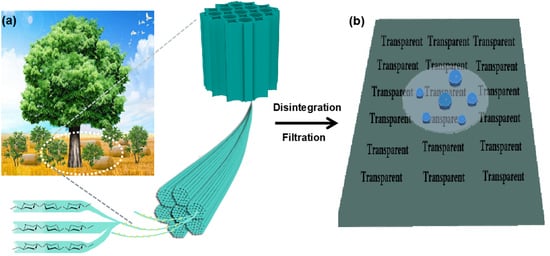
Screening of Nanocellulose from Different Biomass Resources and Its Integration for Hydrophobic Transparent Nanopaper
Abstract
1. Introduction
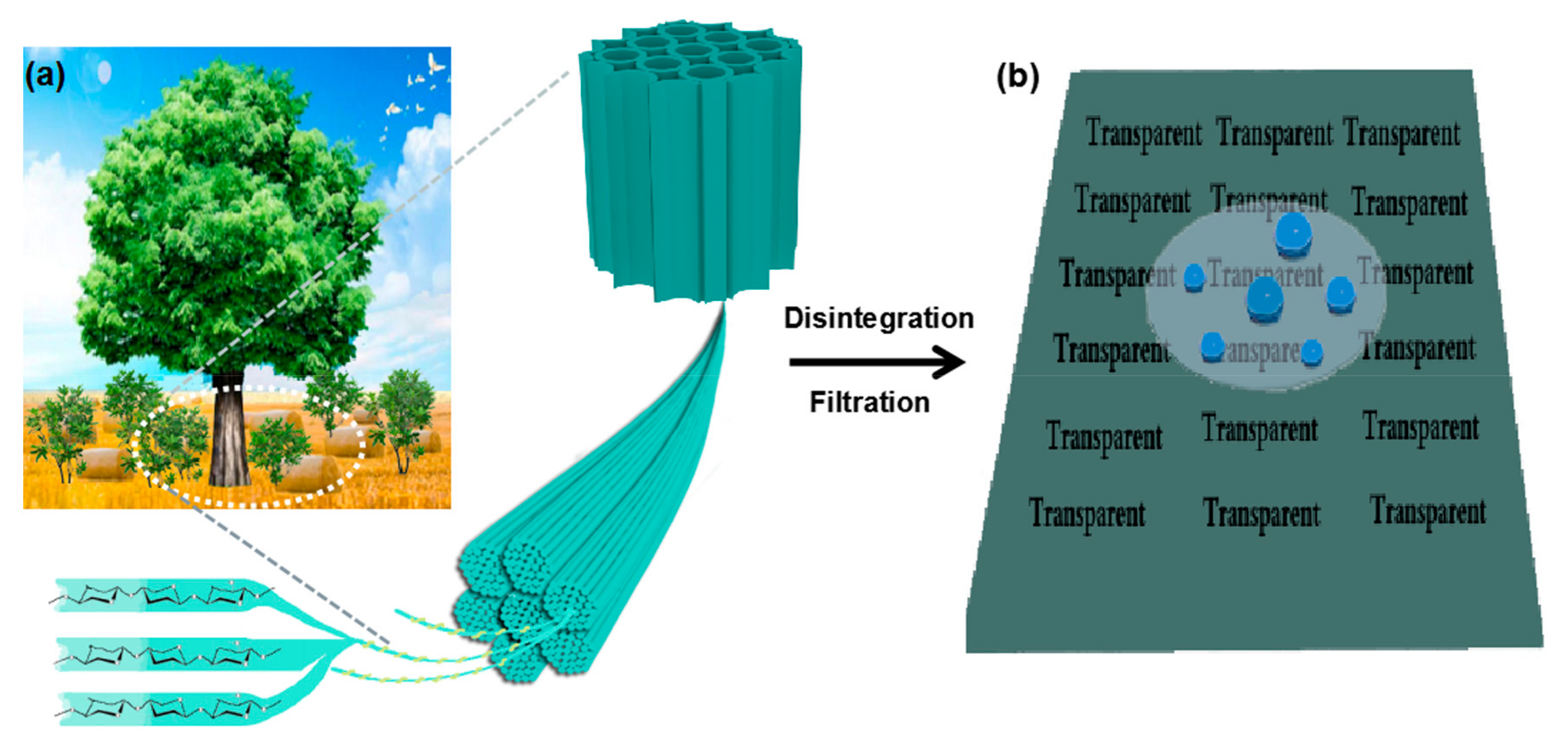
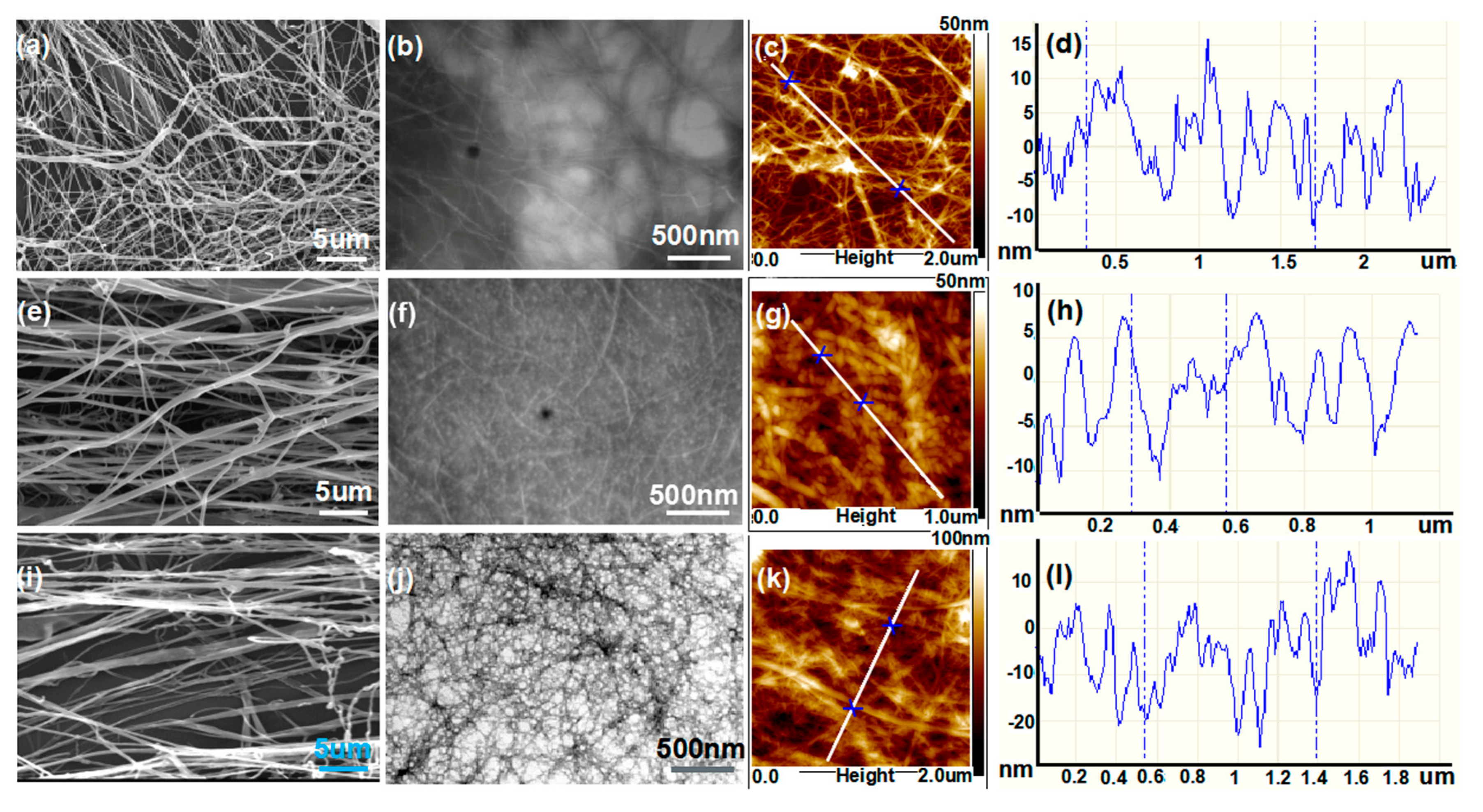
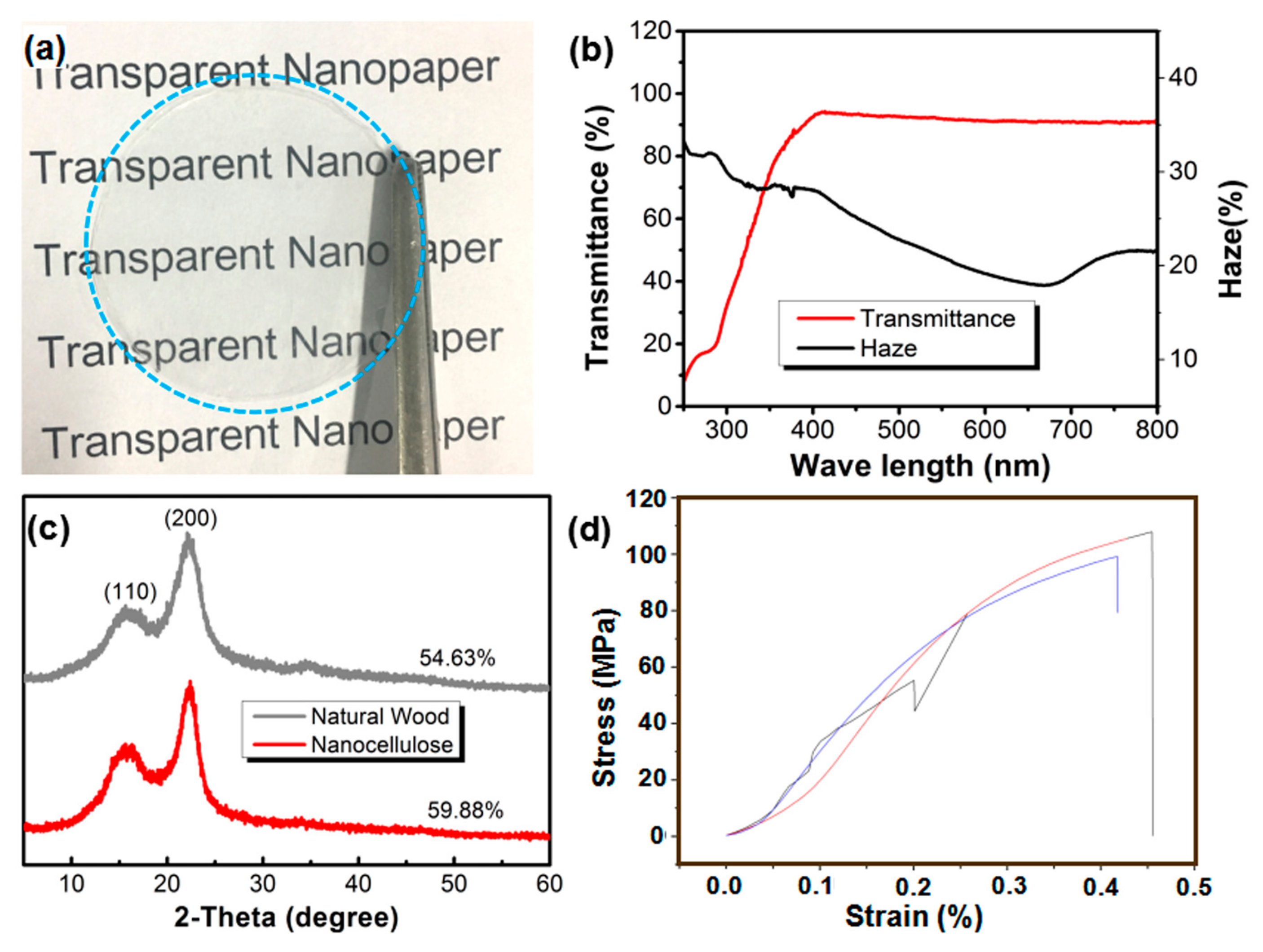
2. Results and Discussion
3. Materials and Methods
3.1. Experiment Materials
3.2. Experiment Methods
3.2.1. Preparation of the Chemically Purified Cellulose
3.2.2. Mechanical Treatment Method
3.2.3. Preparation of the Nano-Silica
3.2.4. Preparation of Nanopaper
3.3. Characterization Test Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiajia, Z.; Sangwon, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar]
- Roland, G.; Jambeck, J.R.; Kara, L.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar]
- Bo, J.; Chaoji, C.; Zhiqiang, L.; Shuaiming, H.; Yudi, K.; Jianwei, S.; Ruiyu, M.; Gegu, C.; Miaolun, J.; Liangbing, H. Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Adv. Funct. Mater. 2019, 1906307. [Google Scholar] [CrossRef]
- Bejoy, T.; Midhum, C.R.; Athira, K.B.; Rubiyah, M.H.; Jithin, J.; Audrey, M.; Glenna, L.D.; Clément, S. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar]
- Hongli, Z.; Wei, L.; Peter, N.C.; Zhiqiang, F.; Junyong, Z.; Gunnar, H.; Michael, E.H.; Liangbing, H. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar]
- Jie, W.; Shihan, G.; Junhua, W.; Dandan, X.; Yun, S.; Xiaoying, D.; Yangyong, D.; Yongfeng, L. Nanocellulose-graphene oxide hybrid aerogel towards water purification. Appl. Environ. Biotechnol. 2019, 4, 11–17. [Google Scholar]
- Oleksandr, N.; Mohamed, N.B.; Julien, B. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar]
- Xiao, Z.; Chang, L.; Rutan, P.; Xiaoying, D.; Yongfeng, L. Nanocellulose mechanically isolated from Amorpha fruticosa linn. ACS Sustain. Chem. Eng. 2017, 5, 4414–4420. [Google Scholar]
- Tian, L.; Xin, Z.; Steven, D.L.; Ruiyu, M.; Xinpeng, Z.; Feng, J.; Jianwei, S.; Zhongqi, L.; Guang, C.; Jiaqi, D.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 2019, 608–613. [Google Scholar]
- Reza, M.; Samaneh, K.; Siavash, I.; Rajender, S.V. Plant-derived nanostructures: Types and applications. Green Chem. 2016, 18, 20–52. [Google Scholar]
- Tian, L.; Yao, Z.; Shuaiming, H.; Wentao, G.; Zhiyuan, W.; Mohammad, H.; Daniel, D.; Ruiyu, M.; Xinpeng, Z.; Jianwei, S.; et al. A radiative cooling structural material. Science 2019, 364, 760–763. [Google Scholar]
- Trache, D.; Hussin, M.H.; Haafizc, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Orlando, J.R.; Lucian, A.L.; Mohini, S. Cellulosic Nanocomposites: A review. BioResources 2008, 3, 929–980. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chemi. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Edit. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Gamelas, J.A.F. The surface properties of cellulose and lignocellulosic materials assessed by inverse gas chromatography: A review. Cellulose 2013, 20, 2675–2693. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose–Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Wen, H.; Zhiqiang, F.; Yu, L.; Yicong, Z.; Yudi, K.; Honglong, N.; Bo, L.; Gang, C.; Yingyao, L. Protonation process to enhance the water resistance of transparent and hazy paper. ACS Sustain. Chem. Eng. 2018, 6, 12385–12392. [Google Scholar]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications. Cellulose 2010, 17, 835–848. [Google Scholar] [CrossRef]
- Akira, I. Wood nanocelluloses: Fundamentals and applications as new bio-based nanomaterials. J. Wood Sci. 2013, 59, 449–459. [Google Scholar]
- Yang, L.; Dmitry, G.; Qian, L.; Xiaonan, L.; Jie, X. Recent advancements in functionalized paper-based electronics. ACS Appl. Mater. Inter. 2016, 8, 20501–20515. [Google Scholar]
- Khalil, H.P.S.A.; Bhat, A.H.; Yusra, A.F.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohyd. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Jinwu, W.; Douglas, J.G.; Nicole, M.S.; Douglas, W.B.; Mehdi, T.; Zhiyong, C. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: A review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Youssef, H.; Lucian, A.L.; Orlando, J.R. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar]
- Masaya, N.; Shinichiro, I.; Antonio, N.N.; Hiroyuki, Y. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar]
- Xiaoying, D.; Xiao, Z.; Jie, W.; Gang, Z.; Yongfeng, L. Wood-based nanocomposite derived by in-situ formation of organic-inorganic hybrid polymer within wood via a sol-gel method. ACS Appl. Mater. Inter. 2017, 9, 9070–9078. [Google Scholar]
- Yongfeng, L.; Xiaoying, D. A Preparation Method of Superhydrophobic, Oleophobic, Transparent Nanocellulose-Based Paper. ZL201610114924.3, 10 November 2017. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Zhang, H.; Xu, D.; He, Z.; Pan, X.; Gui, S.; Dai, X.; Fan, J.; Dong, X.; Li, Y. Screening of Nanocellulose from Different Biomass Resources and Its Integration for Hydrophobic Transparent Nanopaper. Molecules 2020, 25, 227. https://doi.org/10.3390/molecules25010227
Qi Y, Zhang H, Xu D, He Z, Pan X, Gui S, Dai X, Fan J, Dong X, Li Y. Screening of Nanocellulose from Different Biomass Resources and Its Integration for Hydrophobic Transparent Nanopaper. Molecules. 2020; 25(1):227. https://doi.org/10.3390/molecules25010227
Chicago/Turabian StyleQi, Yanran, Hao Zhang, Dandan Xu, Zaixin He, Xiya Pan, Shihan Gui, Xiaohan Dai, Jilong Fan, Xiaoying Dong, and Yongfeng Li. 2020. "Screening of Nanocellulose from Different Biomass Resources and Its Integration for Hydrophobic Transparent Nanopaper" Molecules 25, no. 1: 227. https://doi.org/10.3390/molecules25010227
APA StyleQi, Y., Zhang, H., Xu, D., He, Z., Pan, X., Gui, S., Dai, X., Fan, J., Dong, X., & Li, Y. (2020). Screening of Nanocellulose from Different Biomass Resources and Its Integration for Hydrophobic Transparent Nanopaper. Molecules, 25(1), 227. https://doi.org/10.3390/molecules25010227