Micelles Mediated Zone Fluidics Method for Hydrazine Determination in Environmental Samples
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of the ZF Method
2.1.1. Preliminary Studies
2.1.2. Effect of Temperature and Time
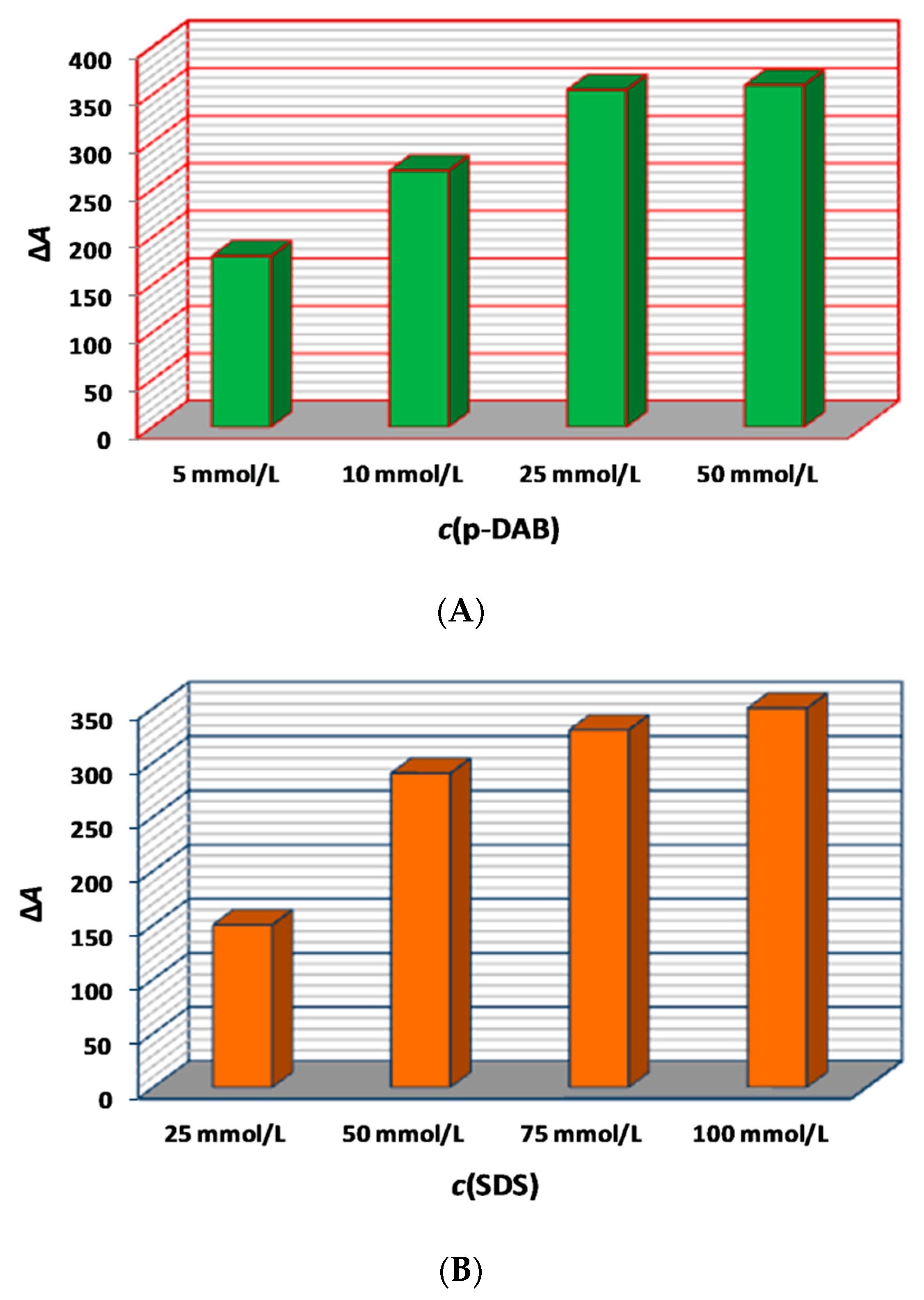
2.1.3. Effect of the Amount Concentrations of p-DAB and SDS
2.1.4. Effect of Sample and Reagent Volumes
2.2. Method Validation/Analytical Figures of Merit
2.3. Interferences and Matrix Effect
2.4. Analysis of Samples
3. Materials and Methods
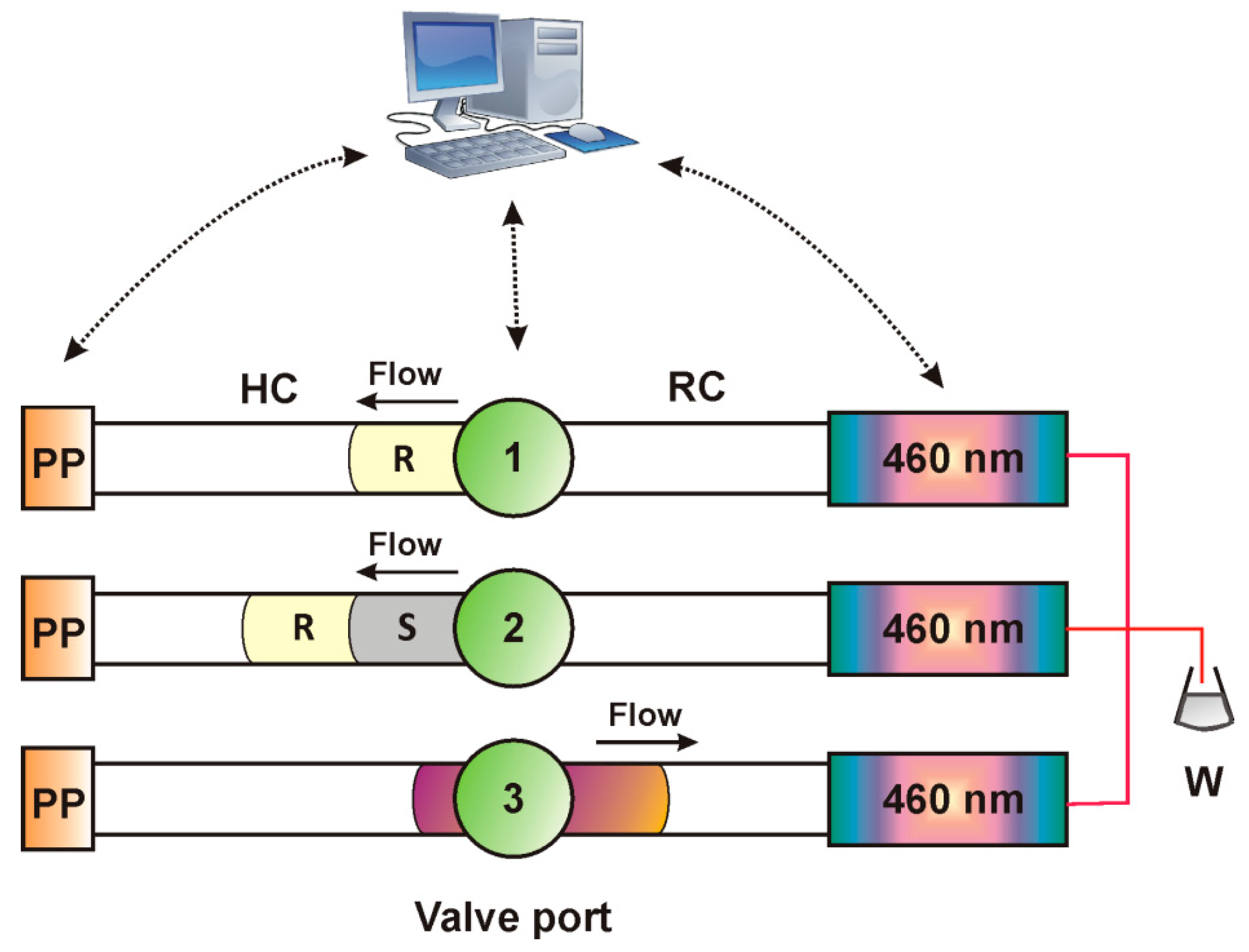
3.1. Instrumentation
3.2. Reagents and Solutions
3.3. Analytical Procedure
3.4. Preparation of Samples
3.5. UPLC-FL Corroborative Method
3.5.1. Instrumentation and Reagents
3.5.2. Derivatization and Chromatography
4. Conclusions
- (i)
- The reaction of p-DAB with hydrazine has been automated under zone fluidics conditions offering flow automation with minimum reagents consumption and waste generation.
- (ii)
- The highly acidic environment typical for p-DAB reactions has efficiently been replaced with micellar medium (SDS) with equal performance and figures of merit.
- (iii)
- The developed method offers adequate sensitivity and selectivity for the determination of hydrazine at the low micromolar level and an analysis throughput of 15 h−1.
- (iv)
- The results were validated by comparison to a UPLC-FL method based on pre-column derivatization with OPA.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SLomiak, K.; Lazarenkow, A.; Chęcińska, L.; Kusz, J.; Ochocki, J.; Nawrot-Modranka, J. Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-formylchromone. Molecules 2018, 23, 2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buecker, B. Water Treatment Recommendations. Available online: https://www.power-eng.com/1995/04/01/water-treatment-recommendations/#gref (accessed on 15 November 2019).
- Matsumoto, M.; Kano, H.; Suzuki, M.; Katagiri, T.; Umeda, Y.; Fukushima, S. Carcinogenicity and Chronic Toxicity of Hydrazine Monohydrate in Rats and Mice by Two-Year Drinking Water Treatment. Regul. Toxicol. Pharmacol. 2016, 76, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Smolenkov, A.D.; Rodin, I.A.; Shpigun, O.A. Spectrophotometric and Fluorometric Methods for the Determination of Hydrazine and its Methylated Analogues. J. Anal. Chem. 2012, 67, 98–113. [Google Scholar] [CrossRef]
- Smolenkov, A.D.; Shpigun, O. Direct Liquid Chromatographic Determination of Hydrazines: A Review. Talanta 2012, 102, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tzanavaras, P.D.; Themistokleous, S.; Zacharis, C.K. Automated Fluorimetric Determination of the Genotoxic Impurity Hydrazine in Allopurinol Pharmaceuticals Using Zone Fluidics and on-line Solid Phase Extraction. J. Pharm. Biomed. Anal. 2020, 177, 112887. [Google Scholar] [CrossRef] [PubMed]
- Teoman, İ.; Karakaya, S.; Dilgin, Y. Sensitive and Rapid Flow Injection Amperometric Hydrazine Sensor using an Electrodeposited Gold Nanoparticle Graphite Pencil Electrode. Anal. Lett. 2019, 52, 2041–2056. [Google Scholar] [CrossRef]
- Ayaz, S.; Dilgin, Y. Flow Injection Amperometric Determination of Hydrazine Based on its Electrocatalytic Oxidation at Pyrocatechol Violet Modified Pencil Graphite Electrode. Electrochim. Acta 2017, 258, 1086–1095. [Google Scholar] [CrossRef]
- Channon, R.B.; Joseph, M.B.; Bitziou, E.; Bristow, A.W.T.; Ray, A.D.; Macpherson, J.V. Electrochemical Flow Injection Analysis of Hydrazine in an Excess of an Active Pharmaceutical Ingredient: Achieving Pharmaceutical Detection Limits Electrochemically. Anal. Chem. 2015, 87, 10064–10071. [Google Scholar] [CrossRef]
- Barathi, P.; Kumar, A.S. Quercetin Tethered Pristine-Multiwalled Carbon Nanotube Modified Glassy Carbon Electrode as an Efficient Electrochemical Detector for Flow Injection Analysis of Hydrazine in Cigarette Tobacco Samples. Electrochim. Acta 2014, 135, 1–10. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Tai, C.-Y.; Lee, J.-F.; Chan, T.S.; Zen, J.-M. A nanostructured AuCu3 alloy electrode for highly sensitive detection of hydrazine at low potential in neutral medium. Electrochim. Acta 2013, 104, 104–109. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Yu, L. Flow Injection Spectrophotometric Determination of Hydrazine in Environmental Water Samples. Adv. Mater. Res. 2012, 396–398, 130–133. [Google Scholar] [CrossRef]
- Richard Prabakar, S.J.; Sriman Narayanan, S. Amperometric Determination of Hydrazine Using a Surface Modified Nickel Hexacyanoferrate Graphite Electrode Fabricated Following a New Approach. J. Electroanal. Chem. 2008, 617, 111–120. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Homayoonfard, M.; Taheri, A. Indirect Flow Injection Determination of Hydrazine Using PbO 2-packed Column. Chem. Anal. 2007, 52, 491–499. [Google Scholar]
- Pinter, J.S.; Brown, K.L.; DeYoung, P.A.; Peaslee, G.F. Amperometric Detection of Hydrazine by Cyclic Voltammetry and Flow Injection Analysis Using Ruthenium Modified Glassy Carbon Electrodes. Talanta 2007, 71, 1219–1225. [Google Scholar] [CrossRef]
- Yang, C.-C.; Kumar, A.S.; Kuo, M.-C.; Chien, S.-H.; Zen, J.-M. Copper-Palladium Alloy Nanoparticle Plated Electrodes for the Electrocatalytic Determination of Hydrazine. Anal. Chim. Acta 2005, 554, 66–73. [Google Scholar] [CrossRef]
- Sue, J.-W.; Kumar, A.S.; Chung, H.-H.; Zen, J.-M. Barrel Plating Rhodium Electrode: Application to Flow Injection Analysis of Hydrazine. Electroanalysis 2005, 17, 1245–1250. [Google Scholar] [CrossRef]
- Du, J.; Lu, J. Hydrazine-Induced Post-Chemiluminescence Phenomenon of Permanganate-Luminol Reaction and its Applications. Luminescence 2004, 19, 328–332. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Chamjangali, M.A. Flow Injection Spectrophotometric Determination of Trace Amounts of Hydrazine by the Inhibition of the Pyrogallol Red-Iodate Reaction. J. Anal. Chem. 2004, 59, 129–133. [Google Scholar] [CrossRef]
- Watt, G.W.; Chrisp, J.D. A Spectrophotometric Method for the Determination of Hydrazine. Anal. Chem. 1952, 24, 2006–2008. [Google Scholar] [CrossRef]
- Gamble, S.D. Photometric Analysis of Trace Amounts of Hydrazine with p-dimethylaminobenzaldehyde. Chemical Equilibria. Can. J. Chem. Part II 1968, 46, 1365–1371. [Google Scholar] [CrossRef]
- Basson, W.D.; Van Staden, J.F. Low-level Determination of Hydrazine in Boiler Feed Water with an Unsegmented High-Speed Continuous-Flow System. Analyst 1978, 103, 998–1001. [Google Scholar] [CrossRef]
- Hach Company. Hydrazine Method 8141; DOC316.53.01046; Hach Company: Loveland, CO, USA, 2019. [Google Scholar]
- Cid, A.; Moldes, O.A.; Mejuto, J.C.; Simal-Gandara, J. Interaction of Caffeic Acid with SDS Micellar Aggregates. Molecules 2019, 24, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veisi, H.; Azadbakht, R.; Ezadifar, M.; Hemmati, S. An Efficient and Green Procedure for Synthesis of Pyrrole Derivatives by Paal-Knorr Condensation Using Sodium Dodecyl Sulfate in Aqueous Micellar. J. Heterocycl. Chem. 2013, 50, E241–E246. [Google Scholar] [CrossRef]
- Hernández Torres, M.A.; Khaledi, M.G.; Dorsey, J.G. Micellar-Catalyzed Reactions for Flow-Injection Systems. Determination of Pyridoxal. Anal. Chim. Acta 1987, 201, 67–76. [Google Scholar] [CrossRef]
- Czernel, G.; Matwijczuk, A.; Karcz, D.; Górecki, A.; Niemczynowicz, A.; Szcześ, A.; Gładyszewski, G.; Matwijczuk, A.; Gładyszewska, B.; Niewiadomy, A. Spectroscopic Studies of Dual Fluorescence in 2-(4-fluorophenylamino)-5-(2,4-dihydroxybenzeno)-1,3,4-thiadiazole: Effect of Molecular Aggregation in a Micellar System. Molecules 2018, 23, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.; Huo, Y.; Yang, L.; Yang, X.; He, Y.; Wu, J. Determination of Trace Nickel in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Mixed Micelle-Mediated cloud Point Extraction. Molecules 2018, 23, 2597. [Google Scholar] [CrossRef] [Green Version]
- De la Guardia, M.; Peris-Cardells, E. Use of Flow Injection in the Study of Micellar Interactions. Anal. Chim. Acta 1992, 256, 225–230. [Google Scholar] [CrossRef]
- Memon, N.; Tzanavaras, P.D. Utilization of Organized Surfactant Assemblies as Solvents in Flow Injection Analysis with Emphasis to Automated Derivatization of Organic Analytes. Curr. Anal. Chem. 2014, 10, 338–348. [Google Scholar] [CrossRef]
- Afkhami, A.; Zarei, A.R. Simultaneous Spectrophotometric Determination of Hydrazine and Phenylhydrazine based on their Condensation Reactions with Different Aromatic Aldehydes in Micellar Media Using H-Point Standard Addition Method. Talanta 2004, 62, 559–565. [Google Scholar] [CrossRef]
- Tzanavaras, P.D.; Papadimitriou, S.; Zacharis, C.K. Automated Stopped-Flow Fluorimetric Sensor for Biologically Active Adamantane Derivatives Based on Zone Fluidics. Molecules 2019, 24, 3975. [Google Scholar] [CrossRef] [Green Version]
- Martinek, K.; Yatsimirski, A.K.; Osipov, A.P.; Berezin, I.V. Micellar Effects on Kinetics and Equilibrium of Synthesis and Hydrolysis of Benzylideneaniline. A General Kinetic Conception of Micellar Catalysis. Tetrahedron 1973, 29, 963–969. [Google Scholar] [CrossRef]
- Yatsimirskaya, N.T.; Sosnovskaya, I.N.; Yatsimirsky, A.K. Spectrophotometric Determination of 6-Aminopenicillanic and 7- aminocephalosporanic Acids as the Schiff Bases with para- dimethylaminobenzaldehyde in the Presence of Sodium Dodecyl Sulfate Micelles. Anal. Biochem. 1995, 229, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Matsuda, R.; Ito, K.; Nishimura, W.; Imai, K.; Maeda, M. Detection Limit Estimated from Slope of Calibration Curve: An Application to Competitive ELISA. Anal. Sci. 2005, 21, 167–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzanavaras, P.D.; Themelis, D.G. Development and Validation of a High-Throughput High-Performance Liquid Chromatographic Assay for the Determination of Caffeine in Food Samples Using a Monolithic Column. Anal. Chim. Acta 2007, 581, 89–94. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds mentioned in the text are not available from the authors but they can be purchased from the manufacturers mentioned in the “reagents and solutions” section. |

| Calibration Curve | Slope | SD | Matrix Effect (%) a |
|---|---|---|---|
| Aqueous | 85.6 | 0.6 | – |
| Tap Water 1 | 81.1 | 0.8 | −5.3 |
| Tap Water 2 | 81.6 | 0.8 | −4.7 |
| Mineral Water 1 | 88.9 | 1.1 | +3.9 |
| Mineral Water 2 | 89.9 | 1.0 | +5.0 |
| Table Water 1 | 89.5 | 1.1 | +4.6 |
| Table Water 2 | 80.6 | 0.9 | −5.8 |
| Boiler water 1 | 91.9 | 1.6 | +7.4 |
| Boiler water 2 | 79.1 | 1.3 | −7.6 |
| Sample | Hydrazine Found (μmol L−1) | UPLC-FL |
|---|---|---|
| Boiler water 1 | 2.1 | 1.9 |
| Boiler water 2 | 2.8 | 2.5 |
| Tap Water 1 | N.D. | N.D. |
| Tap Water 2 | N.D. | N.D. |
| Mineral Water 1 | N.D. | N.D. |
| Mineral Water 2 | N.D. | N.D. |
| Table Water 1 | N.D. | N.D. |
| Table Water 2 | N.D. | N.D. |
| Sample | Hydrazine Added (μmol L−1) | % Recovery ZF | % Recovery UPLC-FL |
|---|---|---|---|
| Boiler water 1 | 1.0 | 86 | 92 |
| 2.0 | 88 | 102 | |
| 5.0 | 92 | 95 | |
| Boiler water 2 | 1.0 | 82 | 94 |
| 2.0 | 110 | 89 | |
| 5.0 | 99 | 106 | |
| Tap Water 1 | 1.0 | 114 | 86 |
| 2.0 | 91 | 89 | |
| 5.0 | 92 | 89 | |
| Tap Water 2 | 1.0 | 108 | 96 |
| 2.0 | 96 | 99 | |
| 5.0 | 110 | 91 | |
| Mineral Water 1 | 1.0 | 89 | 92 |
| 2.0 | 86 | 109 | |
| 5.0 | 87 | 106 | |
| Mineral Water 2 | 1.0 | 102 | 108 |
| 2.0 | 95 | 110 | |
| 5.0 | 112 | 103 | |
| Table Water 1 | 1.0 | 111 | 91 |
| 2.0 | 84 | 84 | |
| 5.0 | 85 | 113 | |
| Table Water 2 | 1.0 | 109 | 91 |
| 2.0 | 113 | 90 | |
| 5.0 | 95 | 108 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakosta, T.D.; Christophoridis, C.; Fytianos, K.; Tzanavaras, P.D. Micelles Mediated Zone Fluidics Method for Hydrazine Determination in Environmental Samples. Molecules 2020, 25, 174. https://doi.org/10.3390/molecules25010174
Karakosta TD, Christophoridis C, Fytianos K, Tzanavaras PD. Micelles Mediated Zone Fluidics Method for Hydrazine Determination in Environmental Samples. Molecules. 2020; 25(1):174. https://doi.org/10.3390/molecules25010174
Chicago/Turabian StyleKarakosta, Theano D., Christophoros Christophoridis, Konstantinos Fytianos, and Paraskevas D. Tzanavaras. 2020. "Micelles Mediated Zone Fluidics Method for Hydrazine Determination in Environmental Samples" Molecules 25, no. 1: 174. https://doi.org/10.3390/molecules25010174
APA StyleKarakosta, T. D., Christophoridis, C., Fytianos, K., & Tzanavaras, P. D. (2020). Micelles Mediated Zone Fluidics Method for Hydrazine Determination in Environmental Samples. Molecules, 25(1), 174. https://doi.org/10.3390/molecules25010174







