlich Ascites Carcinoma Model
4.7.5. Quantification of Nitrite Levels in the Ehrlich Ascites Carcinoma Model
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Cancer Today. Cancer Fact Sheets. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 4 November 2019).
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Li, B.; Gao, C.; Jiang, Y. Acridine and its derivatives: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 647–664. [Google Scholar] [CrossRef]
- Belmont, P.; Bosson, J.; Godet, T.; Tiano, M. Acridine and acridone derivatives, anticancer properties and synthetic methods: Where are we now? Anti-Cancer Agents Med. Chem. 2007, 7, 139–169. [Google Scholar] [CrossRef]
- Crenshaw, J.M.; Graves, D.E.; Denny, W.A. Interactions of acridine antitumor agents with DNA: Binding energies and groove preferences. Biochemistry 1995, 34, 13682–13687. [Google Scholar] [CrossRef]
- Kao-Shan, C.S.; Micetich, K.; Zwelling, L.A.; Whang-Peng, J. Cytogenetic effects of amsacrine on human lymphocytes in vivo and in vitro. Cancer Treat. Rep. 1984, 68, 989–997. [Google Scholar]
- Gramec, D.; Peterlin Mašič, L.; Sollner Dolenc, M. Bioactivation potential of thiophene-containing drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358. [Google Scholar] [CrossRef] [PubMed]
- Joshi, E.M.; Heasley, B.H.; Chordia, M.D.; Macdonald, T.L. In vitro metabolism of 2-acetylbenzothiophene: Relevance to zileuton hepatotoxicity. Chem. Res. Toxicol. 2004, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Lopez-Cara, C.; Salvador, M.K.; Preti, D.; Tabrizi, M.A.; Balzarini, J.; Nussbaumer, P.; Bassetto, M.; Brancale, A.; et al. Design, synthesis and biological evaluation of 3,5-disubstituted 2-amino thiophene derivatives as a novel class of antitumor agents. Bioorg. Med. Chem. 2014, 22, 5097–5109. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Bashandy, M.S.; Alsaid, M.S. Novel thiophene derivatives with sulfonamide, isoxazole, benzothiazole, quinoline and anthracene moieties as potential anticancer agents. Acta Pharmaceutica 2014, 64, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.A.; Pereira, M.C.; De Oliveira, T.B.; Mendonça, F.J.B.; Do Carmo Alves De Lima, M.; Da Rocha Pitta, M.G.; Da Rocha Pitta, I.; De Melo Rêgo, M.J.B.; Da Rocha Pitta, M.G. Anticancer properties of thiophene derivatives in breast cancer MCF-7 cells. Anti-Cancer Drugs 2017, 29, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cui, Y.; Zhao, L.; Zhu, T.; Lee, R.J.; Liao, W.; Sun, F.; Li, Y.; Teng, L. Thiophene derivatives as new anticancer agents and their therapeutic delivery using folate receptor-targeting nanocarriers. ACS Omega 2019, 4, 8874–8880. [Google Scholar] [CrossRef] [PubMed]
- Gensicka-Kowalewska, M.; Cholewiński, G.; Dzierzbicka, K. Recent developments in the synthesis and biological activity of acridine/acridone analogues. RSC Adv. 2017, 7, 15776–15804. [Google Scholar] [CrossRef]
- De Lima Serafim, V.; Félix, M.B.; Frade Silva, D.K.; da Rodrigues, K.A.F.; Andrade, P.N.; de Almeida, S.M.V.; de Albuquerque dos Santos, S.; de Oliveira, J.F.; do Carmo Alves de Lima, M.; Mendonça-Junior, F.J.B.; et al. New thiophene–acridine compounds: Synthesis, antileishmanial activity, DNA binding, chemometric, and molecular docking studies. Chem. Biol. Drug Des. 2018, 91, 1141–1155. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Q.; Zhou, Y.; Wang, J.; Liu, Q.; Xu, H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017, 11, 87–104. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Chen, C.C.; Neugut, A.I.; Rotterdam, H. Risk factors for adenocarcinomas and malignant carcinoids of the small intestine: Preliminary findings. Cancer Epidemiol. Biomark. Prev. 1994, 3, 205–209. [Google Scholar]
- Logan, I.E.; Miranda, C.L.; Lowry, M.B.; Maier, C.S.; Stevens, J.F.; Gombart, A.F. Antiproliferative and cytotoxic activity of xanthohumol and its non-estrogenic derivatives in colon and hepatocellular carcinoma cell lines. Int. J. Mol. Sci. 2019, 20, 1203. [Google Scholar] [CrossRef]
- Fu, W.; Li, X.; Lu, X.; Zhang, L.; Li, R.; Zhang, N.; Liu, S.; Yang, X.; Wang, Y.; Zhao, Y.; et al. A novel acridine derivative, LS-1-10 inhibits autophagic degradation and triggers apoptosis in colon cancer cells. Cell Death Dis. 2017, 8, 3086–3099. [Google Scholar] [CrossRef]
- Sánchez, I.; Reches, R.; Caignard, D.H.; Renard, P.; Pujol, M.D. Synthesis and biological evaluation of modified acridines: The effect of N- and O- substituent in the nitrogenated ring on antitumor activity. Eur. J. Med. Chem. 2006, 41, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.W.A.; Silva, T.G.; Da Rocha Pitta, M.G.; Bezerra, D.P.; Costa-Lotufo, L.V.; De Moraes, M.O.; Pessoa, C.; De Moura, M.A.F.B.; De Abreu, F.C.; De Lima, M.D.C.A.; et al. Synthesis and cytotoxic activity of new acridine-thiazolidine derivatives. Bioorganic Med. Chem. 2012, 20, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Véras of Aguiar, A.C.; Of Moura, R.O.; Bezerra Mendonça, J.F.; de Oliveira Rocha, H.A.; Gomes Câmara, R.B.; dos Santos Carvalho Schiavon, M. Evaluation of the antiproliferative activity of 2-amino thiophene derivatives against human cancer cells lines. Biomed. Pharmacother. 2016, 84, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar]
- Ducharme, N.A.; Reif, D.M.; Gustafsson, J.A.; Bondesson, M. Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod. Toxicol. 2015, 55, 3–10. [Google Scholar] [CrossRef]
- Sipes, N.S.; Padilla, S.; Knudsen, T.B. Zebrafish—As an integrative model for twenty-first century toxicity testing. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 256–267. [Google Scholar] [CrossRef]
- Huiting, L.N.; Laroche, F.; Feng, H. The zebrafish as a tool to cancer drug discovery. Austin J. Pharmacol. Ther. 2015, 3, 1069–1087. [Google Scholar]
- Hallare, A.V.; Köhler, H.R.; Triebskorn, R. Developmental toxicity and stress protein responses in zebrafish embryos after exposure to diclofenac and its solvent, DMSO. Chemosphere 2004, 56, 659–666. [Google Scholar] [CrossRef]
- Vieira, L.R.; Sousa, A.; Frasco, M.F.; Lima, I.; Morgado, F.; Guilhermino, L. Acute effects of Benzo[a]pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Sci. Total Environ. 2008, 395, 87–100. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D structure to function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kais, B.; Stengel, D.; Batel, A.; Braunbeck, T. Acetylcholinesterase in zebrafish embryos as a tool to identify neurotoxic effects in sediments. Environ. Sci. Pollut. Res. 2015, 22, 16329–16339. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Aguilar, B.; Vidal, C.J.; Palomec, G.; García-Dolores, F.; Gutiérrez-Ruiz, M.C.; Bucio, L.; Gómez-Olivares, J.L.; Gómez-Quiroz, L.E. Acetylcholinesterase is associated with a decrease in cell proliferation of hepatocellular carcinoma cells. Biochimica et Biophysica Acta Mol. Basis Dis. 2015, 1852, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, Z.; Xiao, J.; Yang, Y.; Huang, W.; Zhou, Z.; Shen, J.; Zhu, Y.; Liu, X.-Y.; Chu, L. Acetylcholinesterase overexpression mediated by oncolytic adenovirus exhibited potent anti-tumor effect. BMC Cancer 2014, 14, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, L.; Zhang, X.; Zhang, B.; Wu, J. Synaptic acetylcholinesterase targeted by microRNA-212 functions as a tumor suppressor in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2013, 45, 2530–2540. [Google Scholar]
- Xie, Y.; Zhou, X.; Chen, L.; Zhang, Z.; Wang, C.; Gu, X.; Wang, T.; Peng, X.; Yang, G. Cloning and characterization of a novel sigma-like glutathione S-transferase from the giant panda parasitic nematode, Baylisascaris schroederi. Parasites Vectors 2015, 8, 44–57. [Google Scholar] [CrossRef]
- Sturchio, E.; Ficociello, B.; Minoia, C.; Biamonti, G.; Signorini, S.; Moccaldi, A.; Imbriani, M. Gene expression and environmental exposure to xenobiotics: Overview and applications. Giornale Italiano Medicina Lavoro Ergonomia 2008, 30, 101–114. [Google Scholar]
- Amelo, W.; Nagpal, P.; Makonnen, E. Antiplasmodial activity of solvent fractions of methanolic root extract of Dodonaea angustifolia in Plasmodium berghei infected mice. BMC Complement. Altern. Med. 2014, 14, 462–469. [Google Scholar] [CrossRef]
- Egawa, J.; Ishioka, K.; Ogata, T. Effect of irradiation and chemotherapeutic agents on the capillaries of Ehrlich ascites carcinoma of mice. Acta Radiol. Oncol. Radiat. Phys. Biol. 1979, 18, 535–543. [Google Scholar] [CrossRef]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich ascites carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 2018, 8, 5599–5610. [Google Scholar] [CrossRef]
- Mangueira, V.M.; Batista, T.M.; Brito, M.T.; de Sousa, T.K.G.; da Cruz, R.M.D.; de Abrantes, R.A.; Veras, R.C.; de Medeiros, I.A.; de Paula Medeiros, K.K.; da Costa Pereira, A.L.; et al. A new acridine derivative induces cell cycle arrest and antiangiogenic effect on Ehrlich ascites carcinoma model. Biomed. Pharmacother. 2017, 90, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, K.; Jagadish, S.; Swaroop, T.; Mohan, C.; Ashwini, N.; Harsha, K.; Zameer, F.; Girish, K.; Rangappa, K. Anti-cancer activity of 2,4-disubstituted thiophene derivatives: Dual inhibitors of lipoxygenase and cyclooxygenase. Med. Chem. 2015, 11, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Korde Choudhari, S.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S. Nitric oxide for cancer therapy. Futur. Sci. OA 2015, 1, 44–53. [Google Scholar] [CrossRef]
- Melo, P.S.; De Medeiros Cavalcante, H.M.; Barbosa-Filho, J.M.; De Fátima Formiga Melo Diniz, M.; De Medeiros, I.A.; Haun, M. Warifteine and milonine, alkaloids isolated from Cissampelos sympodialis Eichl: Cytotoxicity on rat hepatocyte culture and in V79 cells. Toxicol. Lett. 2003, 142, 143–151. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Guidelines for the Testing of Chemicals: Fish Embryo Acute Toxicity (FET); Test No. 236; OECD: Paris, France, 2013; pp. 1–22. [Google Scholar]
- Domingues, I.; Oliveira, R.; Lourenço, J.; Grisolia, C.K.; Mendo, S.; Soares, A.M.V.M. Biomarkers as a tool to assess effects of chromium (VI): Comparison of responses in zebrafish early life stages and adults. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 338–345. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Guidelines for the Testing of Chemicals: Acute Oral Toxicity; Test No. 423; OECD: Paris, France, 2001; pp. 1–14. [Google Scholar]
- Dolai, N.; Karmakar, I.; Suresh Kumar, R.B.; Kar, B.; Bala, A.; Haldar, P.K. Evaluation of antitumor activity and in vivo antioxidant status of Anthocephalus cadamba on Ehrlich ascites carcinoma treated mice. J. Ethnopharmacol. 2012, 142, 865–870. [Google Scholar] [CrossRef]
- Santos, J.; Brito, M.; Ferreira, R.; Moura, A.; Sousa, T.; Batista, T.; Mangueira, V.; Leite, F.; Cruz, R.; Vieira, G.; et al. Th1-biased immunomodulation and in vivo antitumor effect of a novel piperine analogue. Int. J. Mol. Sci. 2018, 19, 2594. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971; p. 1432. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Cell Lines * | ACS03 IC50 (µM) |
|---|---|
| HCT-116 | 23.11 ± 1.03 |
| HeLa | >50 |
| MCF-7 | >50 |
| K562 | >50 |
| HL-60 | >50 |
| HaCat | 62.18 ± 1.15 a |
| PBMC | 115.2 ± 5.82 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisboa, T.; Silva, D.; Duarte, S.; Ferreira, R.; Andrade, C.; Lopes, A.L.; Ribeiro, J.; Farias, D.; Moura, R.; Reis, M.; et al. Toxicity and Antitumor Activity of a Thiophene–Acridine Hybrid. Molecules 2020, 25, 64. https://doi.org/10.3390/molecules25010064
Lisboa T, Silva D, Duarte S, Ferreira R, Andrade C, Lopes AL, Ribeiro J, Farias D, Moura R, Reis M, et al. Toxicity and Antitumor Activity of a Thiophene–Acridine Hybrid. Molecules. 2020; 25(1):64. https://doi.org/10.3390/molecules25010064
Chicago/Turabian StyleLisboa, Thaís, Daiana Silva, Sâmia Duarte, Rafael Ferreira, Camyla Andrade, Ana Luiza Lopes, Juliana Ribeiro, Davi Farias, Ricardo Moura, Malu Reis, and et al. 2020. "Toxicity and Antitumor Activity of a Thiophene–Acridine Hybrid" Molecules 25, no. 1: 64. https://doi.org/10.3390/molecules25010064
APA StyleLisboa, T., Silva, D., Duarte, S., Ferreira, R., Andrade, C., Lopes, A. L., Ribeiro, J., Farias, D., Moura, R., Reis, M., Medeiros, K., Magalhães, H., & Sobral, M. (2020). Toxicity and Antitumor Activity of a Thiophene–Acridine Hybrid. Molecules, 25(1), 64. https://doi.org/10.3390/molecules25010064






