Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats
Abstract
1. Introduction
2. Results
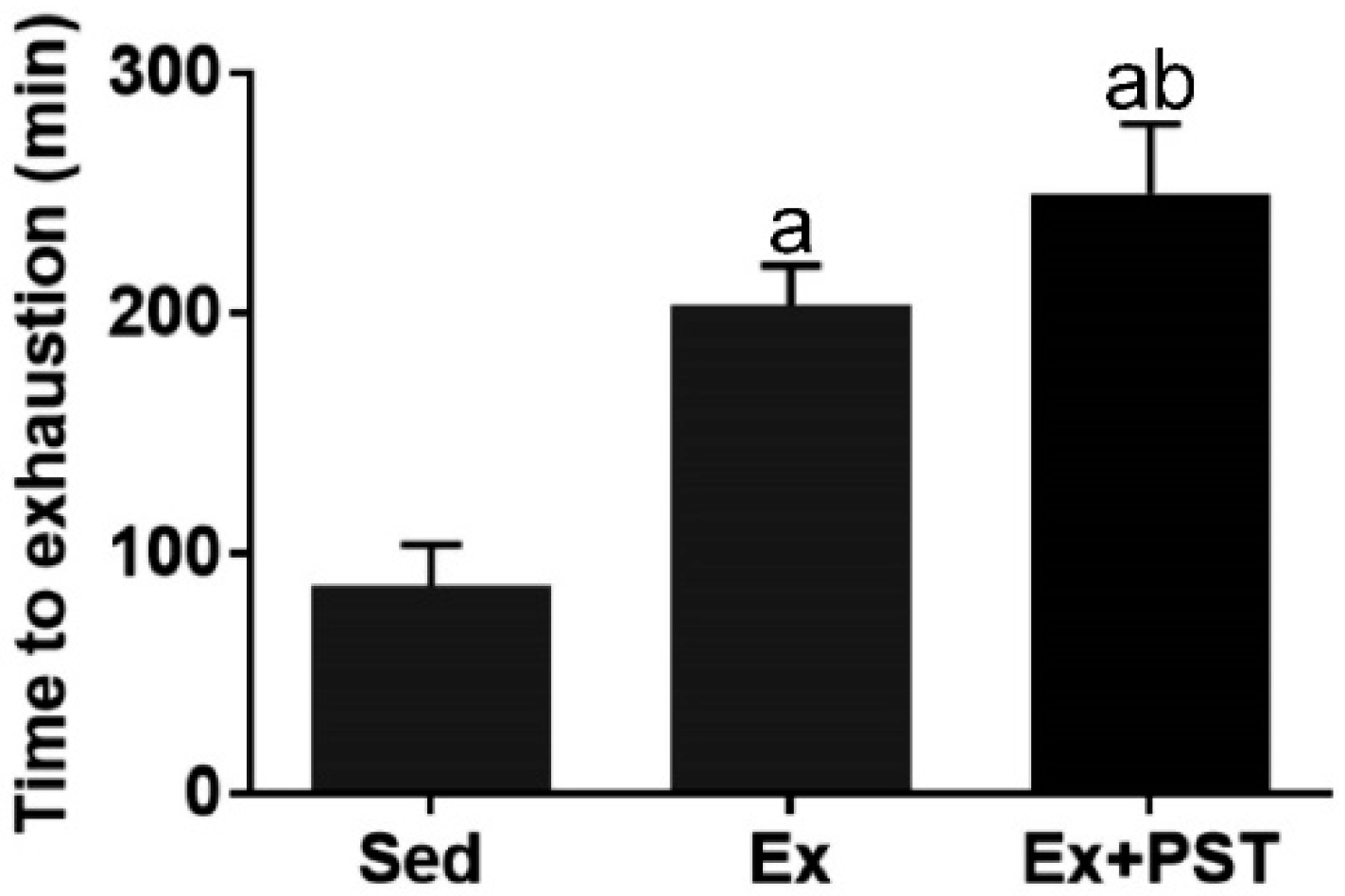
2.1. PST Promotes Exercise Training-Induced Endurance Capacity
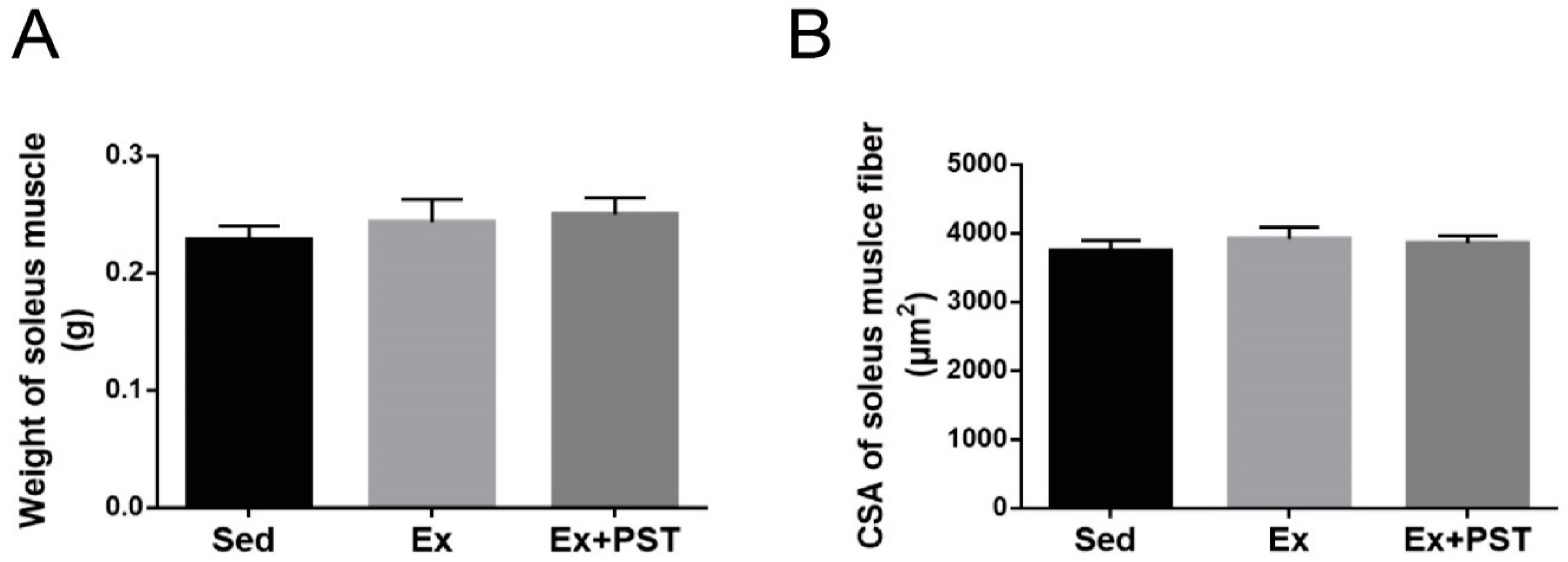
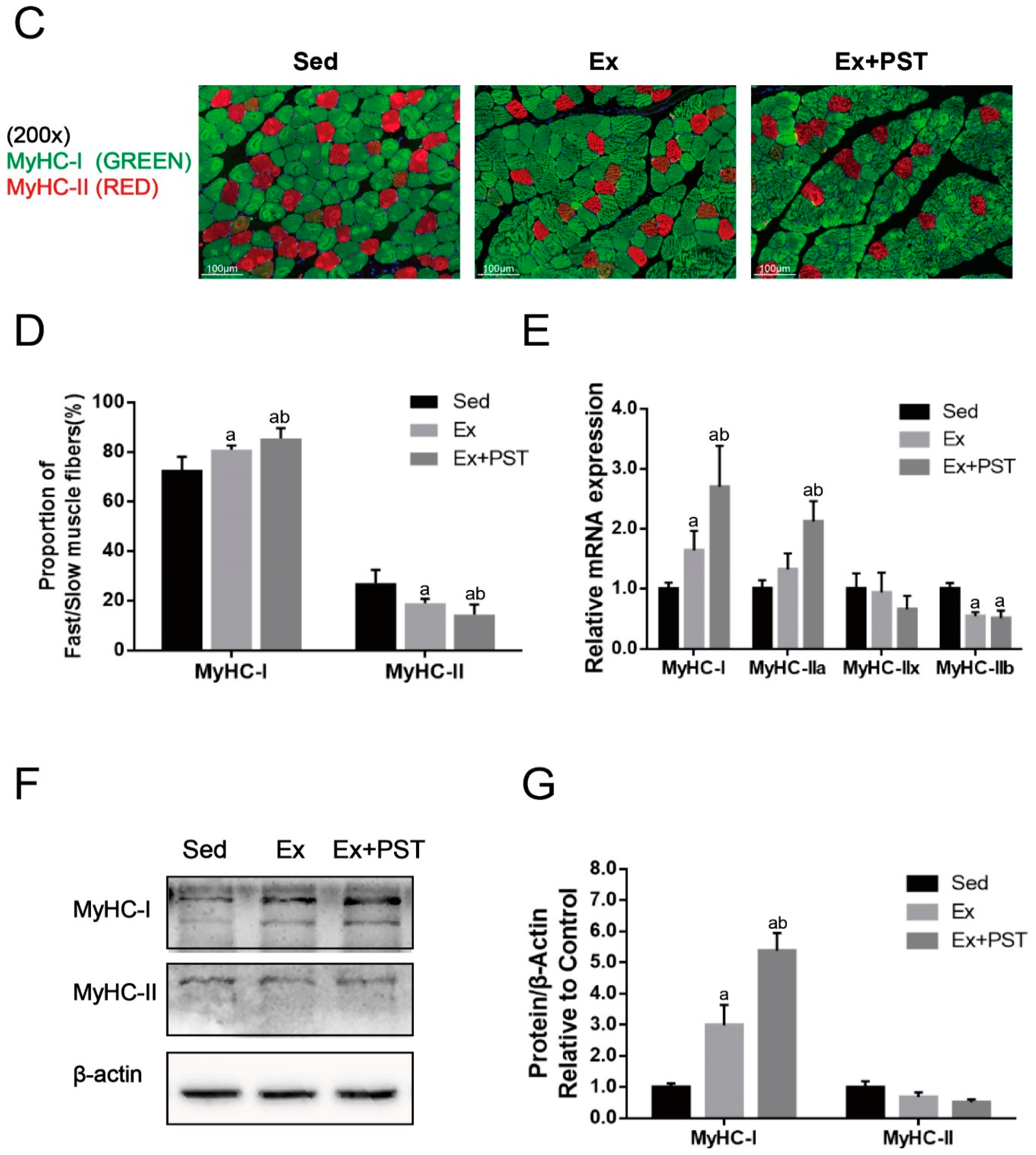
2.2. PST Increases Proportion of Slow-Twitch Fibers in Exercise Training Rats
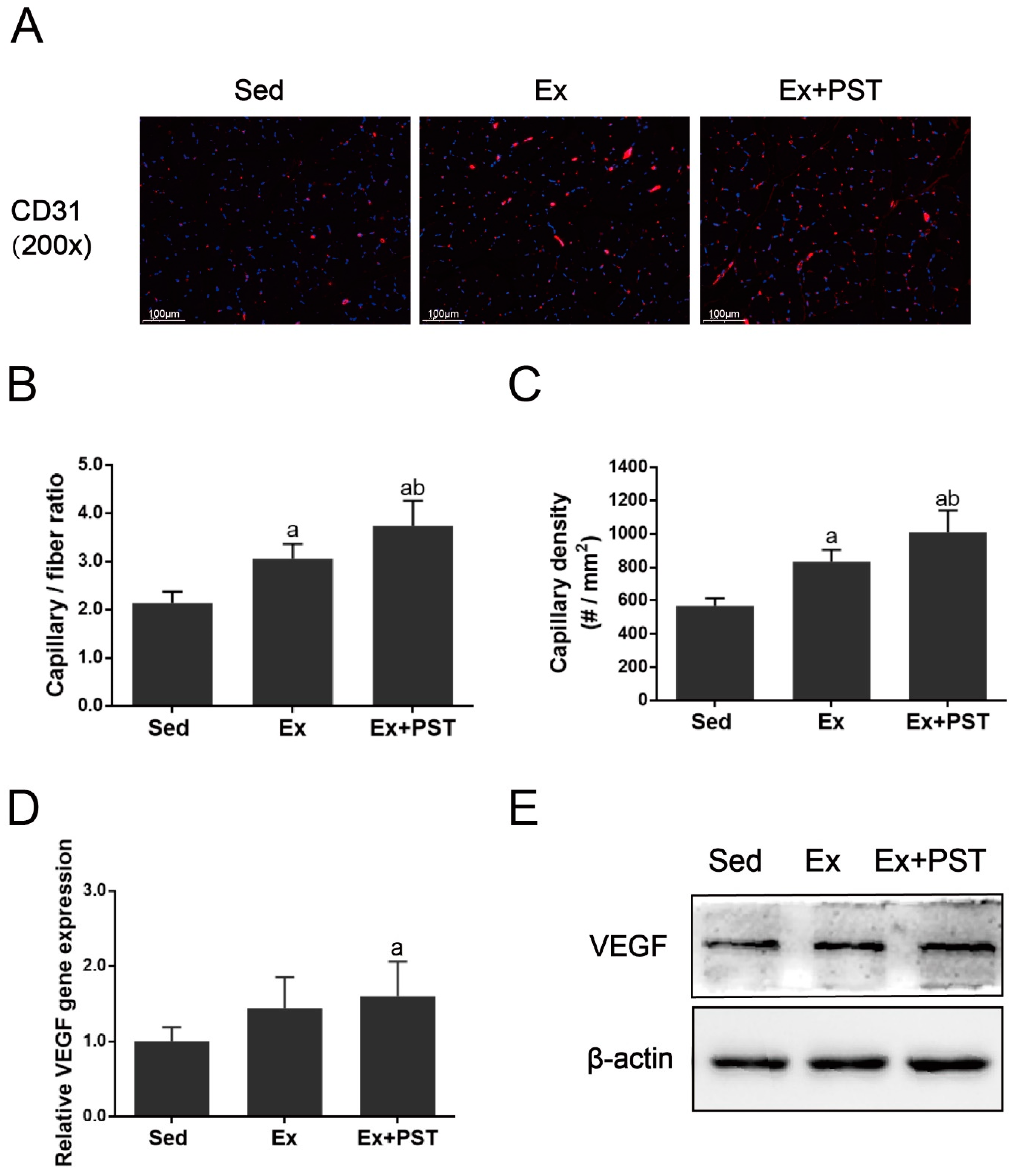
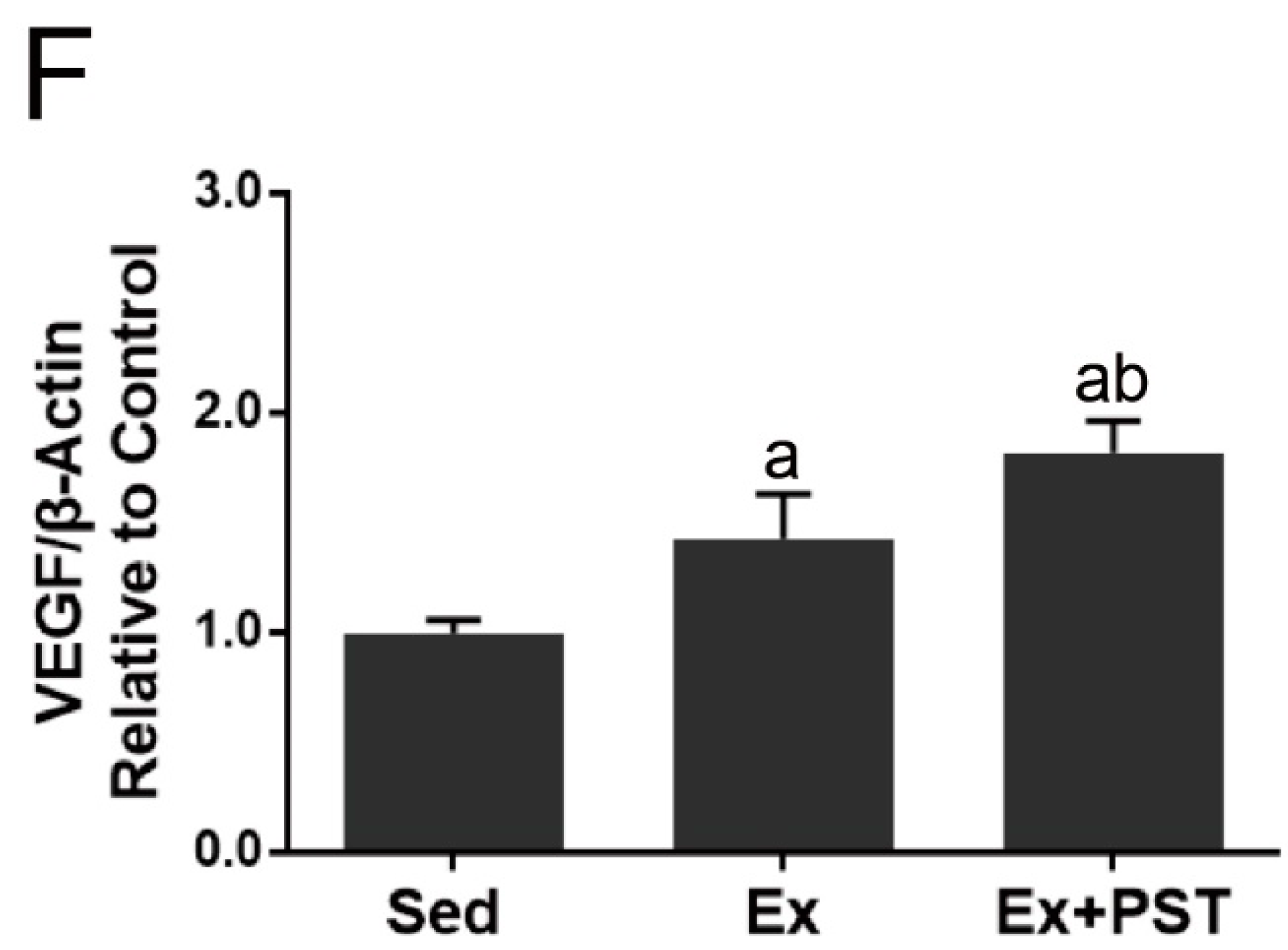
2.3. PST Enhances Muscular Angiogenesis in Exercise Training Rats
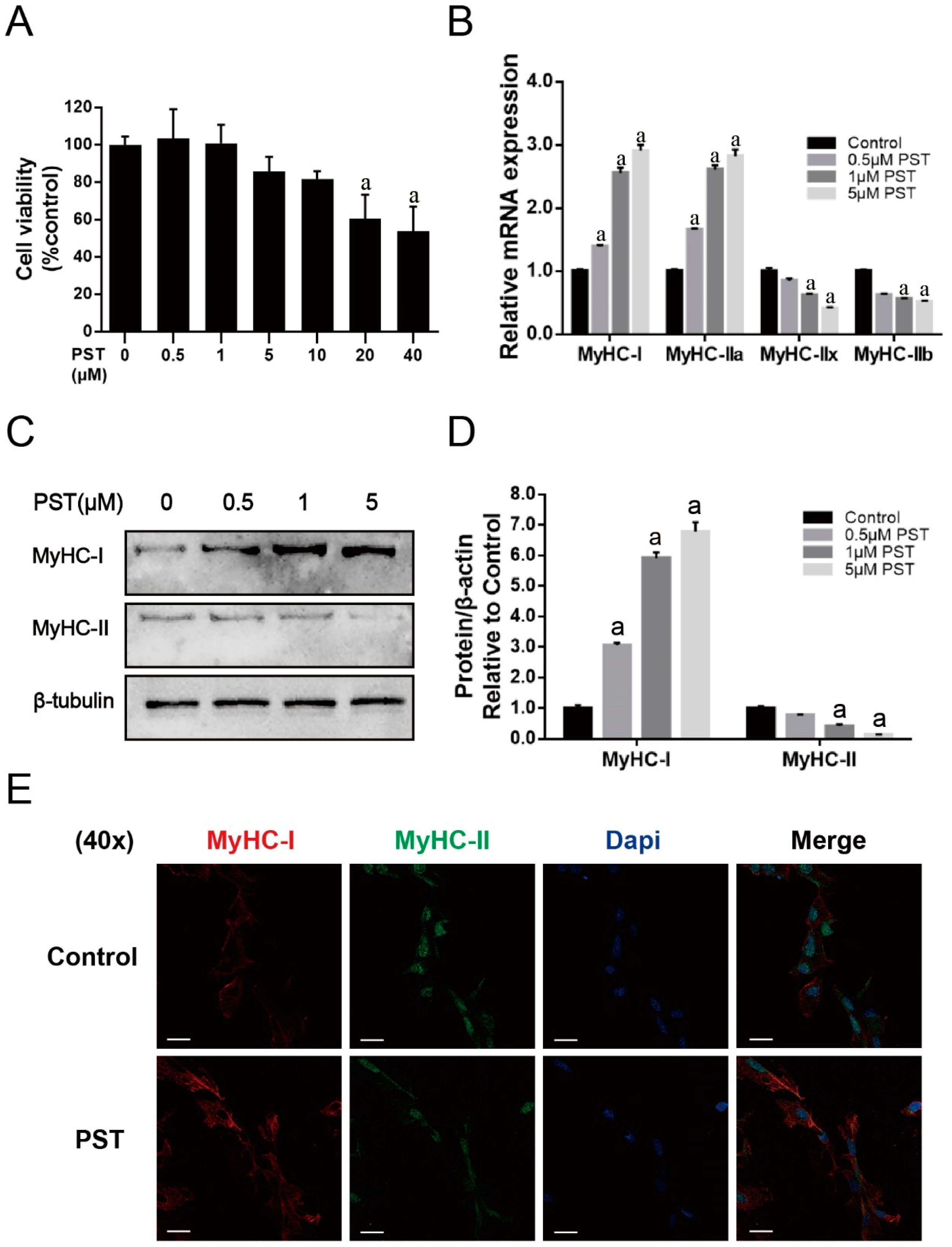
2.4. PST Promotes Slow-Twitch Fiber Formation in C2C12 Myotubes
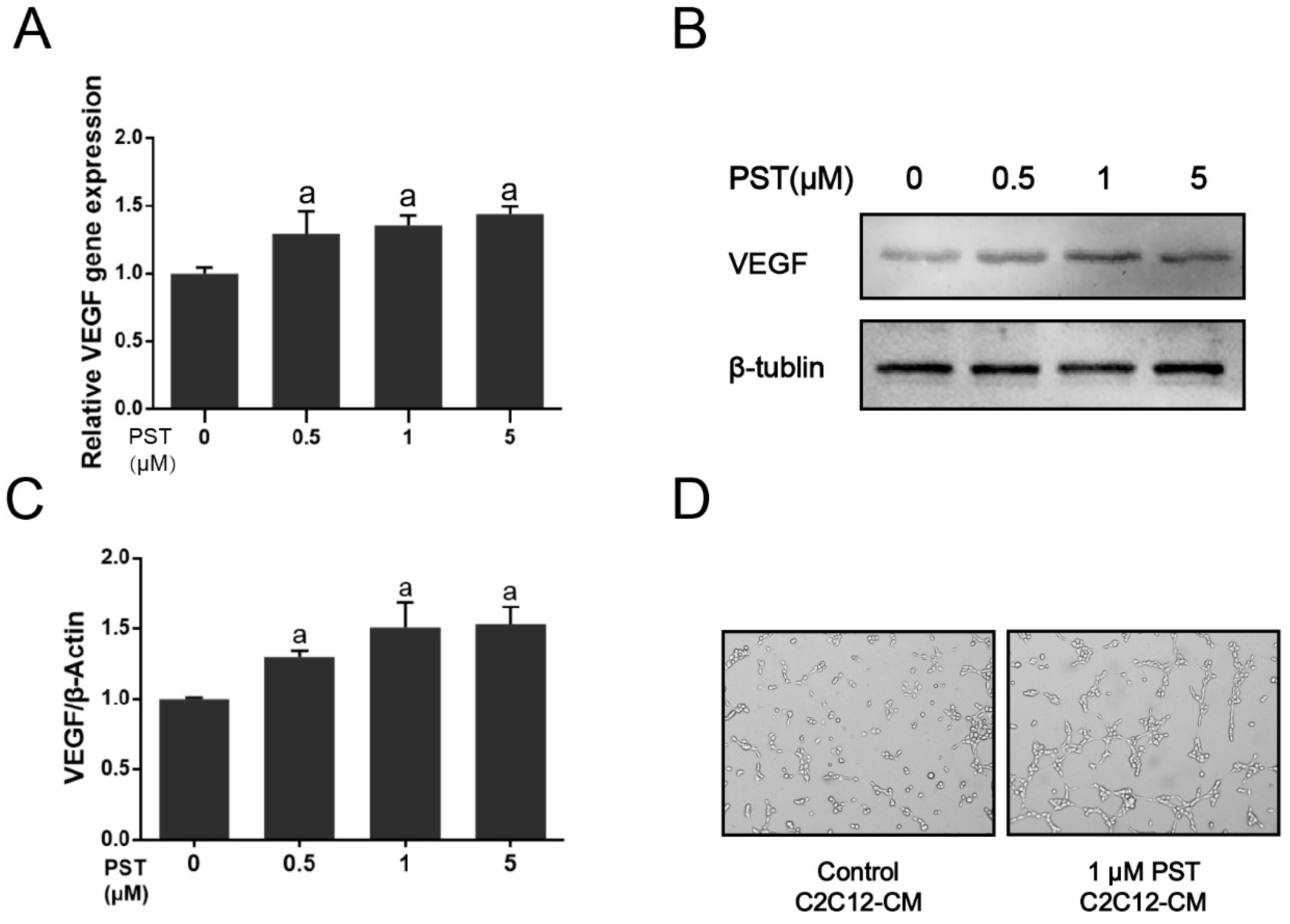
2.5. PST Increases VEGF Production in C2C12 Myotubes
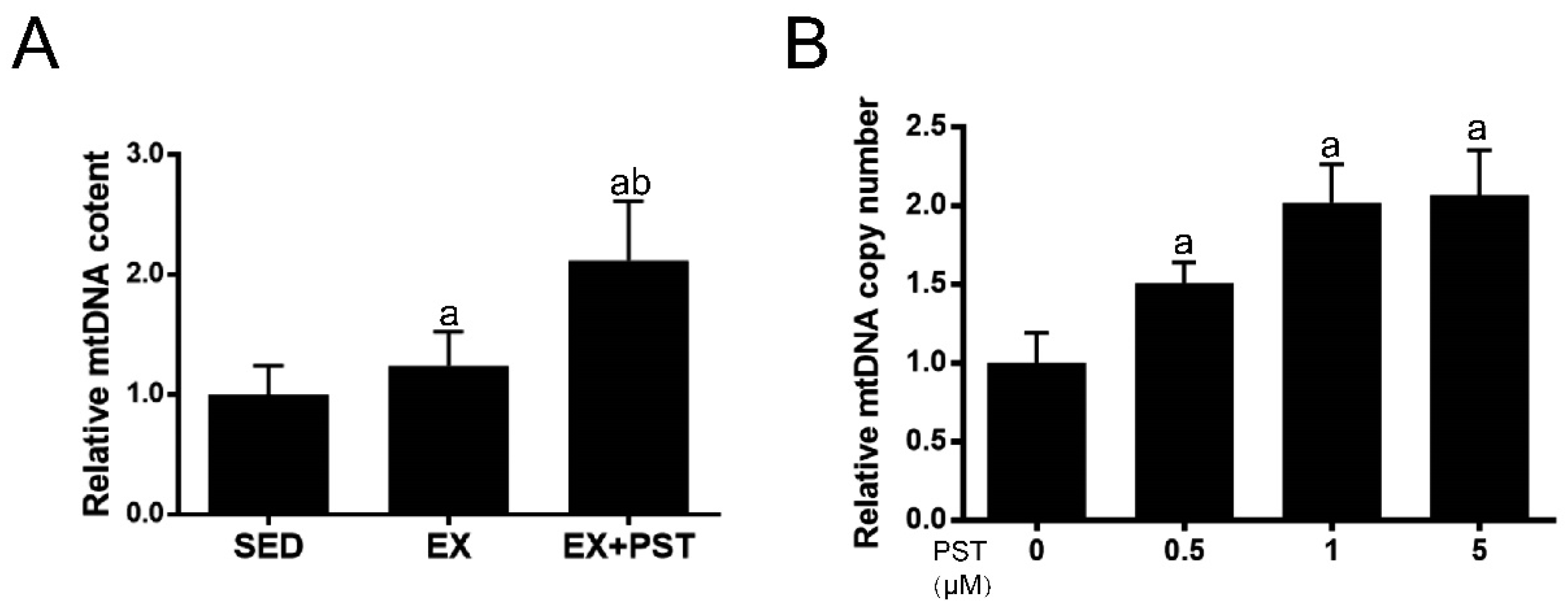
2.6. PST Increases Muscle mtDNA Copy Numbers Both In Vivo and In Vitro
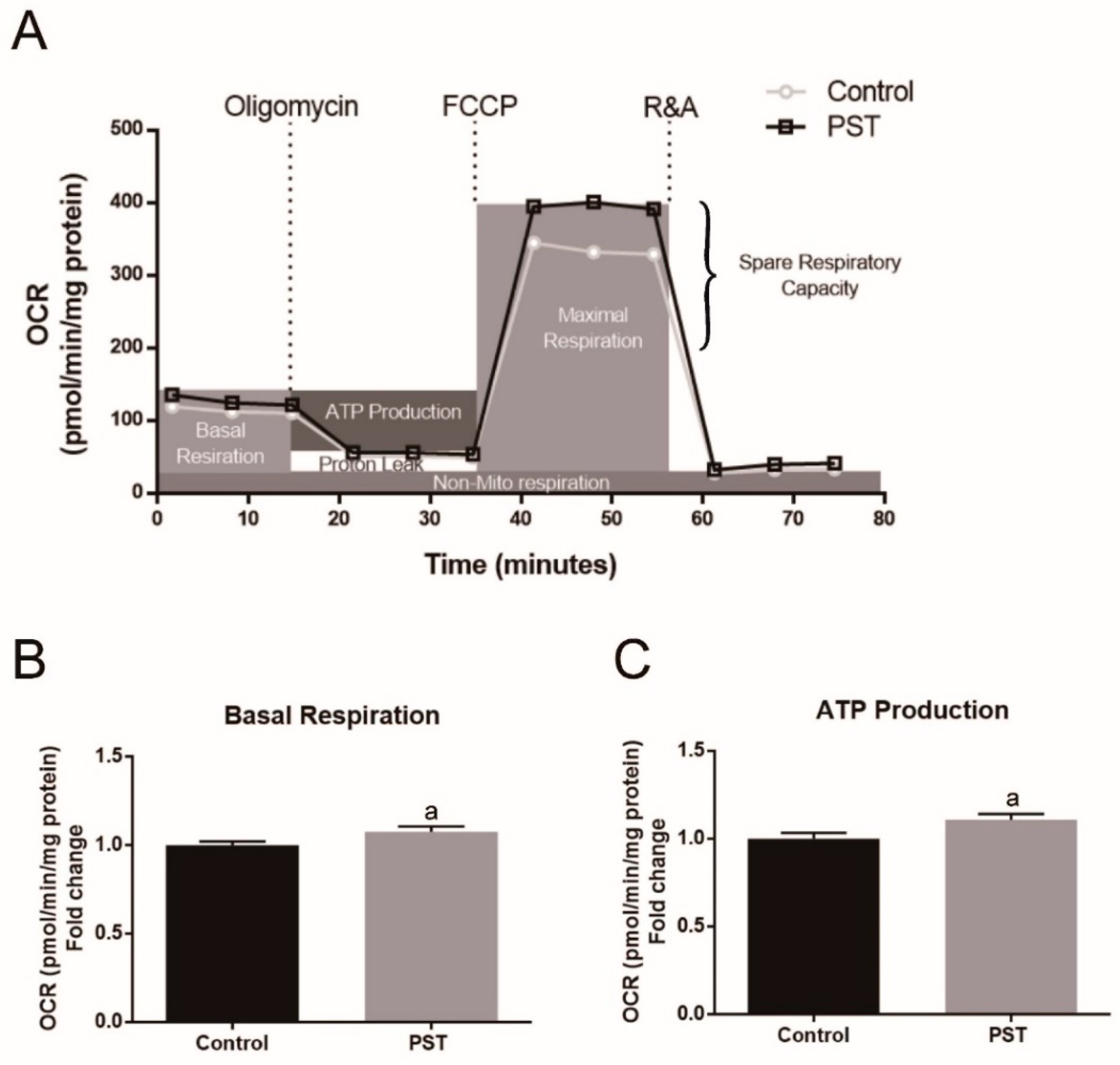
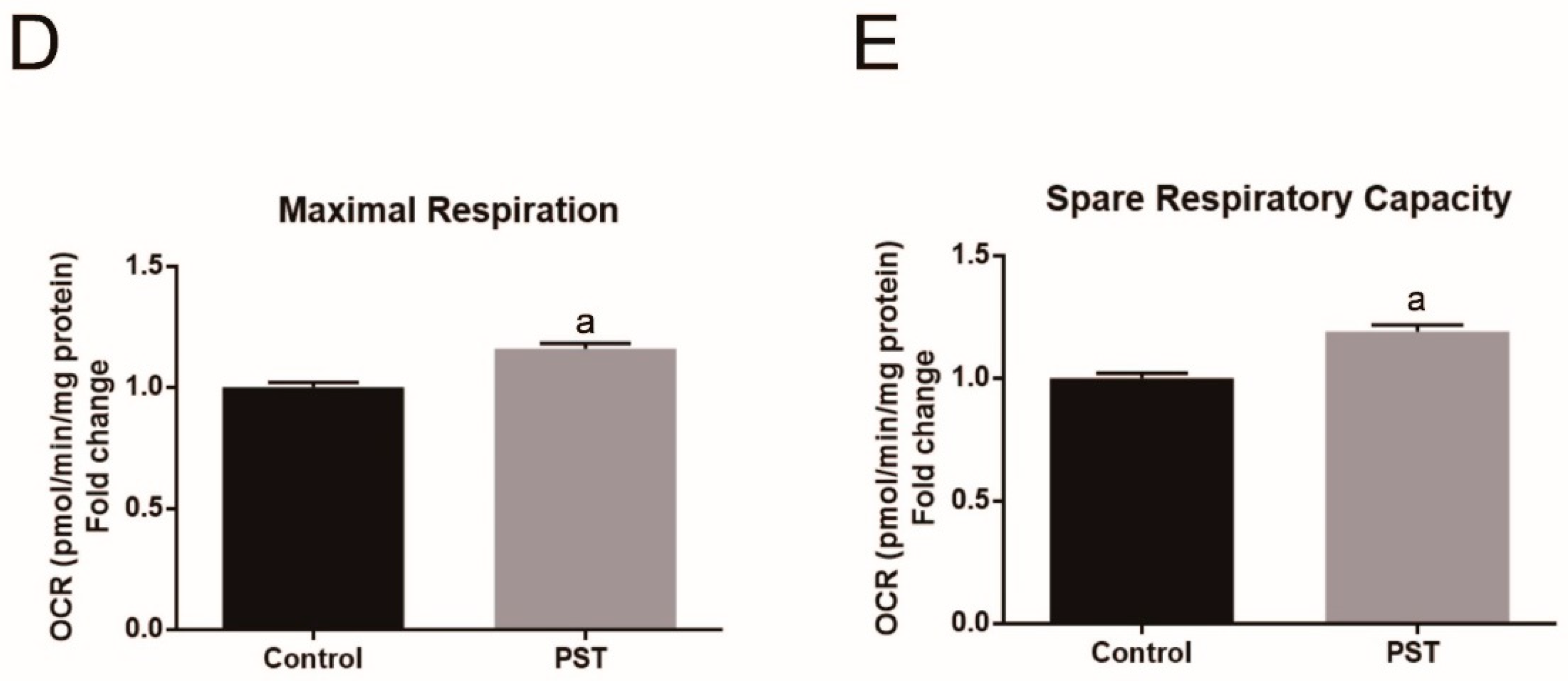
2.7. PST Enhances Mitochondrial Oxidative Metabolism in C2C12 Myotubes
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance
4.2. Exercise Training and Exercise Endurance Test
4.3. Cell Culture and Treatment
4.4. Cell Viability Assay
4.5. In Vitro Angiogenesis Assay
4.6. RNA Extraction, Reverse Transcription, and Real-Time PCR
4.7. Determination of Mitochondrial DNA Copy Number
4.8. Western Blot
4.9. Immunofluorescence Staining
4.10. Mitochondrial Function Assay Using the Seahorse XFp Analyzer
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camera, D.M.; Smiles, W.J.; Hawley, J.A. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic. Biol. Med. 2016, 98, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Donà, S.; Negri, C. Metabolic Effects of Exercise. Front Horm. Res. 2016, 47, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Evans, R.M. Exercise Mimetics: Impact on Health and Performance. Cell Metab. 2017, 25, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.S. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength. Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Mosole, S.; Carraro, U.; Kern, H.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Mayr, W.; Krenn, M.; Paternostro-Sluga, T.; et al. Long-term high-level exercise promotes muscle reinnervation with age. J. Neuropathol. Exp. Neurol. 2014, 73, 284–294. [Google Scholar] [CrossRef]
- Borina, E.; Pellegrino, M.A.; D’Antona, G.; Bottinelli, R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand. J. Med. Sci. Sports 2010, 20, 65–73. [Google Scholar] [CrossRef]
- Mu, L.; Sobotka, S.; Chen, J.; Su, H.; Sanders, I.; Adler, C.H.; Shill, H.A.; Caviness, J.N.; Samanta, J.E.; Beach, T.G. Altered pharyngeal muscles in Parkinson disease. J. Neuropathol. Exp. Neurol. 2012, 71, 520–530. [Google Scholar] [CrossRef]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Coenen-Schimke, J.M.; Rys, P.; Nair, K.S. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J. Appl. Physiol. (1985) 2005, 99, 95–102. [Google Scholar] [CrossRef]
- Hyatt, J.P.; Nguyen, L.; Hall, A.E.; Huber, A.M.; Kocan, J.C.; Mattison, J.A.; de Cabo, R.; LaRocque, J.R.; Talmadge, R.J. Muscle-Specific Myosin Heavy Chain Shifts in Response to a Long-Term High Fat/High Sugar Diet and Resveratrol Treatment in Nonhuman Primates. Front. Physiol. 2016, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, A.; Patel, K. Skeletal muscle fibre plasticity in response to selected environmental and physiological stimuli. Histol. Histopathol. 2009, 24, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Demirel, H.A.; Powers, S.K.; Naito, H.; Hughes, M.; Coombes, J.S. Exercise-induced alterations in skeletal muscle myosin heavy chain phenotype: Dose-response relationship. J. Appl. Physiol. (1985) 1999, 86, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.A.; Fulton, J.E.; Schoenborn, C.A.; Loustalot, F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am. J. Prev. Med. 2010, 39, 305–313. [Google Scholar] [CrossRef]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front. Physiol. 2015, 6, 296. [Google Scholar] [CrossRef]
- Hüttemann, M.; Lee, I.; Perkins, G.A.; Britton, S.L.; Koch, L.G.; Malek, M.H. (–)-Epicatechin is associated with increased angiogenic and mitochondrial signalling in the hindlimb of rats selectively bred for innate low running capacity. Clin. Sci. 2013, 124, 663–674. [Google Scholar] [CrossRef]
- Lee, I.; Huttemann, M.; Kruger, A.; Bollig-Fischer, A.; Malek, M.H. (−)-Epicatechin combined with 8 weeks of treadmill exercise is associated with increased angiogenic and mitochondrial signaling in mice. Front. Pharmacol. 2015, 6, 43. [Google Scholar] [CrossRef]
- Montesano, A.; Luzi, L.; Senesi, P.; Mazzocchi, N.; Terruzzi, I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J. Transl. Med. 2013, 11, 310. [Google Scholar] [CrossRef]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef]
- Xiao, N.N. Effects of Resveratrol Supplementation on Oxidative Damage and Lipid Peroxidation Induced by Strenuous Exercise in Rats. Biomol. Ther. (Seoul.) 2015, 23, 374–378. [Google Scholar] [CrossRef]
- Kan, N.W.; Ho, C.S.; Chiu, Y.S.; Huang, W.C.; Chen, P.Y.; Tung, Y.T.; Huang, C.C. Effects of Resveratrol Supplementation and Exercise Training on Exercise Performance in Middle-Aged Mice. Molecules 2016, 21, 661. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Ljubicic, V.; Burt, M.; Lunde, J.A.; Jasmin, B.J. Resveratrol induces expression of the slow, oxidative phenotype in mdx mouse muscle together with enhanced activity of the SIRT1-PGC-1alpha axis. Am. J. Physiol. Cell Physiol. 2014, 307, C66–C82. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, H.F.; Hughes, D.C.; Allan, R.; Deane, C.S.; Coxon, C.R.; Morton, J.P.; Stewart, C.E.; Sharples, A.P. The role of resveratrol on skeletal muscle cell differentiation and myotube hypertrophy during glucose restriction. Mol. Cell. Biochem. 2018, 444, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lin, H.S.; Ho, P.C.; Ng, K.Y. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef]
- Szekeres, T.; Fritzer-Szekeres, M.; Saiko, P.; Jager, W. Resveratrol and resveratrol analogues--structure-activity relationship. Pharm. Res. 2010, 27, 1042–1048. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Ho, C.T.; Chen, Y.K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3’-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Yan, Z.; Okutsu, M.; Akhtar, Y.N.; Lira, V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. (1985) 2011, 110, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, B.J.; Park, D.R.; Kim, U.H. Exercise induces muscle fiber type switching via transient receptor potential melastatin 2-dependent Ca(2+) signaling. J. Appl. Physiol. (1985) 2018, 124, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Leandro, C.G.; Da, S.R.W.; Dos, S.J.; Bento-Santos, A.; Lima-Coelho, C.H.; Falcao-Tebas, F.; Lagranha, C.J.; Lopes-de-Souza, S.; Manhaes-de-Castro, R.; Toscano, A.E. Moderate physical training attenuates muscle-specific effects on fibre type composition in adult rats submitted to a perinatal maternal low-protein diet. Eur. J. Nutr. 2012, 51, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Koziel, A.; Woyda-Ploszczyca, A.; Celichowski, J.; Jarmuszkiewicz, W. Endurance training increases the efficiency of rat skeletal muscle mitochondria. Pflug. Arch. 2016, 468, 1709–1724. [Google Scholar] [CrossRef]
- Ju, J.S.; Jeon, S.I.; Park, J.Y.; Lee, J.Y.; Lee, S.C.; Cho, K.J.; Jeong, J.M. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J. Physiol. Sci. 2016, 66, 417–430. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef]
- Yamashita, H.; Sato, N.; Yamamoto, M.; Gasa, S.; Izawa, T.; Komabayashi, T.; Ishikawa, M.; Sato, Y.; Ohno, H. Effect of endurance training on angiogenic activity in skeletal muscles. Pflug. Arch. 1993, 422, 332–338. [Google Scholar] [CrossRef]
- Murias, J.M.; Kowalchuk, J.M.; Ritchie, D.; Hepple, R.T.; Doherty, T.J.; Paterson, D.H. Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 957–964. [Google Scholar] [CrossRef]
- Baum, O.; Sollberger, C.; Raaflaub, A.; Odriozola, A.; Spohr, G.; Frese, S.; Tschanz, S.A. Increased capillary tortuosity and pericapillary basement membrane thinning in skeletal muscle of mice undergoing running wheel training. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef]
- Olfert, I.M.; Howlett, R.A.; Wagner, P.D.; Breen, E.C. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1059–R1067. [Google Scholar] [CrossRef] [PubMed]
- Delavar, H.; Nogueira, L.; Wagner, P.D.; Hogan, M.C.; Metzger, D.; Breen, E.C. Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R586–R595. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.B.; Richter, E.A.; Wojtaszewski, J.F. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J. Physiol. 2006, 574, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.; Sundelin, E.I.O.; Møller, A.B. AMP kinase in exercise adaptation of skeletal muscle. Drug Discov. Today 2014, 19, 999–1002. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Weihrauch, M.; Handschin, C. Pharmacological targeting of exercise adaptations in skeletal muscle: Benefits and pitfalls. Biochem. Pharmacol. 2018, 147, 211–220. [Google Scholar] [CrossRef]
- Niederberger, E.; King, T.S.; Russe, O.Q.; Geisslinger, G. Activation of AMPK and its Impact on Exercise Capacity. Sports Med. 2015, 45, 1497–1509. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Qian, Y.; Luo, J.; Leonard, S.S.; Harris, G.K.; Millecchia, L.; Flynn, D.C.; Shi, X. Hydrogen peroxide formation and actin filament reorganization by Cdc42 are essential for ethanol-induced in vitro angiogenesis. J. Boil. Chem. 2003, 278, 16189–16197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gu, Y.; Lang, H.; Wang, X.; Chen, K.; Gong, X.; Zhou, M.; Ran, L.; Zhu, J.; Mi, M. Dihydromyricetin prevents obesity-induced slow-twitch-fiber reduction partially via FLCN/FNIP1/AMPK pathway. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds pterostilbene are available from the authors. |
| Gene | Forward Primer (5′-3′), Reverse Primer (5′-3′) | Gene | Forward Primer (5′-3′), Reverse Primer (5′-3′) |
|---|---|---|---|
| R-MyHC-I | CAGTCATGGCGGATCGAGAG CGGATTCTCCGGTGATGAGG | M-MyHC-I | GCCTGGGCTTACCTCTCTATCAC CTTCTCAGACTTCCGCAGGAA |
| R-MyHC-IIa | GCGACAGACACCTCCTTCAAGAAC GTCCAGCCAGCCAGTGATGTTG | M-MyHC-IIa | AAGTGACTGTGAAAACAGAAGCA GCAGCCATTTGTAAGGGTTGAC |
| R-MyHC-IIx | GCGACAGACACCTCCTTCAAGAAC CCAGCCAGCCAGCGATGTTG | M-MyHC-IIx | GCGAATCGAGGCTCAGAACAA GTAGTTCCGCCTTCGGTCTTG |
| R-MyHC-IIb | CCATCACTGACGCCGCCATG GTTCTTCTTCATCCGCTCCAGGTG | M-MyHC-IIb | CTTGGTGGACAAACTACAGACT TGCAGAATTTATTTCCGTGAT |
| R-VEGFA | CAAGGCAGACTATTCAACGG GGCACGATTTAAGAGGGGAA | M-VEGFA | ACCCTGGCTTTACTGCTGTACCT TCATGGGACTTCTGCTCTCCTT |
| R-β-actin | CCACCATGTACCCAGGCATT CGGACTCATCGTACTCCTGC | M-β-actin | TGGAATCCTGTGGCATCCATGAAAC TAAAACGCAGCTCAGTAACAGTCCG |
| R-COX2 | TGAGCCATCCCTTCACTAGG GTTCATCCTGTTCCTGCTCC | M-COX2 | TTTTCAGGCTTCACCCTAGATGA GAAGAATGTTATGTTATGTTTACTCCTA |
| R-18s rRNA | CACGGGTGACGGGGAATCAG CGGGTCGGGAGTGGGTAATTTG | M-18s rRNA | TAGAGGGACAAGTGGCGTTC CGCTGAGCCAGTCAGTGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Liu, W.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M.; Zhu, J. Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats. Molecules 2020, 25, 186. https://doi.org/10.3390/molecules25010186
Zheng J, Liu W, Zhu X, Ran L, Lang H, Yi L, Mi M, Zhu J. Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats. Molecules. 2020; 25(1):186. https://doi.org/10.3390/molecules25010186
Chicago/Turabian StyleZheng, Jiawei, Wujian Liu, Xiaohui Zhu, Li Ran, Hedong Lang, Long Yi, Mantian Mi, and Jundong Zhu. 2020. "Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats" Molecules 25, no. 1: 186. https://doi.org/10.3390/molecules25010186
APA StyleZheng, J., Liu, W., Zhu, X., Ran, L., Lang, H., Yi, L., Mi, M., & Zhu, J. (2020). Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats. Molecules, 25(1), 186. https://doi.org/10.3390/molecules25010186




