Enniatin A1, A Natural Compound with Bactericidal Activity against Mycobacterium tuberculosis In Vitro
Abstract
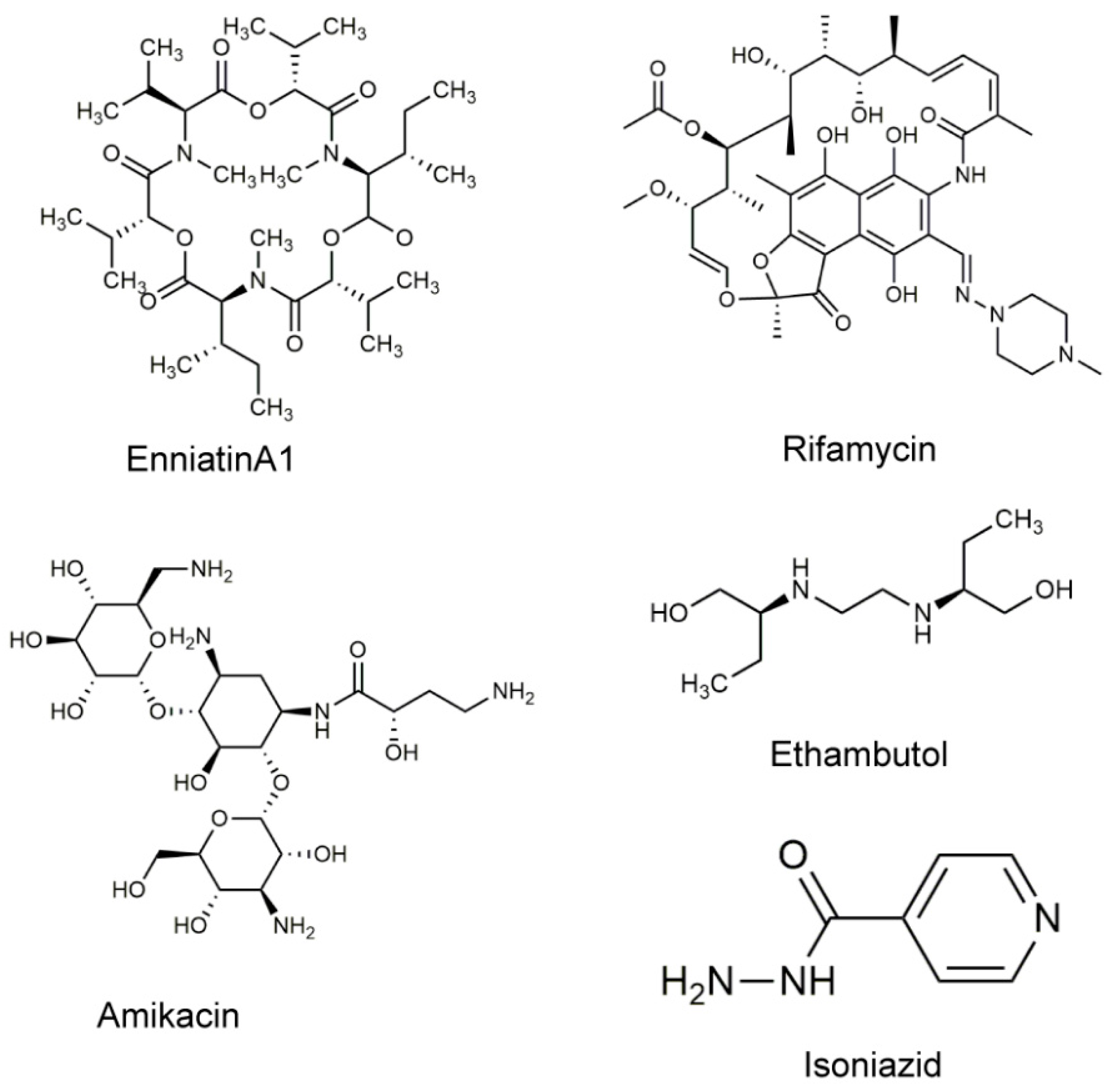
1. Introduction
2. Results
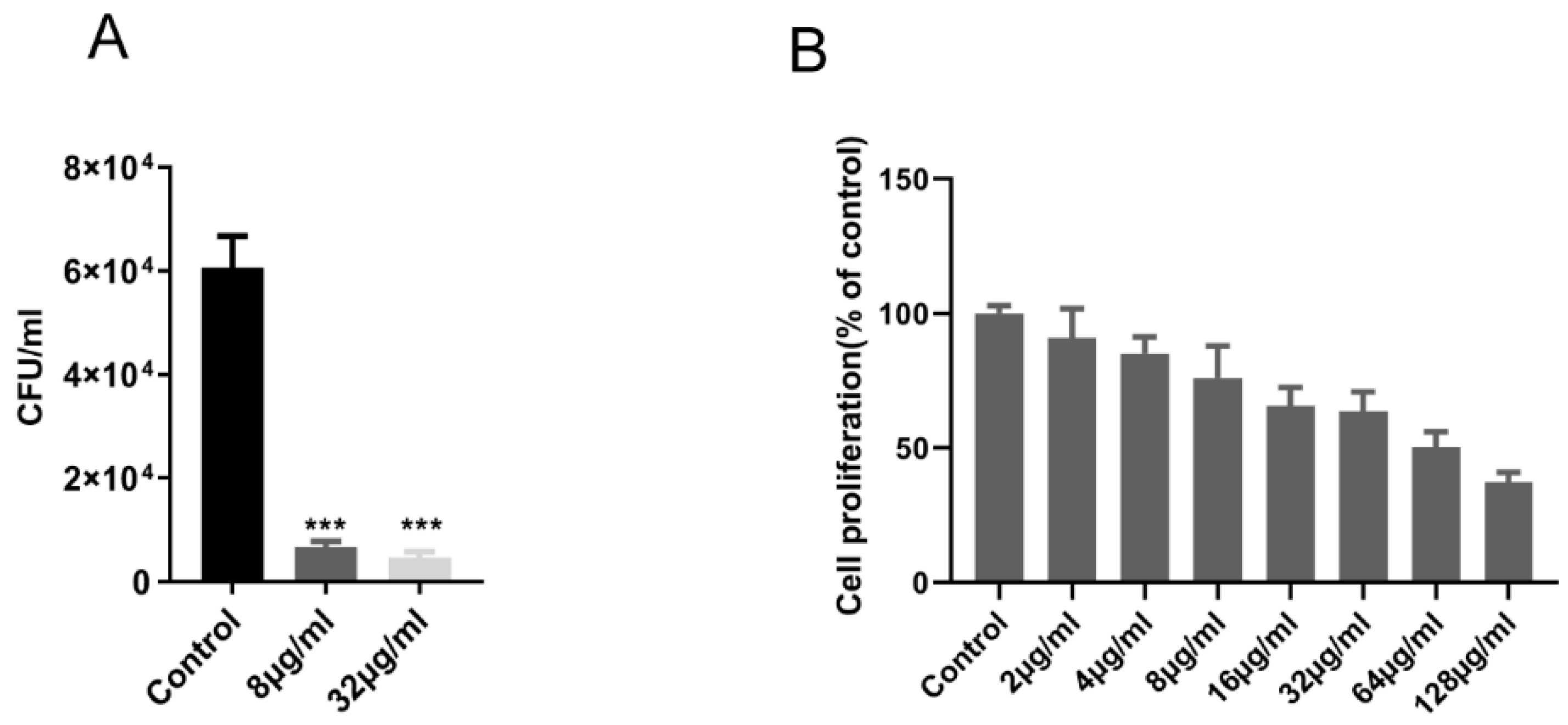
2.1. EnniatinA1 Was Active Against M. Tuberculosis in vitro and Intracellular
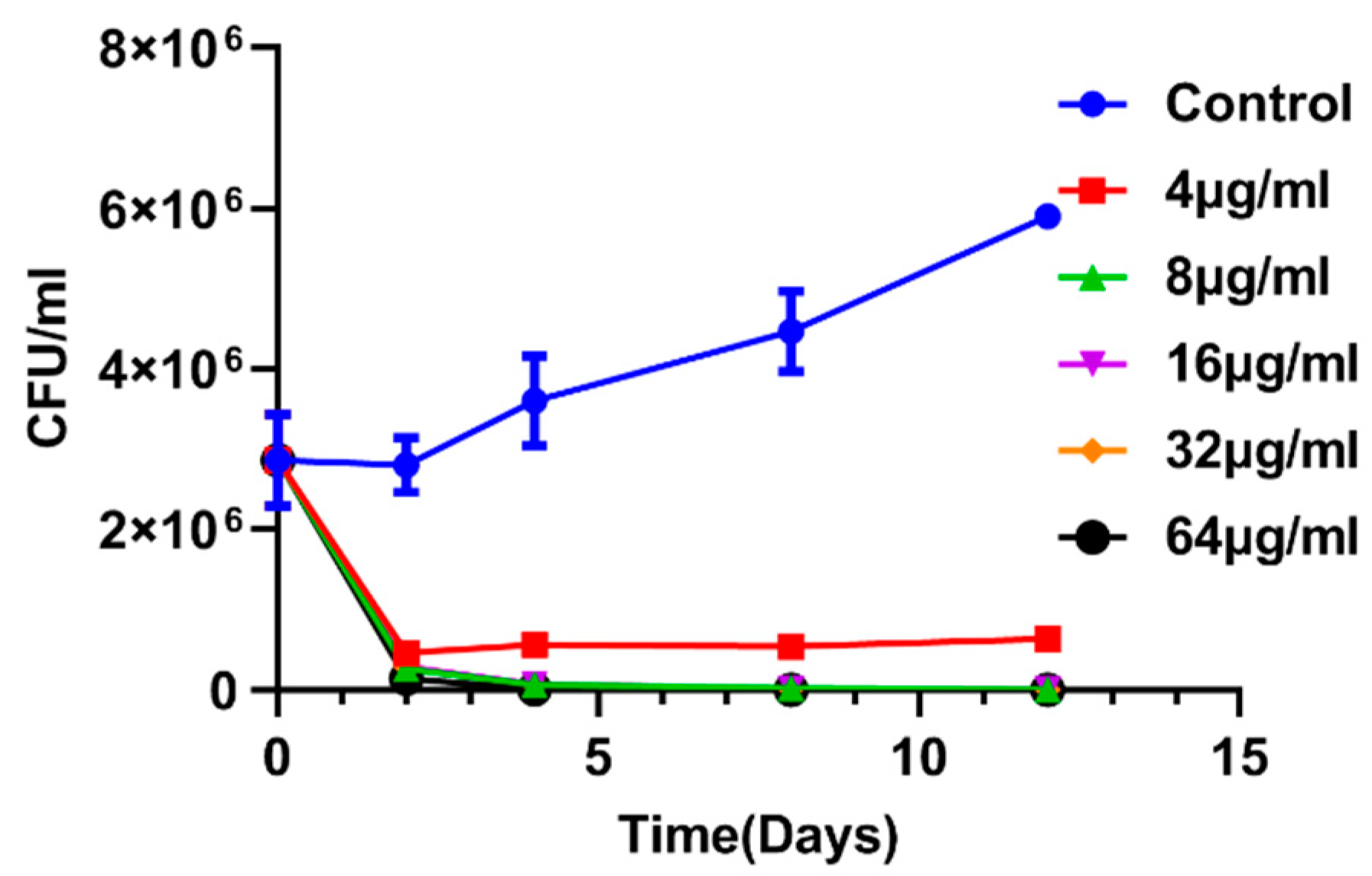
2.2. Enniatin A1 Was Able to Kill M. Tuberculosis
2.3. Antimicrobial Activity of Enniatin A1 in Combination with Classical Anti-Tuberculosis Drugs
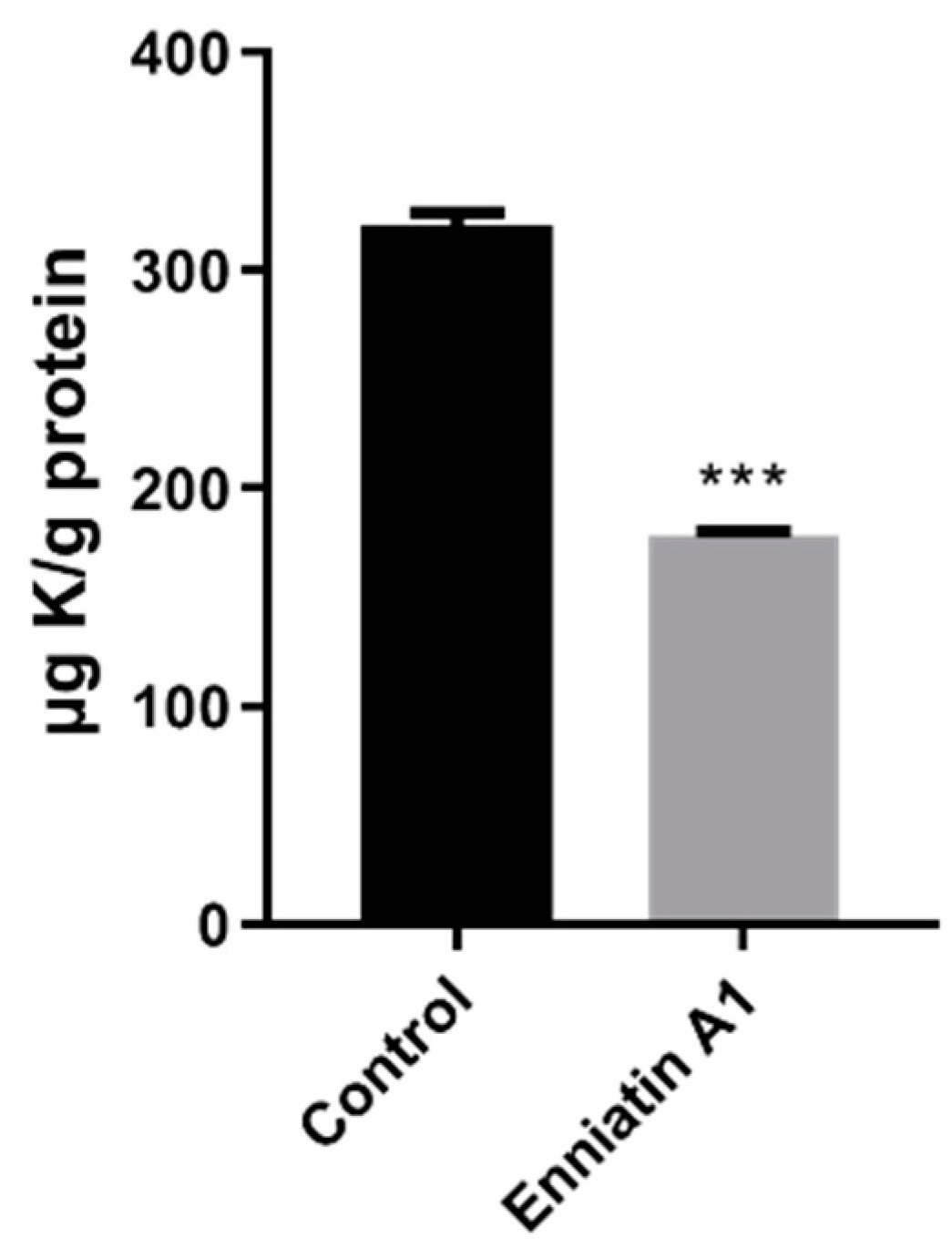
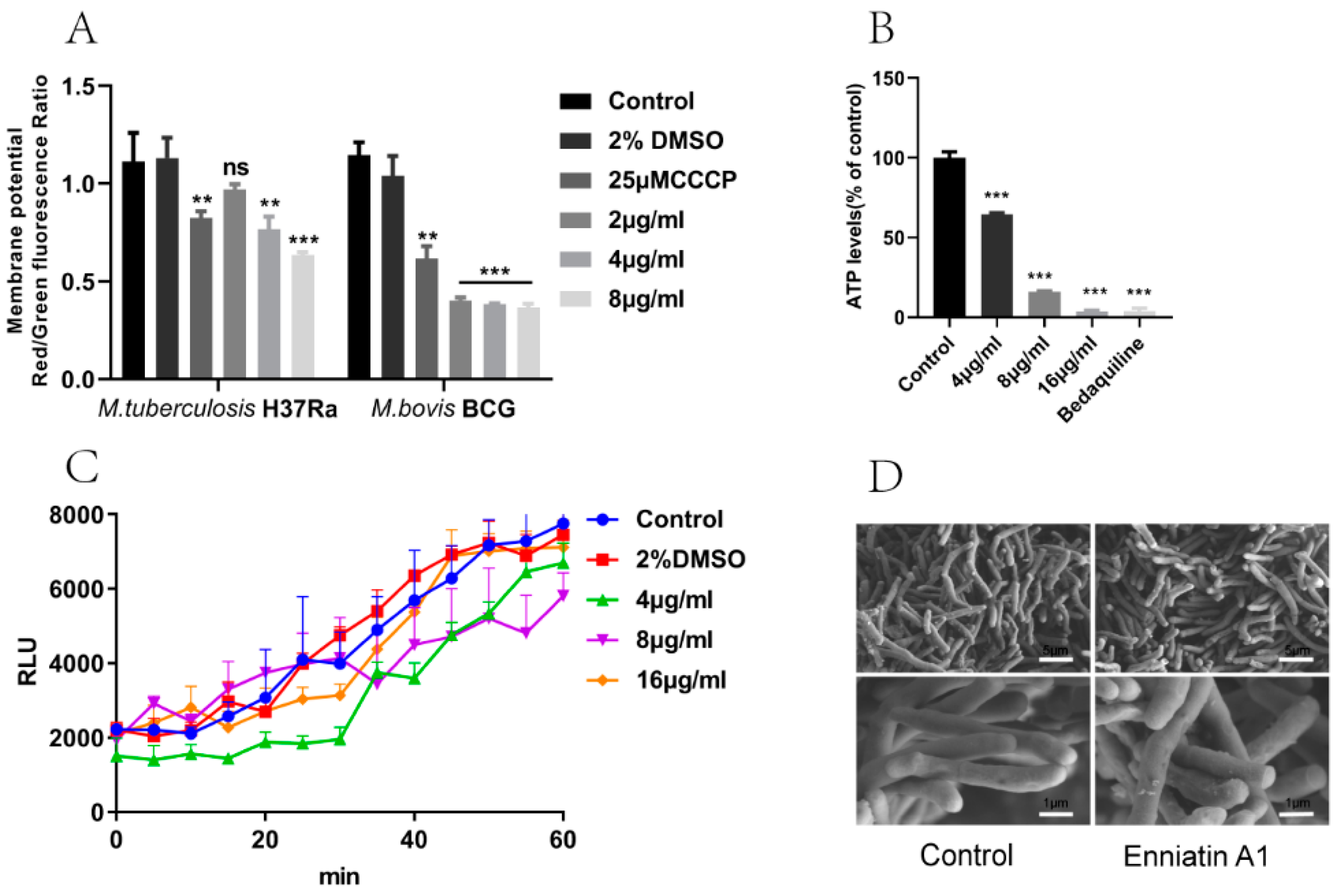
2.4. Enniatin A1 Disrupted Membrane Potential and Affected ATP Synthesis
2.5. Enniatin A1 didn’t Alter the Morphology and the Membrane Permeability of M. tuberculosis
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals Used in This Study
4.2. Bacterial Strains and Eukaryotic Cell Lines
4.3. Determination of MIC and Measurements of Synergy
4.4. Cytotoxicity for Vero Cells
4.5. Kill Kinetic Assay
4.6. Activity of EnniatinA1 against Intracellular M. Tuberculosis
4.7. Measurement of Intracellular Ion Content
4.8. Determination of Membrane Potential
4.9. Determination of Membrane Permeability
4.10. Scanning Electron Microscopy of M. Bovis BCG
4.11. Quantification of Intracellular ATP
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foo, C.S.; Lupien, A.; Kienle, M.; Vocat, A.; Benjak, A.; Sommer, R.; Lamprecht, D.A.; Steyn, A.J.C.; Pethe, K.; Piton, J.; et al. Arylvinylpiperazine Amides, a New Class of Potent Inhibitors Targeting QcrB of Mycobacterium tuberculosis. MBio 2018, 9, e01276-18. [Google Scholar] [CrossRef] [PubMed]
- Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018.
- Stewart, G.R.; Robertson, B.D.; Young, D.B. Tuberculosis: A problem with persistence. Nat. Rev. Microbiol. 2003, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.S.; Tichauer, E.; Kay, G.L.; Phillips, D.J.; Bailey, T.L.; Harrison, J.; Furze, C.M.; Millard, A.D.; Gibson, M.I.; Pallen, M.J.; et al. Identification of the anti-mycobacterial functional properties of piperidinol derivatives. Br. J. Pharmacol. 2017, 174, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Lun, S.; Guo, H.; Onajole, O.K.; Pieroni, M.; Gunosewoyo, H.; Chen, G.; Tipparaju, S.K.; Ammerman, N.C.; Kozikowski, A.P.; Bishai, W.R. Indoleamides are active against drug-resistant Mycobacterium tuberculosis. Nat. Commun. 2013, 4, 2907. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rather, M.A.; Bhat, Z.S.; Majeed, A.; Maqbool, M.; Shah, A.M.; Aga, M.A.; Shah, A.; Mushtaq, S.; Sangwan, P.L.; et al. In vitro evaluation of dinactin, a potent microbial metabolite against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 2019, 53, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, U.H.; Smith, P.W. Perspective: Challenges and opportunities in TB drug discovery from phenotypic screening. Bioorg. Med. Chem. 2015, 23, 5087–5097. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Gohlmann, H.W.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science (New York NY USA) 2005, 307, 223–227. [Google Scholar] [CrossRef]
- Bedaquiline (Sirturo) for multidrug-resistant tuberculosis. Med. Lett. Drugs Ther. 2013, 55, 66–68.
- Strahl, H.; Errington, J. Bacterial Membranes: Structure, Domains, and Function. Annu. Rev. Microbiol. 2017, 71, 519–538. [Google Scholar] [CrossRef]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
- Hancock, R.E. Peptide antibiotics. Lancet (Lond. Engl.) 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Garudachari, B.; Isloor, A.M.; Satyanarayana, M.N.; Fun, H.K.; Hegde, G. Click chemistry approach: Regioselective one-pot synthesis of some new 8-trifluoromethylquinoline based 1,2,3-triazoles as potent antimicrobial agents. Eur. J. Med. Chem. 2014, 74, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Pyta, K.; Klich, K.; Domagalska, J.; Przybylski, P. Structure and evaluation of antibacterial and antitubercular properties of new basic and heterocyclic 3-formylrifamycin SV derivatives obtained via ’click chemistry’ approach. Eur. J. Med. Chem. 2014, 84, 651–676. [Google Scholar] [CrossRef] [PubMed]
- Pyta, K.; Janas, A.; Szukowska, M.; Pecyna, P.; Jaworska, M.; Gajecka, M.; Bartl, F.; Przybylski, P. Synthesis, docking and antibacterial studies of more potent amine and hydrazone rifamycin congeners than rifampicin. Eur. J. Med. Chem. 2019, 167, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Pyta, K.; Przybylski, P.; Bartl, F. Regioselective long-range proton transfer in new rifamycin antibiotics: A process in which crown ethers act as stronger Bronsted bases than amines. Chemphyschem Eur. J. Chem. Phys. Phys. Chem. 2015, 16, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Pyta, K.; Janas, A.; Skrzypczak, N.; Schilf, W.; Wicher, B.; Gdaniec, M.; Bartl, F.; Przybylski, P. Specific Interactions between Rifamycin Antibiotics and Water Influencing Ability To Overcome Natural Cell Barriers and the Range of Antibacterial Potency. ACS Infect. Dis. 2019, 5, 1754–1763. [Google Scholar] [CrossRef]
- Ashforth, E.J.; Fu, C.; Liu, X.; Dai, H.; Song, F.; Guo, H.; Zhang, L. Bioprospecting for antituberculosis leads from microbial metabolites. Nat. Prod. Rep. 2010, 27, 1709–1719. [Google Scholar] [CrossRef]
- Malachova, A.; Dzuman, Z.; Veprikova, Z.; Vaclavikova, M.; Zachariasova, M.; Hajslova, J. Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: The major mycotoxins found in cereal-based products on the Czech market. J. Agric. Food Chem. 2011, 59, 12990–12997. [Google Scholar] [CrossRef]
- Audhya, T.K.; Russell, D.W. Natural enniatin A, a mixture of optical isomers containing both erythro- and threo-N-methyl-L-isoleucine residues. J. Chem. Soc. Perkin Trans. 1 1974, 7, 743–746. [Google Scholar] [CrossRef]
- Meca, G.; Soriano, J.M.; Gaspari, A.; Ritieni, A.; Moretti, A.; Manes, J. Antifungal effects of the bioactive compounds enniatins A, A(1), B, B(1). Toxicon Off. J. Int. Soc. Toxinol. 2010, 56, 480–485. [Google Scholar] [CrossRef]
- Shemyakin, M.M.; Ovchinnikov, Y.A.; Ivanov, V.T.; Antonov, V.K.; Vinogradova, E.I.; Shkrob, A.M.; Malenkov, G.G.; Evstratov, A.V.; Laine, I.A.; Melnik, E.I.; et al. Cyclodepsipeptides as chemical tools for studying ionic transport through membranes. J. Membr. Biol. 1969, 1, 402–430. [Google Scholar] [CrossRef] [PubMed]
- Kamyar, M.; Rawnduzi, P.; Studenik, C.R.; Kouri, K.; Lemmens-Gruber, R. Investigation of the electrophysiological properties of enniatins. Arch. Biochem. Biophys. 2004, 429, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Russell, J.B.; Strobel, H.J. Effect of ionophores on ruminal fermentation. Appl. Environ. Microbiol. 1989, 55, 1–6. [Google Scholar]
- German, M. Fusafungine an Antimicrobial with Anti-Inflammatory Properties in Respiratory Tract Infections: Review, and Recent Advances in Cellular and Molecular Activity. Clin. Drug Investig. J. 2001, 21, 653–670. [Google Scholar] [CrossRef]
- Dornetshuber-Fleiss, R.; Heilos, D.; Mohr, T.; Richter, L.; Sussmuth, R.D.; Zlesak, M.; Novicky, A.; Heffeter, P.; Lemmens-Gruber, R.; Berger, W. The naturally born fusariotoxin enniatin B and sorafenib exert synergistic activity against cervical cancer in vitro and in vivo. Biochem. Pharmacol. 2015, 93, 318–331. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Peloquin, C.A.; Singh, R.P.; Derendorf, H.; Tyagi, S.; Ginsberg, A.; Grosset, J.H.; Nuermberger, E.L. PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 239–245. [Google Scholar] [CrossRef]
- Patterson, T.F. Combination antifungal therapy. Pediatr. Infect. Dis. J. 2003, 22, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Hohle, T.H.; O’Brian, M.R. Control of bacterial iron homeostasis by manganese. Proc. Nat. Acad. Sci. USA 2010, 107, 10691–10695. [Google Scholar] [CrossRef] [PubMed]
- Champion, O.L.; Karlyshev, A.; Cooper, I.A.; Ford, D.C.; Wren, B.W.; Duffield, M.; Oyston, P.C.; Titball, R.W. Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology (Reading Engl.) 2011, 157, 1115–1122. [Google Scholar] [CrossRef]
Sample Availability: Samples are notavailable from the authors. |
| Microorganisms | MIC (μg/mL) |
|---|---|
| M. tuberculosis H37Rv | 1.0 |
| M. tuberculosis H37Ra | 2.0 |
| M. bovis | 2.0 |
| M. bovis BCG | 2.0 |
| M. smegmatis mc2 155 | 8.0 |
| E. coli | >100 |
| S. aureus | >100 |
| p. aeruginosa | >100 |
| K. pneumonia | >100 |
| L. monocytogenes | >100 |
| Drug | Combination | MIC (μg/mL) | ** FIC | *** ∑FIC | Remarks | |
|---|---|---|---|---|---|---|
| Alone | Combination | |||||
| RIF | 0.00098 | 0.000045 | 0.0459 | 0.2959 | ||
| INH | 0.0625 | 0.0156 | 0.25 | Synergism | ||
| RIF | 0.00098 | 0.00012 | 0.125 | 0.1328 | ||
| * E-A1 | 2.0 | 0.0156 | 0.0078 | Synergism | ||
| INH | 0.0625 | 0.00049 | 0.0078 | 1.0078 | ||
| E-A1 | 2.0 | 2.0 | 1.0 | No interaction | ||
| EMB | 2.0 | 0.125 | 0.0625 | 0.1250 | ||
| E-A1 | 2.0 | 0.125 | 0.0625 | Synergism | ||
| AMK | 1.0 | 0.0625 | 0.0625 | 0.1875 | ||
| E-A1 | 2.0 | 0.25 | 0.125 | Synergism | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Dong, W.; Lu, H.; Lu, W.; Feng, J.; Wang, X.; Chen, H.; Liu, M.; Tan, C. Enniatin A1, A Natural Compound with Bactericidal Activity against Mycobacterium tuberculosis In Vitro. Molecules 2020, 25, 38. https://doi.org/10.3390/molecules25010038
Wang G, Dong W, Lu H, Lu W, Feng J, Wang X, Chen H, Liu M, Tan C. Enniatin A1, A Natural Compound with Bactericidal Activity against Mycobacterium tuberculosis In Vitro. Molecules. 2020; 25(1):38. https://doi.org/10.3390/molecules25010038
Chicago/Turabian StyleWang, Gaoyan, Wenqi Dong, Hao Lu, Wenjia Lu, Jiajia Feng, Xiangru Wang, Huanchun Chen, Manli Liu, and Chen Tan. 2020. "Enniatin A1, A Natural Compound with Bactericidal Activity against Mycobacterium tuberculosis In Vitro" Molecules 25, no. 1: 38. https://doi.org/10.3390/molecules25010038
APA StyleWang, G., Dong, W., Lu, H., Lu, W., Feng, J., Wang, X., Chen, H., Liu, M., & Tan, C. (2020). Enniatin A1, A Natural Compound with Bactericidal Activity against Mycobacterium tuberculosis In Vitro. Molecules, 25(1), 38. https://doi.org/10.3390/molecules25010038






