Tracking Extracellular Matrix Remodeling in Lungs Induced by Breast Cancer Metastasis. Fourier Transform Infrared Spectroscopic Studies
Abstract
1. Introduction
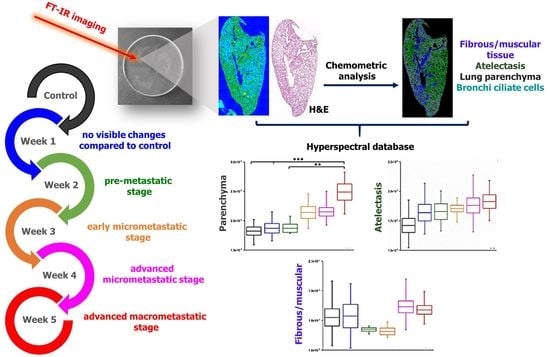
2. Results and Discussion
2.1. Morphological Characterization of Lungs in Metastasis Development
2.2. FTIR-Based Discrimination of Chemical Changes in Lung Tissue Structures due to Metastasis
2.3. A Semi-Quantitative FTIR Approach in an Analysis of Proteins in the Lung Tissue Structures
3. Materials and Methods
3.1. Sample Preparation and Histological Analysis
3.2. An Assessment of the Primary Tumor, Number, and Type of Metastases in the Lungs
3.3. FTIR Spectroscopic Imaging and Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, C.A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef]
- Weigelt, B.; Peterse, J.L.; van ’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Peinado, H.; Lavotshkin, S.; Lyden, D. The secreted factors responsible for pre-metastatic niche formation: Old sayings and new thoughts. Semin. Cancer Biol. 2011, 21, 139–146. [Google Scholar] [CrossRef]
- Alderton, G.K. Metastasis. Exosomes drive premetastatic niche formation. Nat. Rev. Cancer 2012, 12, 447. [Google Scholar] [CrossRef]
- Murata, T. Histological Studies on the Respiratory Portions of the Lungs of Cetacea. Sci. Rep. 1951, 6, 35–47. [Google Scholar]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Dunsmore, S.E.; Rannels, D.E. Extracellular matrix biology in the lung. Am. J. Physiol. 1996, 270, 3–27. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Xiong, G.; Flynn, T.J.; Chen, J.; Trinkle, C.; Xu, R. Development of an ex vivo breast cancer lung colonization model utilizing a decellularized lung matrix. Integr. Biol. 2015, 7, 1518–1525. [Google Scholar] [CrossRef]
- Oskarsson, T.; Acharyya, S.; Zhang, X.H.; Vanharanta, S.; Tavazoie, S.F.; Morris, P.G.; Downey, R.J.; Manova-Todorova, K.; Brogi, E.; Massagué, J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011, 17, 867–874. [Google Scholar] [CrossRef]
- Malanchi, I.; Santamaria-Martínez, A.; Susanto, E.; Peng, H.; Lehr, H.A.; Delaloye, J.F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011, 481, 85–89. [Google Scholar] [CrossRef]
- Bissell, M.J.; Weaver, V.M.; Lelièvre, S.A.; Wang, F.; Petersen, O.W.; Schmeichel, K.L. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999, 59, 1757–1763. [Google Scholar]
- Depciuch, J.; Kaznowska, E.; Golowski, S.; Koziorowska, A.; Zawlik, I.; Cholewa, M.; Szmuc, K.; Cebulski, J. Monitoring breast cancer treatment using a Fourier transform infrared spectroscopy-based computational model. J. Pharm. Biomed. Anal. 2017, 143, 261–268. [Google Scholar] [CrossRef]
- Wang, J.-S.; Shi, J.-S.; Xu, Y.-Z.; Duan, X.-Y.; Zhang, L.; Wang, J.; Yang, L.-M.; Weng, S.-F.; Wu, J.-G. FT-IR spectroscopic analysis of normal and cancerous tissues of esophagus. World J. Gastroenterol. 2003, 9, 1897–1899. [Google Scholar] [CrossRef]
- Mehrotra, R.; Ray, B.; Chaturvedi, H. Biochemical Characterization of Lung Cancer Tissue: A Spectroscopic Investigation. J. Cancer Clin. Oncol. 2016, 2, 1–8. [Google Scholar]
- Lv, J.; Zhang, L.; Feng, J.; Shi, R. Optical observation of lung cancer with attenuated total reflectance-fourier transform infrared microscope (ATR-FTIR) and confocal Raman microscope. Spectrosc. Lett. 2011, 44, 312–317. [Google Scholar] [CrossRef]
- Kaznowska, E.; Depciuch, J.; Łach, K.; Kołodziej, M.; Koziorowska, A.; Vongsvivut, J.; Zawlik, I.; Cholewa, M.; Cebulski, J. The classification of lung cancers and their degree of malignancy by FTIR, PCA-LDA analysis, and a physics-based computational model. Talanta 2018, 186, 337–345. [Google Scholar] [CrossRef]
- Lewis, P.D.; Lewis, K.E.; Ghosal, R.; Bayliss, S.; Lloyd, A.J.; Wills, J.; Godfrey, R.; Kloer, P.; Mur, L.A. Evaluation of FTIR Spectroscopy as a diagnostic tool for lung cancer using sputum. Bmc Cancer 2010, 10, 640. [Google Scholar] [CrossRef]
- Chrabaszcz, K.; Kochan, K.; Fedorowicz, A.; Jasztal, A.; Buczek, E.; Leslie, L.S.; Bhargava, R.; Malek, K.; Chlopicki, S.; Marzec, K.M. FT-IR- and Raman-based biochemical profiling of the early stage of pulmonary metastasis of breast cancer in mice. Analyst 2018, 143, 2042–2050. [Google Scholar] [CrossRef]
- Augustyniak, K.; Chrabaszcz, K.; Jasztal, A.; Smeda, M. High and ultra-high definition of infrared spectral histopathology gives an insight into chemical environment of lung metastases in breast cancer. J. Biophotonics 2019, 12, 201800345. [Google Scholar] [CrossRef]
- Blat, A.; Wiercigroch, E.; Smeda, M.; Wislocka, A.; Chlopicki, S.; Malek, K. Fourier transform infrared spectroscopic signature of blood plasma in the progression of breast cancer with simultaneous metastasis to lungs. J. Biophotonics 2019, 12, 201900067. [Google Scholar] [CrossRef]
- Noreen, R.; Chien, C.C.; Delugin, M.; Yao, S.; Pineau, R.; Hwu, Y.; Moenner, M.; Petibois, C. Detection of collagens in brain tumors based on FTIR imaging and chemometrics. Anal. Bioanal. Chem. 2011, 401, 845–852. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Bromberg, P.S.; Gough, K.M.; Dixon, I.M. Collagen remodeling in the extracellular matrix of the cardiomyopathic Syrian hamster heart as assessed by FTIR attenuated total reflectance spectroscopy. Can. J. Chem. 1999, 77, 1843–1855. [Google Scholar] [CrossRef]
- Varma, V.K.; Kajdacsy-Balla, A.; Akkina, S.; Setty, S.; Walsh, M.J. Predicting Fibrosis Progression in Renal Transplant Recipients Using Laser-Based Infrared Spectroscopic Imaging. Sci. Rep. 2018, 8, 686. [Google Scholar] [CrossRef]
- Al-Mehdi, A.B.; Tozawa, K.; Fisher, A.B.; Shientag, L.; Lee, A.; Muschel, R.J. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Gómez-Cuadrado, L.; Tracey, N.; Ma, R.; Qian, B.; Brunton, V.G. Mouse models of metastasis: Progress and prospects. Dis. Model. Mech. 2017, 10, 1061–1074. [Google Scholar] [CrossRef]
- Smeda, M.; Przyborowski, K.; Proniewski, B.; Zakrzewska, A.; Kaczor, D.; Stojak, M.; Buczek, E.; Nieckarz, Z.; Zoladz, J.A.; Wietrzyk, J.; et al. Breast cancer pulmonary metastasis is increased in mice undertaking spontaneous physical training in the running wheel; a call for revising beneficial effects of exercise on cancer progression. Am. J. Cancer Res. 2017, 7, 1926–1936. [Google Scholar]
- Smeda, M.; Kieronska, A.; Adamski, M.G.; Proniewski, B.; Sternak, M.; Mohaissen, T.; Przyborowski, K.; Derszniak, K.; Kaczor, D.; Stojak, M.; et al. Nitric oxide deficiency and endothelial-mesenchymal transition of pulmonary endothelium in the progression of 4T1 metastatic breast cancer in mice. Breast Cancer Res. 2018, 20, 86. [Google Scholar] [CrossRef]
- Lasch, P. CytospecTM. A Matlab Based Application for Infrared Imaging. 2001. Available online: http://www.cytospec.com (accessed on 3 January 2020).
- Bassan, P.; Kohler, A.; Martens, H.; Lee, J.; Byrne, H.J.; Dumas, P.; Gazi, E.; Brown, M.; Clarke, N.; Gardner, P. Resonant Mie Scattering (RMieS) correction of infrared spectra from highly scattering biological samples. Analyst 2010, 135, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Smeda, M.; Kieronska, A.; Proniewski, B.; Jasztal, A.; Selmi, A.; Wandzel, K.; Zakrzewska, A.; Wojcik, T.; Przyborowski, K.; Derszniak, K.; et al. Dual antiplatelet therapy with clopidogrel and aspirin increases mortality in 4T1 metastatic breast cancer-bearing mice by inducing vascular mimicry in primary tumour. Oncotarget 2018, 9, 17810–17824. [Google Scholar] [CrossRef] [PubMed]
- Townsley, M.I. Structure and composition of pulmonary arteries, capillaries, and veins. Compr. Physiol. 2012, 2, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Pratt, P.C. Basic Anatomy and Histology of the Lung. Toxicol. Ind. Health 1985, 1, 1–5. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Federman, S.; Miller, L.M.; Sagi, I. Following matrix metalloproteinases activity near the cell boundary by infrared micro-spectroscopy. Matrix Biol. 2002, 21, 567–577. [Google Scholar] [CrossRef]
- Cheheltani, R.; McGoverin, C.M.; Rao, J.; Vorp, D.A.; Kiani, M.F.; Pleshko, N. Fourier transform infrared spectroscopy to quantify collagen and elastin in an in vitro model of extracellular matrix degradation in aorta. Analyst 2014, 139, 3039–3047. [Google Scholar] [CrossRef]
- Garlick, P.J.; McNurlan, M.A. Protein metabolism in the cancer patient. Biochimie 1994, 76, 713–717. [Google Scholar] [CrossRef]
- Lim, S.; Yoo, B.K.; Kim, H.S.; Gilmore, H.L.; Lee, Y.; Lee, H.P.; Kim, S.J.; Letterio, J.; Lee, H.G. Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer. BMC Cancer 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Audas, T.E.; Audas, D.E.; Jacob, M.D.; Ho, J.J.; Khachi, M.; Wang, M.; Perera, J.K.; Gardiner, C.; Bennett, C.A.; Head, T.; et al. Adaptation to stressors by systemic protein amyloidogenesis. Dev. Cell 2016, 39, 155–168. [Google Scholar] [CrossRef]
- Mizejewski, G.J. Breast cancer and amyloid bodies: Is there a role for amyloidosis in cancer-cell dormancy? Breast Cancer Targets 2017, 9, 287–291. [Google Scholar] [CrossRef]
- Azzi, S.; Hebda, J.K.; Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013, 3, 211. [Google Scholar] [CrossRef]
- Mazur, A.I.; Marcsisin, E.J.; Bird, B.; Milijkovis, M.; Diem, M. Evaluating different fixation protocols for spectral cytopathology, Part I. Anal. Chem. 2012, 84, 1259–1266. [Google Scholar] [CrossRef][Green Version]
- Verdonck, M.; Wald, N.; Janssis, J.; Yan, P.; Meyer, C.; Legat, A.; Speiser, D.E.; Desmedt, C.; Larsimont, D.; Sotiriou, C.; et al. Breast cancer and melanoma cell line identification by FTIR imaging after formalin-fixation and paraffin-embedding. Analyst 2013, 138, 4083–4091. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |



| Group | Number of Metastasis | Lungs Mass [% of Body Weight] | Primary Tumor Mass [g] |
|---|---|---|---|
| control | 0 | 0.795–0.894 | 0 |
| week 1 | 0 | 0.785–0.903 | 0 |
| week 2 | 0 | 0.731–0.8 | 0–0.024 |
| week 3 | 0–2 | 0.82–0.84 | 0.203–0.257 |
| week 4 | 2–5 | 0.75–0.895 | 0.493–0.700 |
| week 5 | 7–89 | 0.827–0.986 | 0.927–2.562 |
| Vibration Modes | Position [cm−1] | Assignment |
|---|---|---|
| ν(C=O), amide I: β-turns, β-sheets | 1684, 1680 | Proteins |
| ν(C=O), amide I: α-helices | 1655, 1652 | Proteins |
| ν(C=O); amide I, parallel β-sheets | 1631, 1628 | Proteins |
| δ(NH); amide II of proteins | 1547 | Proteins |
| in-plane δ(CH) of phenyl ring | 1516 | Tyrosine residues |
| ν(C-N)/δ(N-H)/ν(CH3-C): amide III | 1310, 1312 | Proteins |
| amide III | 1286–1202 | Fibrous proteins |
| ν(C-OH) | 1166 | Hydroxyproline residues |
| ν(C-O), δ(C-O-H), δ(C-O-C) | 1116, 1081, 1038 | Collagen-associated carbohydrate moieties |
| Spectral Parameter | Lungs Mass [% of Body Weight] | |
|---|---|---|
| Spearman R | Spearman P | |
| PARENCHYMA | ||
| Secondary structure of proteins | 0.82 | 0.001 |
| Fibrillar proteins | 0.81 | 0.001 |
| Carbohydrates moiety | 0.81 | 0.001 |
| Hydroxyproline residues | 0.84 | 0.0006 |
| ATELECTASIS | ||
| Total proteins | 0.84 | 0.0006 |
| Secondary structure of proteins | 0.88 | 0.0002 |
| FIBROUS/MUSCULAR | ||
| Total proteins | 0.87 | 0.0002 |
| Fibrillar proteins | 0.82 | 0.001 |
| Hydroxyproline residues | 0.82 | 0.0009 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrabaszcz, K.; Kaminska, K.; Augustyniak, K.; Kujdowicz, M.; Smeda, M.; Jasztal, A.; Stojak, M.; Marzec, K.M.; Malek, K. Tracking Extracellular Matrix Remodeling in Lungs Induced by Breast Cancer Metastasis. Fourier Transform Infrared Spectroscopic Studies. Molecules 2020, 25, 236. https://doi.org/10.3390/molecules25010236
Chrabaszcz K, Kaminska K, Augustyniak K, Kujdowicz M, Smeda M, Jasztal A, Stojak M, Marzec KM, Malek K. Tracking Extracellular Matrix Remodeling in Lungs Induced by Breast Cancer Metastasis. Fourier Transform Infrared Spectroscopic Studies. Molecules. 2020; 25(1):236. https://doi.org/10.3390/molecules25010236
Chicago/Turabian StyleChrabaszcz, Karolina, Katarzyna Kaminska, Karolina Augustyniak, Monika Kujdowicz, Marta Smeda, Agnieszka Jasztal, Marta Stojak, Katarzyna M. Marzec, and Kamilla Malek. 2020. "Tracking Extracellular Matrix Remodeling in Lungs Induced by Breast Cancer Metastasis. Fourier Transform Infrared Spectroscopic Studies" Molecules 25, no. 1: 236. https://doi.org/10.3390/molecules25010236
APA StyleChrabaszcz, K., Kaminska, K., Augustyniak, K., Kujdowicz, M., Smeda, M., Jasztal, A., Stojak, M., Marzec, K. M., & Malek, K. (2020). Tracking Extracellular Matrix Remodeling in Lungs Induced by Breast Cancer Metastasis. Fourier Transform Infrared Spectroscopic Studies. Molecules, 25(1), 236. https://doi.org/10.3390/molecules25010236