Abstract
Discovering new natural resources of polyphenols is the aim of many recent studies in the field of natural product research. This study tentatively investigated the polyphenols profile of the stems of seven Mammillaria species (M. rhodantha, M. spinosissima, M. hahniana, M. crucigera, M. candida, M. albilanata, and M. muehlenpfordtii) using high performance liquid chromatography with DAD detector (HPLC-DAD) method. Furthermore, the anti-cancer, anti-oxidant, and anti-bacterial potentials of these extracts as well as major identified phenols were explored. The HPLC-DAD study confirmed the availability of six phenolic acids, including gentisic acid, chlorogenic acid, caffeic acid, protocatechuic acid, sinapic acid, and p-hydroxybenzoic acid. The dominant compounds were: gentisic acid in M. rhodantha and M. spinosissima; chlorogenic acid in M. muehlenpfordtii, M. crucigera, and M. rhodantha; and caffeic acid in M. rhodantha, M. crucigera, and M. spinosissima. Stems of Mammillaria sp. showed antiproliferative effects against HeLa, MCF-7, and Jurkat cells. In HeLa and MCF-7 cells, the best antiproliferative activities were found in the treatments with M. rhodantha, M. spinosissima, and M. muehlenpfordtii. The apoptotic assay of M. rhodantha, M. spinosissima, and M. muehlenpfordtii showed accumulation of necrotic cells in the early and late apoptotic phase. M. rhodantha, M. spinosissima, and M. muehlenpfordtii showed the highest anti-oxidant activities using 2,2-diphenyl-1-picrylhydrazyl (DPPH), β-carotene bleaching, and ferric reducing anti-oxidant power (FRAP) assays. M. rhodantha was the best source of antioxidants. Mammillaria sp. showed moderate anti-bacterial effects against bacteria and the highest effects were found using the extracts of M. rhodantha, M. spinosissima, M. crucigera and M. muehlenpfordtii against most bacteria. The anti-bacterial activities were attributed to other phenolic compounds (e.g., chlorogenic acid) than gentisic acid, which was not active against most bacteria. Mammillaria sp. could be considered to be an important natural source of phenolic acids with anti-cancer, anti-bacterial, and anti-oxidant activities.
Keywords:
Mammillaria; stem extract; phenolic acids; anti-cancer; anti-oxidant; anti-bacterial; cytotoxicity 1. Introduction
Polyphenols are commonly found in cereals, fruit, vegetables, and beverages. However, other resources are being explored such as tree barks [1,2] and fungi [3]. Polyphenols play an important role in controlling several diseases including cancer, diabetes, cardiovascular diseases, osteoporosis, neurodegenerative diseases, hypertension, and asthma, and act as antiaging compounds [4]. The application of polyphenols as anti-cancer is mainly attributed to their protective effects and they act as inhibitors for tumor growth and dissemination. The mechanism of action of polyphenols on tumors include antiproliferation, apoptosis, stimulating cell cycle arrest, anti-oxidant mechanism, and induction of detoxification enzymes, molecular regulation of cancer related genes, and anti-inflammatory activity [5,6,7]. One of the most important groups of plant polyphenols are flavonoids and phenolic acids. Some phenolic acids are recognized as strong anti-oxidant, anti-cancer, and antimicrobial metabolites [2,8,9,10,11,12].
Discovering new resources of antioxidants is one of the main objectives of natural product research investigations worldwide. Consumption of polyphenols is strongly associated with reduced levels of lymphocytic DNA oxidative damage; they protect cells and limit the risk of associated degenerative diseases. The anti-cancer effects of polyphenols are closely associated with its anti-oxidant activity. Polyphenols inhibit protein kinase C, cyclooxygenase, hydroperoxidase, Akt, focal adhesion kinase, NFκB, and Bcl-2 phosphorylation [1,13,14,15,16]. Polyphenols as plant secondary metabolites play a pivotal role in control bacterial infections both in plants and humans [17,18,19]. They show strong antimicrobial activities on human pathogenic bacteria by applying several mechanisms including cytoplasmic membrane destabilization, increasing cell membrane permeability, inhibiting microbial enzymes, influencing the metabolism of bacteria, and deprivation of essential minerals [20].
The Mammillaria genus is the largest in its family (Cactaceae), and contains more than 200 species of globose or ball-shaped cacti that carry small spines and flowers. These plants are native to the Western Hemisphere and especially Mexico. The family Cactaceae has some medicinal genera such as Opuntia sp. which are used as food and has important economic value as horticultural crop [21]. Mammillaria sp. are commonly used for the decoration and ornamenting gardens all over the world. However, no studies revealed the phenolic composition and biological activities of the extracts of any species of Mammillaria. Common species such as M. rhodantha, M. spinosissima, M. hahniana, M. crucigera, M. candida, M. albilanata, and M. muehlenpfordtii are widely used in spring flower festival decoration in cactus or desert gardens. They are decorated with plastic red and yellow synthetic flowers, because they rarely flower under the natural conditions which usually do not simulate their natural habitat. However, their natural flowers have different colors and are borne in rings around the column. The polyphenolic profile and biological activities of this genus has not been studied before.
The Mammillaria genus is the most common genus used in the Cactaceae family in ornamenting gardens; however, experimental studies regarding the bioactivity of stem extracts is limited. In this study, the polyphenol profiles (aimed on chosen flavonoids and phenolic acids) of seven Mammillaria cacti were evaluated qualitatively and quantitatively using HPLC-DAD method for the first time. Furthermore, the anti-cancer, anti-oxidant, and anti-bacterial properties were explored.
2. Results
2.1. Polyphenol Profiling of Mammillaria
Seven species from Mammillaria genus—M. rhodantha, M. spinosissima, M. hahniana, M. crucigera, M. candida, M. albilanata, and M. muehlenpfordtii—were tested tentatively with HPLC-DAD method to detect chosen phenolic acids and flavonoids. In the stem extracts, seven phenolic acids were identified: gentisic acid, chlorogenic acid, caffeic acid, protocatechuic acid, sinapic acid, and p-hydroxybenzoic acid (Table 1 and Figure 1) out of the 21 screened. None of 14 tested flavonoids were confirmed. The dominant compounds were: gentisic acid in M. spinosissima (40.44 mg 100 g−1 DW) and M. rhodantha (38.27 mg 100 g−1 DW); chlorogenic acid in M. muehlenpfordtii (30.88 mg 100 g−1 DW), M. crucigera (14.61 mg 100 g−1 DW), M. candida (11.61 mg 100 g−1 DW) and M. rhodantha (10.37 mg 100 g−1 DW); and caffeic acid in M. rhodantha (15.80 mg 100g−1 DW), M. crucigera (7.17 mg 100 g−1 DW), and M. spinosissima (9.66 mg 100 g−1 DW). Protocatechuic acid, sinapic acid, and p-hydroxybenzoic acid were estimated in relatively lower amounts in all studied species. Out of the studied Mammillaria species, M. rhodantha and M. spinosissima could be considered to be the richest source of the studied phenolic acids (Table 1 and Figure 1).

Table 1.
The quantitative (mg 100 g−1 DW ± SD) estimations of phenolic acids in Mammillaria sp. stem extracts.
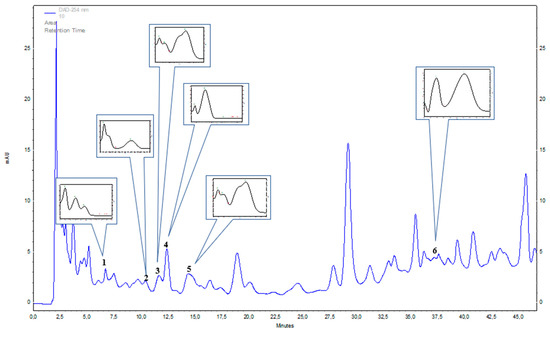
Figure 1.
Representative HPLC-DAD (λ = 254 nm) chromatogram of Mammillaria pringlei stem extracts: 1—protocatechuic acid, 2—gentisic acid, 3—chlorogenic acid, 4—p-hydroxybenzoic acid, 5—caffeic acid, 6—sinapic acid.
2.2. Anti-Cancer Effects
Stems of Mammillaria sp. showed antiproliferative effects against selected cancer cells as shown in Table 2. The highest activities were against HeLa, MCF-7and Jurkat cells. In HeLa and MCF-7 cells, the best antiproliferative activities were found in the treatments with M. rhodantha, M. spinosissima, and M. muehlenpfordtii. The only anti-cancer activity against HT-29 was found in the extracts of M. rhodantha and M. spinosissima. The best anti-cancer effects against Jurkat were found in the treatments of M. rhodantha and M. spinosissima. Gentisic acid showed comparable activities to those of M. rhodantha and M. spinosissima. The apoptotic assay of M. rhodantha, M. spinosissima, and M. muehlenpfordtii showed accumulation of necrotic cells in the early and late apoptotic (Figure 2).

Table 2.
Antiproliferative activity [IC50 (µg mL−1)] of Mammillaria sp. stem extracts (mg mL−1) as well as gentisic acid on cancer cells.
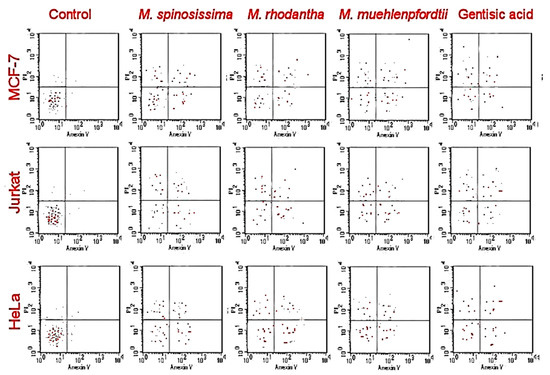
Figure 2.
Apoptotic cell population (IC50) using flow cytometry.
2.3. Anti-oxidant Effects
Mammillaria sp. showed obvious anti-oxidant effects by several methods (Table 3). M. rhodantha, M. spinosissima, and M. muehlenpfordtii showed the highest anti-oxidant activities using DPPH, β-carotene bleaching, and ferric reducing anti-oxidant power (FRAP) assays compared to other species. M. rhodantha was best the source of antioxidants as revealed by lowest IC50. Furthermore, butylated hydroxytoluene (BHT) and Trolox showed higher activities than all stem extracts.

Table 3.
DPPH and β-carotene bleaching acid of Mammillaria sp. stem extracts as well as gentisic acid.
2.4. Anti-Bacterial Activities
Mammillaria sp. showed moderate anti-bacterial effects against all studied bacteria: Bacillus cereus, Listeria monocytogenes, Escherichia coli, Micrococcus flavus, Staphylococcus aureus, and Pseudomonas aeruginosa (Table 4). The highest effects were found using the extracts of M. rhodantha, M. spinosissima, M. crucigera, and M. muehlenpfordtii against most bacteria. Other Mammillaria sp. showed much lower anti-bacterial effects. The gentisic acid did not show activities against most bacteria. The chlorogenic acid showed high anti-bacterial effects which is comparable to antibiotics.

Table 4.
Minimum inhibitory (MIC) and bactericidal concentration (MBC) of Mammillaria sp. stem extracts (mg mL−1) as well as gentisic and chlorogenic acids.
3. Discussion
The seven Mammillaria species—M. rhodantha, M. spinosissima, M. hahniana, M. crucigera, M. candida, M. albilanata, and M. muehlenpfordtii—under HPLC-DAD qualitative and quantitative study showed that six phenols were present in all the species, which are gentisic acid, chlorogenic acid, caffeic acid, protocatechuic acid, sinapic acid, and p-hydroxybenzoic acid. This is the first report on the tentative phenolic composition of the stems of this genus. Indeed, further analyses with more chromatographic techniques would be recommended, for proper confirmation of detected compounds. Previous investigation revealed that the pollen of Mammillaria heyderi Sensu Lato contained quercetin, herbacetin glycoside derivatives, and kaempferol [22]. Under our study we did not detect quercetin or kaempferol in the studied steam extracts. The major phenol in M. rhodantha and M. spinosissima was gentisic acid. Gentisic acid was discovered in a few species such as Momordica charantia leaves (12.75 mg g−1) [23] and Vaccinium oxyccocos fruit (0.3 mg 100 g−1) [24] and is rare in general. Gentisic acid is a strong anti-oxidant and has important health benefits [25,26]. Chlorogenic acid is the ester of caffeic acid and is much more common than gentisic acid. Chlorogenic acid was isolated from different plants species such as Etlingera elatior leaves, coffee, and fruit [27] as well as medicinal plants [17]. The chlorogenic acid is known for strong its anti-oxidant, anti-cancer, anti-inflammatory, and antiviral properties, and modulates the anti-oxidant enzyme activities [28,29]. Protocatechuic acid, sinapic acid and p-hydroxybenzoic acid were relatively low but they are considered to be important anti-oxidant and anti-cancer compounds [30,31,32].
Mammillaria species showed antiproliferative effects against cancer cells. Most activities were against HeLa and MCF-7 cells using M. rhodantha, M. spinosissima, and M. muehlenpfordtii stem extracts. The three species are rich in specific phenolic compounds such gentisic acid, which is dominant in M. rhodantha and M. spinosissima, and chlorogenic acid, which is dominant in M. muehlenpfordtii. Gentisic acid showed comparable antiproliferative and cytotoxic activities to those of M. rhodantha and M. spinosissima. Gentisic acid is a quinonoid phenolic acid that is important for cancer prevention and treatment. These phenols activate the brain chemotherapeutics that help in the reduction of brain tumors [33] and has anti-oxidant activities related to the control of HCT-116 cancer cells [34]. In M. muehlenpfordtii there were strong antiproliferative activities against different cancer cells, which is explained by the activity of the major phenol found, which is chlorogenic acid. This phenol has anti-cancer activities on liver, colorectal, and laryngeal cancer. It influences the expression of specific genes such as GSK-3β and APC (up-regulation) and β-catenin (down-regulation) which promote the apoptosis of tumor cells [35]. The apoptotic activity of protocatechuic acid has been reported on human gastric carcinoma [36]. Sinapic and caffeic acids have been related to anti-cancer activities. Sinapic acid is considered to be a hydroxycinnamic acid derivative which up- and down-regulate specific genes in PC-3 and LNCaP prostate cancer cells [37]. Caffeic acid has anti-cancer activities against different cancer cells, such as human cervical cancer [38].
Mammillaria sp. had anti-oxidant activities as found in M. rhodantha, M. spinosissima, and M. muehlenpfordtii extracts. M. rhodantha was the best source of antioxidants in this study because it was rich in the strong antioxidants of gentisic acid, chlorogenic acid, caffeic acid, and protocatechoic acid. The four of these phenols have been associated with anti-oxidant activities as revealed in several studies [17,38,39]. Previous reports have found that gentisic acid provided protection to human erythrocytes against gamma radiation [25]. In another study, gentisic acid and protochatechuic acid imparted oxidative stability in sardine oil [32]. M. spinosissima is rich in gentisic acid and caffeic acid and both are strong antioxidants. M. muehlenpfordtii is rich in chlorogenic acid, which is responsible for anti-oxidant activities in this species. Previous investigation showed that chlorogenic acid can mitigate oxidative stresses in vitro and in vivo [39]. It was obvious that these dominant phenols were responsible for anti-oxidant activities.
Mammillaria sp. showed anti-bacterial effects against bacteria, and the highest effects were found in the extracts of M. rhodantha, M. spinosissima, M. crucigera and M. muehlenpfordtii against most bacteria. The gentisic acid did not show activities against most bacteria. Gentisic acid showed weak or no activity against E. coli, P. aeruginosa, and S. aureus in the previous study [40]. The anti-bacterial activities of M. rhodantha is mainly attributed to other major phenols including chlorogenic acid, caffeic acid, and protocatechoic acid. Chlorogenic acid has anti-bacterial activities against bacteria such as E. coli [41] as found in the current study. It increases plasma membrane permeability, causing loss of barrier function [42]. Caffeic acid has antimicrobial activities against several microorganisms including S. aureus [43]. In a previous investigation, protocatechoic acid showed strong anti-bacterial activities against E. coli, L. monocytogenes, S. aureus, and B. cereus [44]. Furthermore, it has anti-oxidant, anti-inflammatory, and anti-cancer activities [30]. It was obvious that the anti-bacterial activities of the extracts of M. rhodantha, M. spinosissima, M. crucigera, and M. muehlenpfordtii against most bacteria was attributed to the chlorogenic composition as well as other major phenols.
4. Materials and Methods
4.1. Plant Material and Preparation
The stem of Mammillaria species (M. rhodantha subsp. pringlei (J.M. Coult.) D.R. Hunt M. spinosissima Lem., M. hahniana Werderm., M. crucigera Mart., M. candida Scheidw., M. albilanata Backeb., and M. muehlenpfordtii) were sampled from cactus nurseries in Alexandria, Egypt. Each species was identified by Ahmed Abdelmoneium and Hosam Elansary. Samples were vouchered at Alexandria (Hosam0001030–1037). Stems (3 replications) were dried using lyophilization (freeze dryers Labconco, Kansas City, MO, USA), then powdered [1,2]. The powder (0.2 g DW, dry weight), was put in 15 mL tubes, then subjected to extraction with methanol (10 mL, Chempur, Poland) by sonication (2 × 30 min at 30 °C) in an ultrasonic bath (Sonic-2, POLSONIC). Stem extracts were filtered using Whatman paper and left in crystallizers to evaporate methanol at room temperature. The residue was dissolved in methanol (1 mL, Merck for liquid chromatography LiChrosolv®, Merck KGaA, Darmstadt, Germany). The samples were maintained in deep freeze (−80 °C). A rotary evaporator was used to eliminate the methanol for bioassays. The bacterial and fungal cultures were obtained from the Faculty of Agriculture, Alexandria, Egypt. Cell cultures of breast adenocarcinoma (MCF-7), cervical adenocarcinoma (HeLa), T-cell lymphoblast (Jurkat), colon adenocarcinoma (HT-29), and HEK-293 (human normal cells). Cells were purchased from American-Type Culture Collection (ATCC).
4.2. Chemicals
The following standards were used for phenolic acid qualification and quantification: benzoic acid and its derivatives (3,4-dihydroxyphenylacetic, ellagic, gallic, gentisic, p-hydroxybenzoic, protocatechuic, salicylic, syringic, and vanillic acids); cinnamic acid and its derivatives (caffeic, o-coumaric, m-coumaric, p-coumaric, ferulic, hydrocaffeic, isoferulic, and sinapic acids); and depsides (chlorogenic, neochlorogenic, and rosmarinic acids). To quantify the flavonoids, aglycone (kaempferol, luteolin, myricetin, quercetin and rhamnetin) and glycoside (apigetrin, cynaroside, hyperoside, isoquercetin, quercitrin, robinin, rutoside, trifolin, vitexin) standards were used. All the substances were acquired from Sigma–Aldrich, Germany.
4.3. Analyses of Phenolic Compounds
The stem extracts were studied by HPLC-DAD method [1,2,45,46] using the Merck-Hitachi liquid chromatograph (LaChrom Elite) with a DAD detector (L-2455) in a ultraviolet (UV)—visible spectra range of 200–400 nm (detection wavelength for all compounds was set at 254 nm). The Purospher RP-18e (250 × 4 mm; 5 μm, Merck) column was used and the temperature was set at 25 °C. The mobile phase consisted of A—methanol, B—methanol: 0.5% acetic acid 1:4 (v/v). The gradient was as follows: 100% B for 0–20 min; 100%–80% B for 20–35 min; 80%–60% B for 35–55 min; 60%–0% B for 55–70 min; 0% B for 70–75 min; 0%–100% B for 75–80 min; 100% B for 80–90 min with a flow rate (1 mL min−1). The injection volume was 20 µL and the compounds of interest were detected at 254 nm. The applied HPLC method was previously validated by our group [45,46]. The tested parameters were the following: accuracy; precision at three levels of standard substance concentrations in solution, 50%, 100%, and 150%; linearity; limit of detection (LOD); and limit of quantification (LOQ) [45,46]. Identification of compounds was performed either by comparison with UV spectra and retention times of reference substances or using co-chromatography. The compounds were quantified using the calibration curves method [45,46,47].
4.4. Anti-Cancer Activities
Antiproliferative and cytotoxic effects of stem extracts were tested on MCF-7, HeLa, Jurkat, and HT-29, as well as HEK-293 (human normal cells) [1,2,16]. Extracts were solubilized in DMSO (1%). Vinblastine sulfate and taxol were considered positive controls. A microplate reader (Thermo, Waltham, MA, USA) was used at a 570 nm wavelength. The percentage of activity inhibition was calculated in triplicate:
% Inhibition = (Abs. 570 nm control‒Abs. 570 nm sample)/Abs. 570 nm control × 100. Furthermore, IC50 values were obtained by plotting the percentage of cell viability against extract concentration and expressed in µg/mL. The IC30 and IC50 were determined in the apoptotic cell population (flow cytometry, FAC Scan, Becton Dickinson, Iowa, USA) following [1,15,16]. Control cells were untreated with extracts or methanol standards.
4.5. Anti-Oxidant Activity
DPPH, β-carotene bleaching [48] and FRAP [2,49] assays were used. For DPPH, the wavelength of 517 nm was used to determine the absorbance. During the β-carotene bleaching assay, the wavelength of 470 nm was used. Samples amount required to scavenged 50% of the DPPH/β-carotene bleaching solutions (IC50 in µg/mL) was determined by plotting the inhibition percent against extract concentration. The butylated hydroxytoluene (BHT) was incorporated as control. The FRAP reagent was prepared as described in previous studies (e.g., [43]) using tripyridyl triazine (TPTZ, Sigma–Aldrich, Berlin, Germany). Aliquots (100 μL) of bark extracts or Trolox (Sigma–Aldrich, Berlin, Germany) were added to FRAP reagent (3 mL), mixed, incubated for half an hour at 37 °C and the absorbance was measured at 593 nm. Aqueous solutions of known serial concentrations of Trolox (0–0.5 Mmol/L) were used for the calibration. Two sets of triplicate replications were conducted for all experiments.
4.6. Anti-Bacterial Activity
stem extracts effects against Bacillus cereus (ATCC 14579), Listeria monocytogenes (clinical isolate), Escherichia coli (ATCC 35210), Micrococcus flavus (ATCC 10240), Staphylococcus aureus (ATCC 6538), and Pseudomonas aeruginosa (ATCC 27853) using the micro-dilution method [17,50]. Microtiter plates (96-well) containing a serial concentration of the stem extracts (0.05–200 mg mL−1) in each well mixed with bacterial inoculum (1.0 × 104 CFU per well) in 100 μL tryptic soy broth were incubated at 37 °C for 24 h in a rotary shaker. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of the stem extract that exhibited no visible growth using a binocular microscope and was determined following the incubation period of the microtiter plates. The minimum bactericide concentration (MBC), which was defined as the lowest concentration that caused no visible growth and indicated the killing of 99.5% of the inoculum, was determined using serial sub-culturing of the stem extract or standard. The optical density was determined at a wavelength of 655 nm. A positive control was used (streptomycin, 0.01–10 mg/mL), as well as a negative one (DMSO, 1%). The optical density was determined at a wavelength of 655 nm. A positive control was used (streptomycin, 0.01–10 mg/mL), as well as a negative one (DMSO, 1%).
4.7. Statistical Analyses
The SPSS (Version 21.0) was used to determine least significance difference (LSD). The standard deviation (SD) of means of three replicates was used.
5. Conclusions
This is the first report exploring seven Mammillaria species giving information about phenolic acid composition, as well as anti-cancer, anti-oxidant, and anti-bacterial activities. In all studied species, gentisic, chlorogenic, caffeic, protocatechuic, sinapic, and p-hydroxybenzoic acids were estimated qualitatively and quantitatively. The dominant compounds were gentisic acid in M. rhodantha and M. spinosissima, chlorogenic acid in M. muehlenpfordtii, M. crucigera, and M. rhodantha, and caffeic acid in M. rhodantha, M. crucigera, and M. spinosissima. The quantitative dominant were confirmed as gentisic acid in M. spinosissima (40.44 mg 100g−1 DW) and chlorogenic acid in M. muehlenpfordtii (30.88 mg 100g−1 DW). Stem extracts of Mammillaria sp. showed antiproliferative effects against HeLa, MCF-7, and Jurkat cells. In HeLa and MCF-7 cells, the best antiproliferative activities were found in treatments with M. rhodantha, M. spinosissima, and M. muehlenpfordtii. The only anti-cancer activity against HT-29 was found in the extracts of M. rhodantha and M. spinosissima. The apoptotic assay of M. rhodantha, M. spinosissima, and M. muehlenpfordtii showed the accumulation of necrotic in the early and late apoptotic (Figure 2). M. rhodantha, M. spinosissima, and M. muehlenpfordtii showed the highest anti-oxidant activities using DPPH, β-carotene bleaching, and FRAP assays compared to other species. M. rhodantha was the best source of antioxidants as revealed by lowest IC50. Mammillaria sp. showed moderate anti-bacterial effects against bacteria, and the highest effects were found in the extracts of M. rhodantha, M. spinosissima, M. crucigera, and M. muehlenpfordtii against B. cereus, L. monocytogenes, E. coli, M. flavus, S. aureus and P. aeruginosa. Gentisic acid did not show activities against most bacteria. Chlorogenic acid showed high anti-bacterial effects, which was comparable to the antibiotic used. Mammillaria sp. could be considered to be a new natural resource of phenols with biological activities.
Author Contributions
Conceptualization, H.O.E., A.S., H.E. and E.A.M.; Data curation, H.O.E., A.S., K.J., A.A.B. and D.O.E.-A.; Formal analysis, H.O.E., A.S., M.K.-S., K.J., H.E. and E.A.M.; Funding acquisition, H.O.E., A.S. and A.A.B.; Investigation, H.O.E., A.S., M.K.-S., K.J., H.E., E.A.M. and A.A.B.; Methodology, A.S., E.A.M., A.A.B. and D.O.E.-A.; Project administration, H.O.E., H.E. and D.O.E.-A.; Resources, A.S.; Software, D.O.E.-A.; Supervision, A.S., H.E.; Validation, A.S. and M.K.-S.; Writing—original draft, H.O.E., and A.S.; Writing—review & editing, H.O.E. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by King Saud University, Researchers Supporting Project number (RSP-2019/118).
Acknowledgments
The authors extend their appreciation to King Saud University, Researchers Supporting Project for funding this work through research group (RSP-2019-118).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| HPLC-DAD | High-Performance Liquid Chromatography with Diode-Array Detection |
| MCF-7 | cell cultures of breast adenocarcinoma |
| HeLa | cell cultures of cervical adenocarcinoma |
| Jurkat | cell cultures of T-cell lymphoblast |
| HT-29 | cell cultures of colon adenocarcinoma |
| ATCC | American-Type Culture Collection |
References
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; El-Abedin, T.K.A.Z.; Mattar, M.A.; Ekiert, H. Phenolic compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have antioxidant and anticancer activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.A.; Mattar, M.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol profile and pharmaceutical potential of Quercus spp. bark extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Negreiros de Carvalho, P.L.; Silva Ede, O.; Chagas-Paula, D.A.; Hortolan Luiz, J.H.; Ikegaki, M. Importance and implications of the production of phenolic secondary metabolites by endophytic fungi: A mini-review. Mini Rev. Med. Chem. 2016, 16, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Orfali, G.d.C.; Duarte, A.C.; Bonadio, V.; Martinez, N.P.; de Araújo, M.E.M.B.; Priviero, F.B.M.; Carvalho, P.O.; Priolli, D.G. Review of anticancer mechanisms of isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.K.; El-Maghraby, M.L.; Elansary, H.; Hafez, M.Y.; Ibrahim, I.E.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef]
- Elansary, H.O.; Zin El-Abedin, T.K. Omeprazole alleviates water stress in peppermint and modulates the expression of menthol biosynthesis genes. Plant Physiol. Bioch. 2019, 139, 578–586. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; Hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, S.M.; Amorós, A.; Carbonell-Barrachina, A.Á.; Hernández, F. Polyphenol compounds and biological activity of caper (Capparis spinosa L.) flowers buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef]
- Fejér, J.; Kron, I.; Pellizzeri, V.; Pľuchtová, M.; Eliašová, A.; Campone, L.; Gervasi, T.; Bartolomeo, G.; Cicero, N.; Babejová, A.; et al. First report on evaluation of basic nutritional and antioxidant properties of Moringa oleifera Lam. from Caribbean Island of Saint Lucia. Plants 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupo, M.; Burlando, B.; Denaro, M.; Barreca, D.; Trombetta, D.; Smeriglio, A.; Cornara, L. Polyphenol characterization and skin-preserving properties of hydroalcoholic flower extract from Himantoglossum robertianum (Orchidaceae). Plants 2019, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharm. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic transformation and hairy root induction enhance the antioxidant potential of Lactuca serriola L. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. Bmc Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Yessoufou, K.; Elansary, H.O.; Mahmoud, E.A.; Skalicka-Wozniak, K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind Crop Prod. 2015, 74, 752–758. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of traditional medicinal plants in Alexandria. Evid. Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K. In vitro antioxidant, antifungal and antibacterial activities of five international Calibrachoa cultivars. Nat. Prod. Res. 2016, 30, 1339–1342. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Mahmoud, E.A.; Skalicka-Wozniak, K. In vitro Antioxidant and Antimicrobial Effects of Ceratostigma plumbaginoides. Nat. Prod. Commun. 2016, 11, 1455–1458. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Brinker, F. Prickly pear as food and medicine. J. Diet. Suppl. 2009, 6, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Almaraz-Abarca, N.; Campos, M.G.; Delgado-Alvarado, E.A.; Ávila-Reyes, J.A.; Herrera-Corral, J.; González-Valdez, L.S.; Naranjo-Jiménez, N.; Frigerio, C.; Tomatas, A.F.; Almeida, A.J.; et al. Uribe-Soto, pollen flavonoid/phenolic acid composition of four species of Cactaceae and its taxonomic significance. Am. J. Agric. Biol. Sci. 2008, 3, 534–543. [Google Scholar]
- Zhang, M.; Hettiarachchy, N.S.; Horax, R.; Chen, P.; Over, K.F. Effect of maturity stages and drying methods on the retention of selected nutrients and phytochemicals in bitter melon (Momordica charantia) leaf. 2009, 74, C441–C448. J. Food Sci. 2009, 74, C441–C448. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive compounds, antioxidant activity, and biological effects of European cranberry (Vaccinium oxycoccos). Molecules 2018, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gangabhagirathi, R.; Venu, S.; Adhikari, S.; Mukherjee, T. Antioxidant activity and free radical scavenging reactions of gentisic acid: In-vitro and pulse radiolysis studies. Free Radic. Res. 2012, 46, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ashidate, K.; Kawamura, M.; Mimura, D.; Tohda, H.; Miyazaki, S.; Teramoto, T.; Yamamoto, Y.; Hirata, Y. Gentisic acid, an aspirin metabolite, inhibits oxidation of low-density lipoprotein and the formation of cholesterol ester hydroperoxides in human plasma. Eur. J. Pharm. 2005, 513, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lim, Y.Y.; Tan, S.P. Standardised herbal extract of chlorogenic acid from leaves of Etlingera elatior (Zingiberaceae). Pharmacogn. Res. 2011, 3, 178–184. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kovayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on protocatechuic acid and its pharmacological potential. J. ISRN Pharmacol. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. J. Oxid. Med. Cell. Longev. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, V.; Belur, P.D.; Regupathi, I. Effect of hydroxybenzoic acids antioxidants on the oxidative stability of sardine oil. Resour. Effic. Technol. 2016, 2, S114–S118. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Elmaci, I.; Ozpinar, A.A. Gentisic acid, a quinonoid aspirin metabolite in cancer prevention and treatment. New horizons in management of brain tumors and systemic cancers. J. Cancer Res. Oncobiol. 2018, 1, 109. [Google Scholar] [CrossRef]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing anticancer potential of blueberry flavonoids, quercetin, kaempferol, and gentisic acid, through oxidative stress and apoptosis parameters on HCT-116 cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kang, Q.; Ren, J.; Li, Z.; Xu, X. Antitumor Molecular Mechanism of Chlorogenic Acid on Inducting Genes GSK-3 β and APC and Inhibiting Gene β -Catenin. J. Anal. Methods Chem. 2013, 2013, 951319. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Chen, J.-H.; Huang, C.-C.; Wang, C.-J. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int. J. Cancer 2007, 120, 2306–2316. [Google Scholar] [CrossRef]
- Eroğlu, C.; Avcı, E.; Vural, H.; Kurar, E. Anticancer mechanism of Sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene 2018, 671, 127–134. [Google Scholar] [CrossRef]
- Kanimozhi, G.; Prasad, N.R. Anticancer effect of caffeic acid on human cervical cancer cells. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 655–661. [Google Scholar]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Vandal, J.; Abou-Zaid, M.M.; Ferroni, G.; Leduc, L.G. Antimicrobial activity of natural products from the flora of Northern Ontario, Canada. Pharm. Biol. 2015, 53, 800–806. [Google Scholar] [CrossRef]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial effects of chlorogenic acid and related compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Kepa, M.; Miklasinska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smolen-Dzirba, J.; Wasik, T.J. Antimicrobial potential of caffeic acid against staphylococcus aureus clinical strains. J Biol. Med. Res. Int. 2018, 2018, 9. [Google Scholar]
- Chao, C.-Y.; Yin, M.-C. Antibacterial effects of Roselle Calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2008, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Sulkowska-Ziaja, K.; Maslanka, A.; Szewczyk, A.; Muszynska, B. Physiologically active compounds in four species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD- HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krośniak, M.; Marzec-Wróblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Technology, comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A. ×prunifolia and their antioxidant activities. Eur. Food. Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance salam turfgrass performance during prolonged irrigation intervals and saline shock. Front Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Sousa, V.B.; Pereira, H. Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS ONE 2018, 13, e0197135. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skaicka-Wozniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crop. Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).