Secondary Metabolites from Gorgonian Corals of the Genus Eunicella: Structural Characterizations, Biological Activities, and Synthetic Approaches
Abstract
1. Introduction
2. Terpenoids
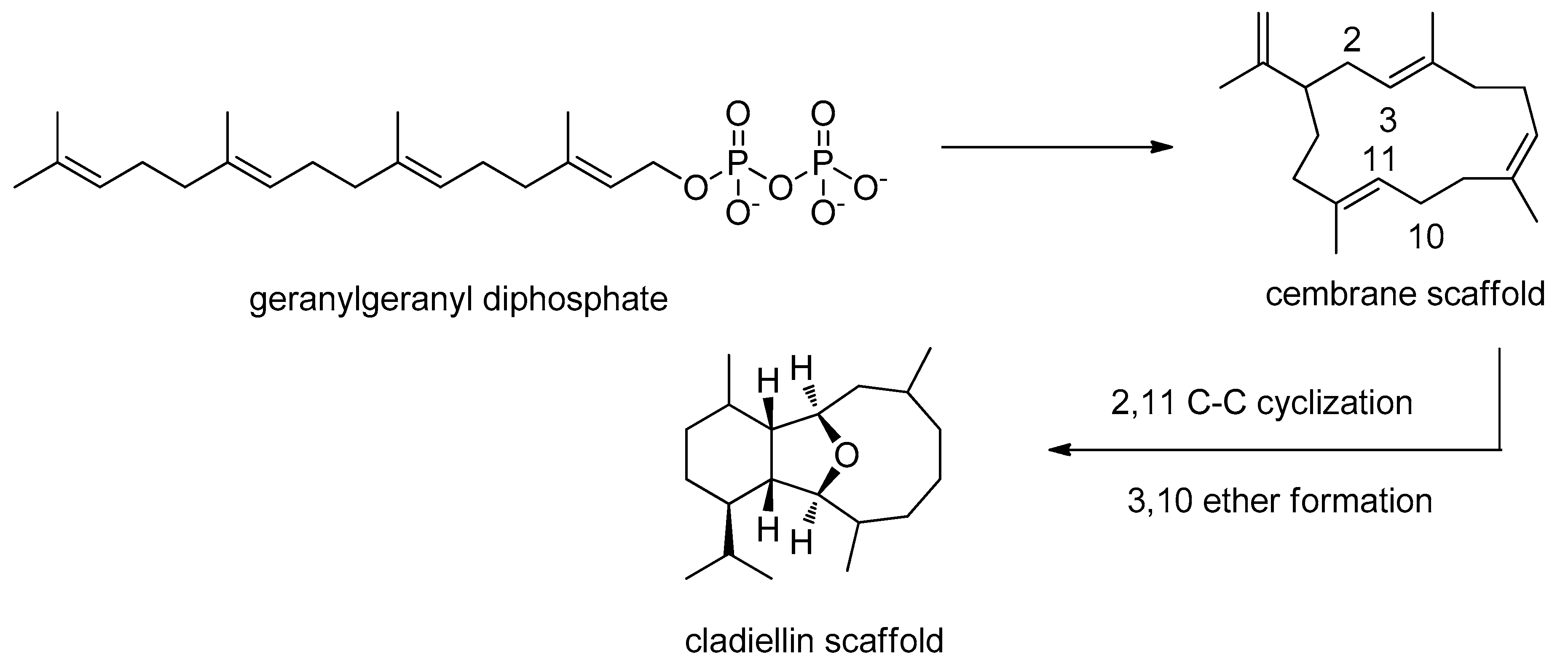
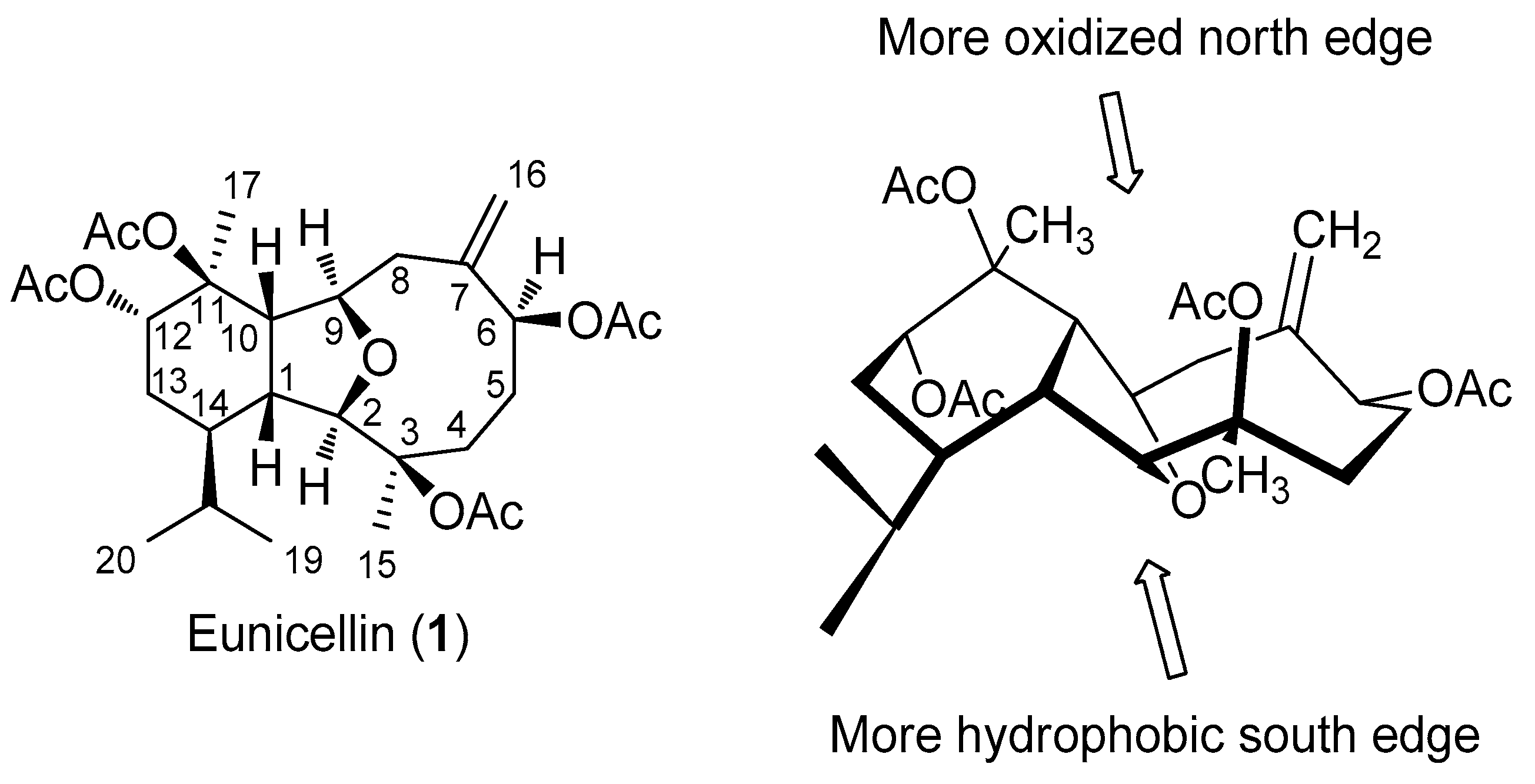
2.1. Biogenesis and Structural Analysis of Eunicellins
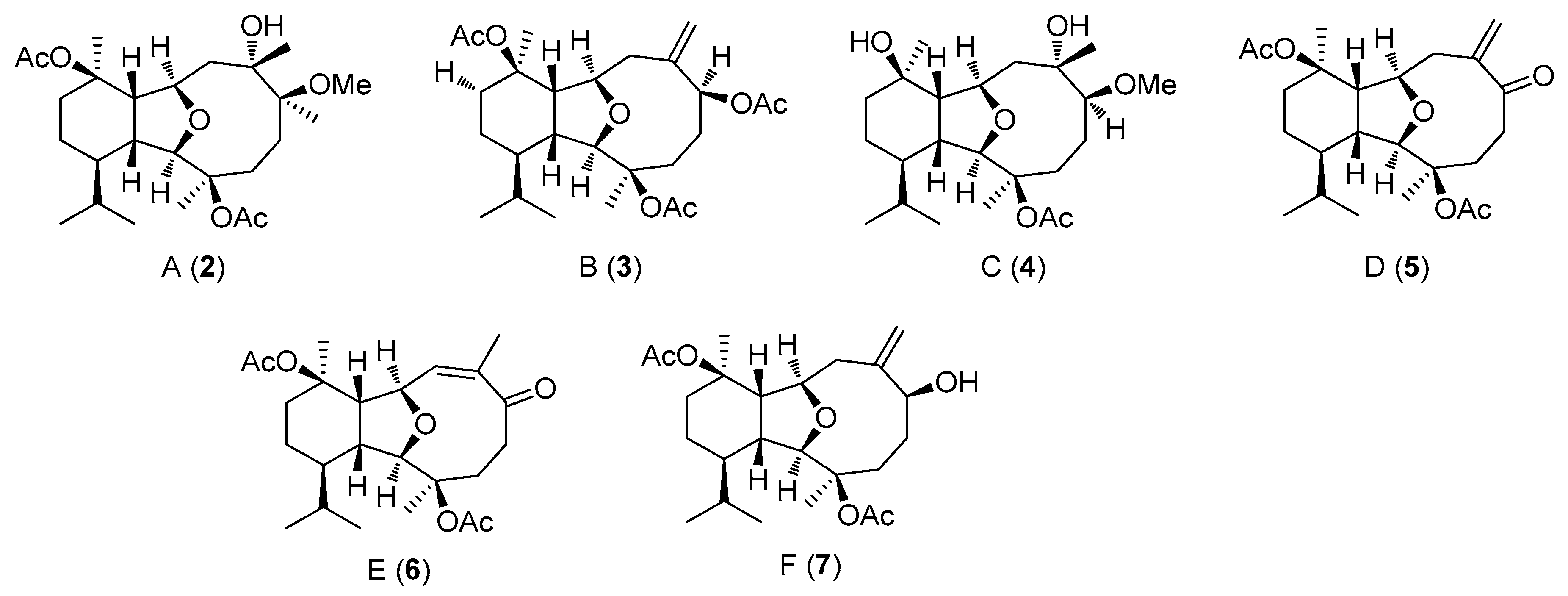
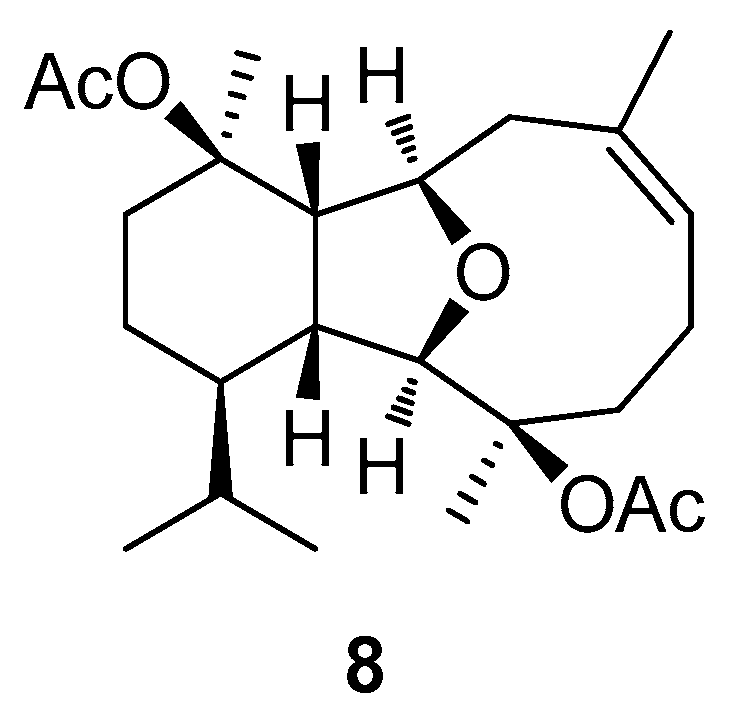
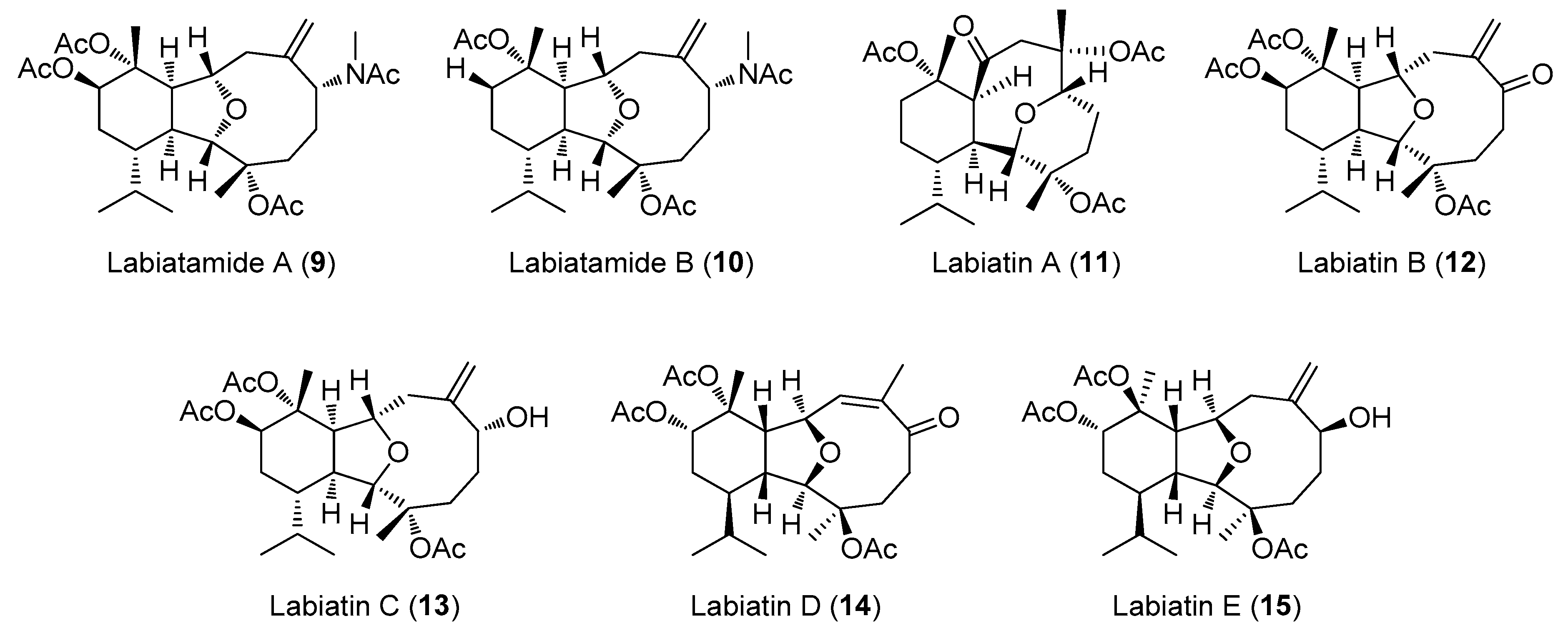
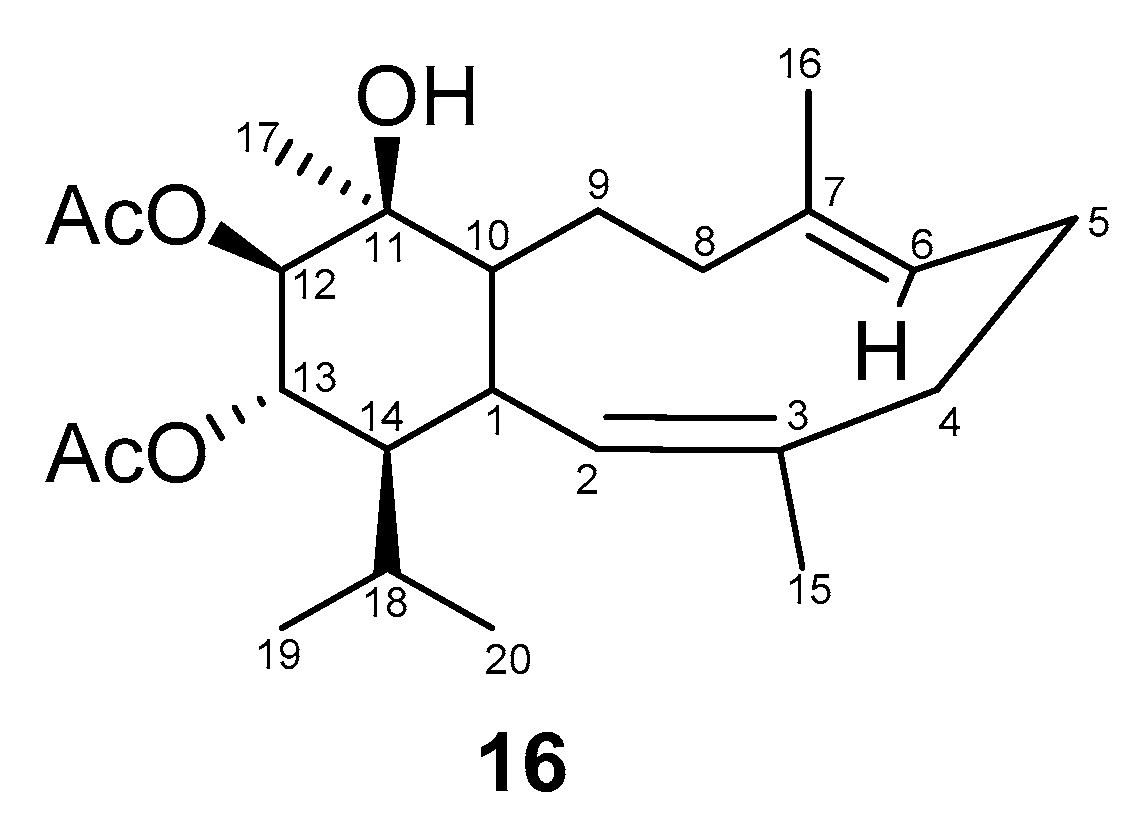
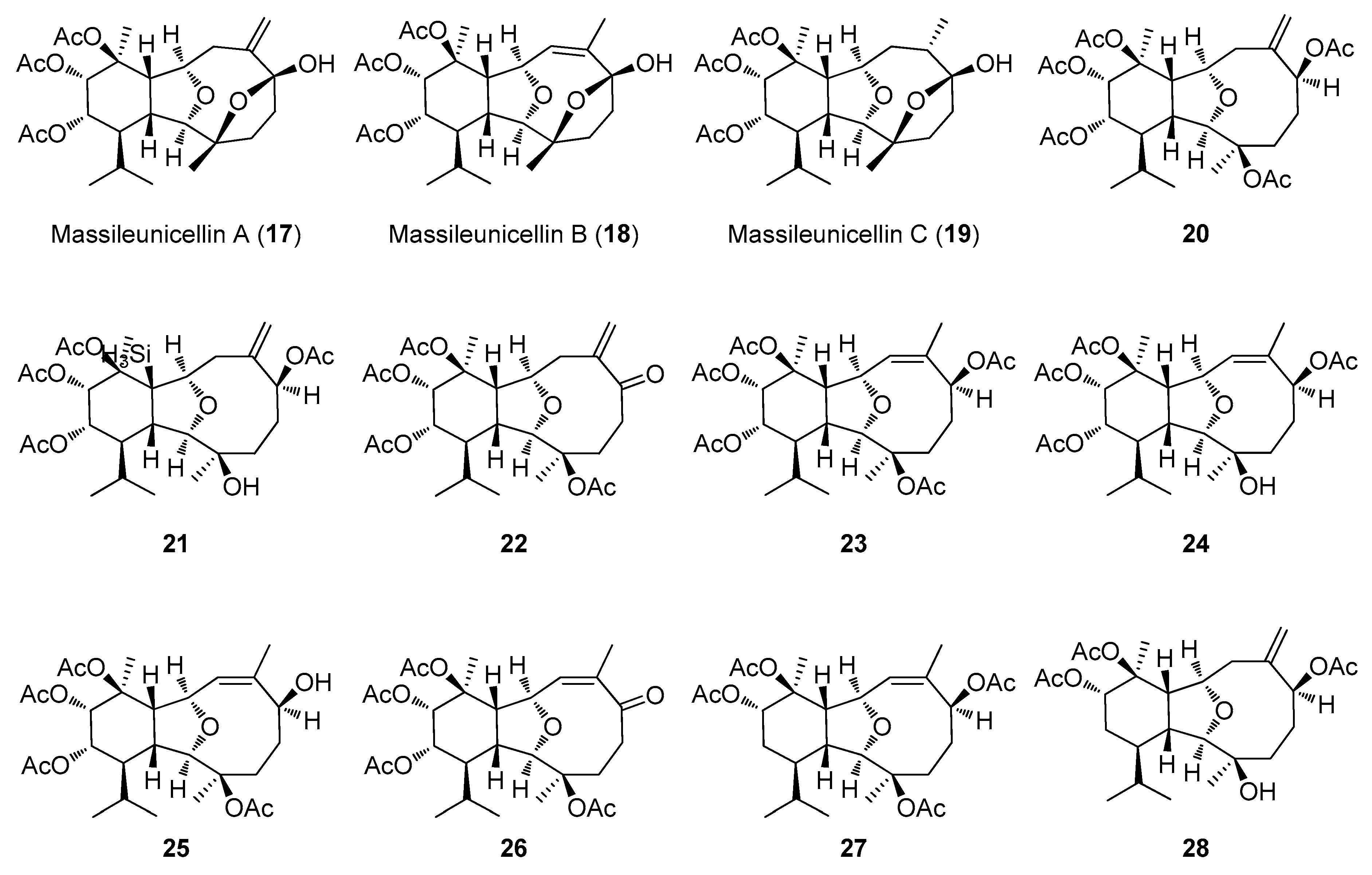
2.2. Structural Diversity of Terpenoids from Eunicella sp.
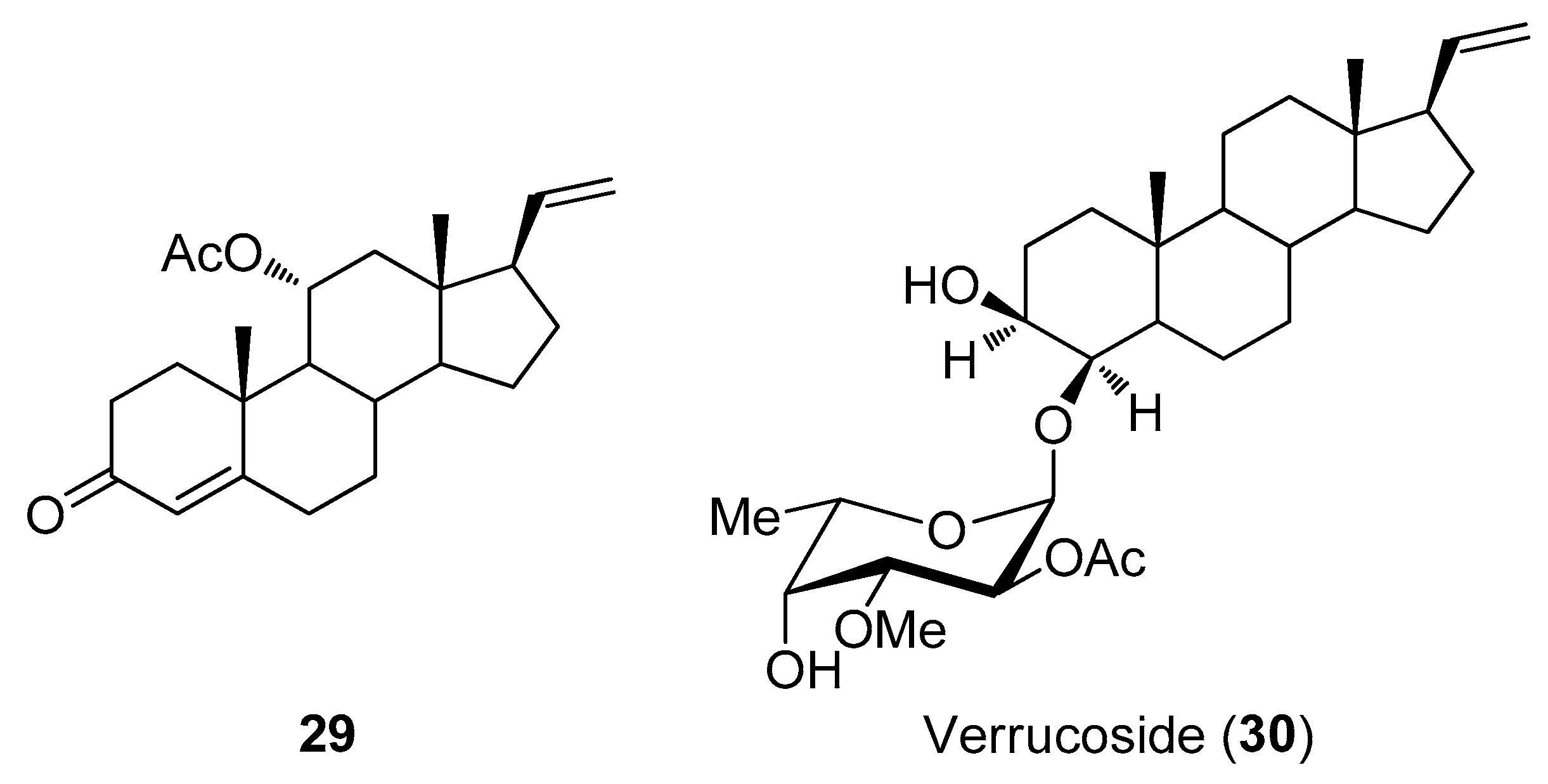
2.3. Steroids
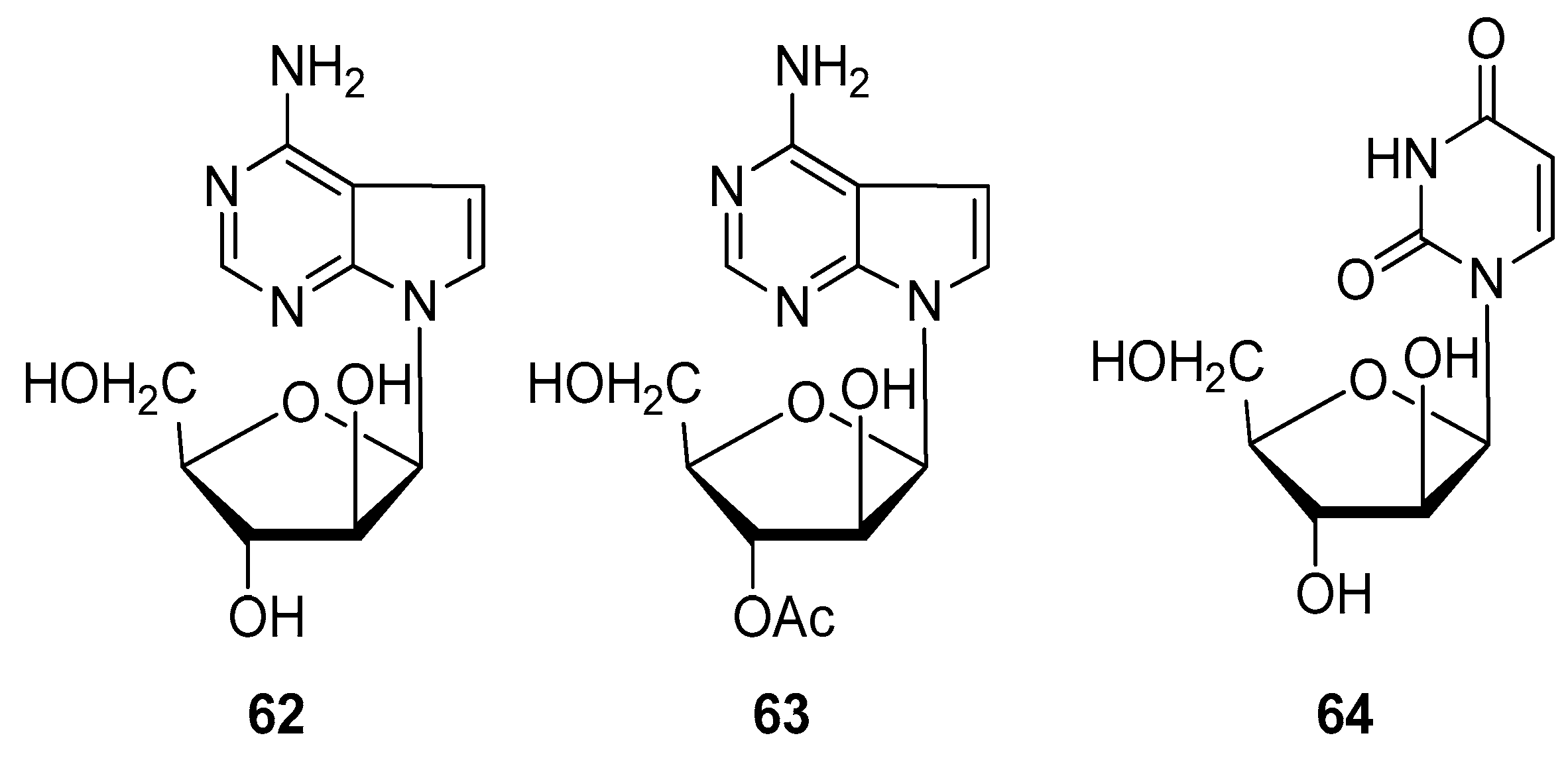
2.4. Alkaloids and Nucleosides
3. Biological Activities
3.1. Cytotoxic Activity
3.2. Anti-Inflammatory Activity
3.3. Antimicrobial Activity
4. Synthetic Approaches
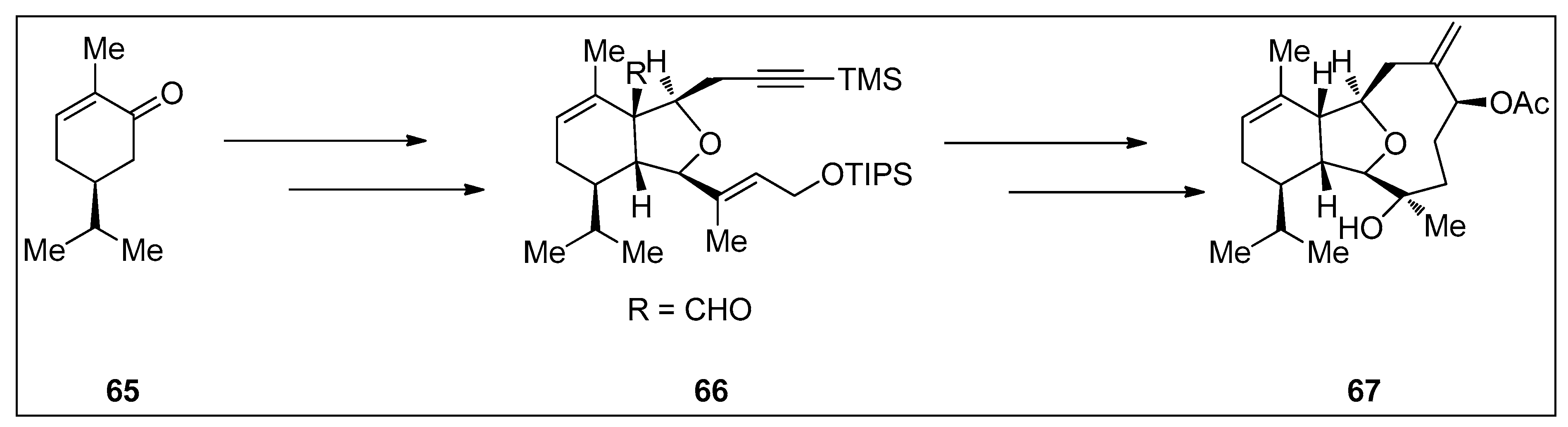
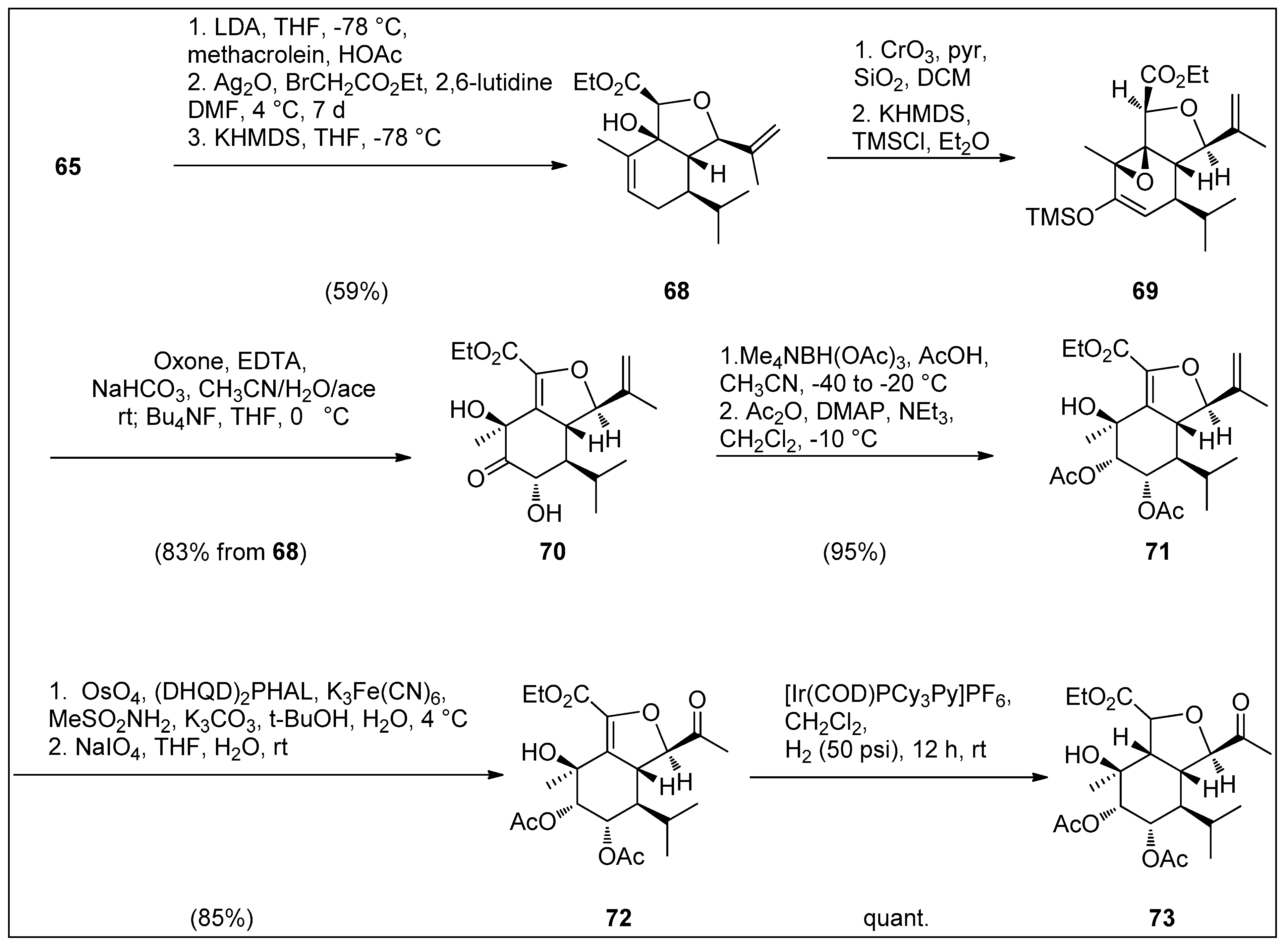
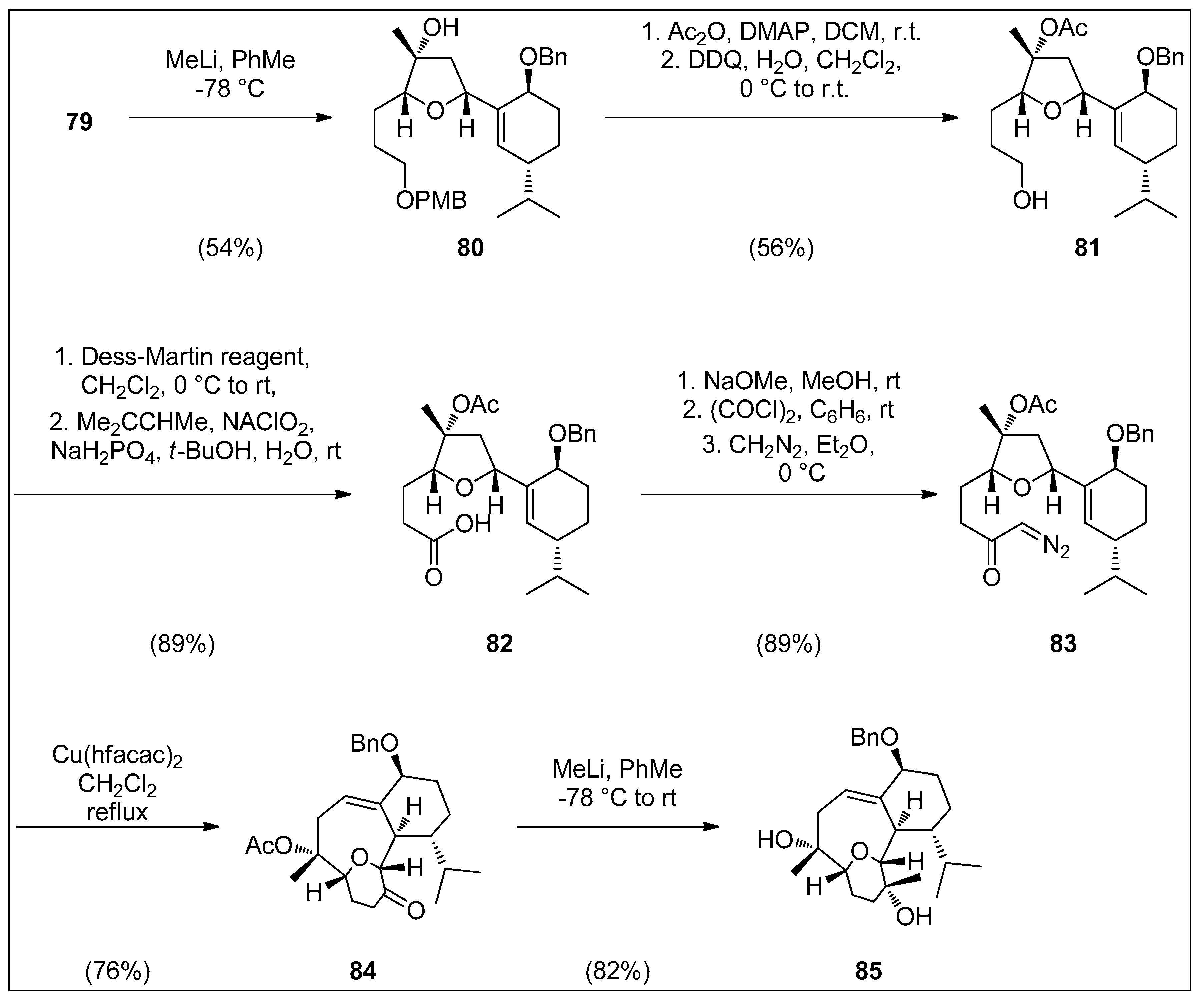
4.1. Synthetic Approaches toward the Synthesis of Terpenoids Isolated from Eunicella Species
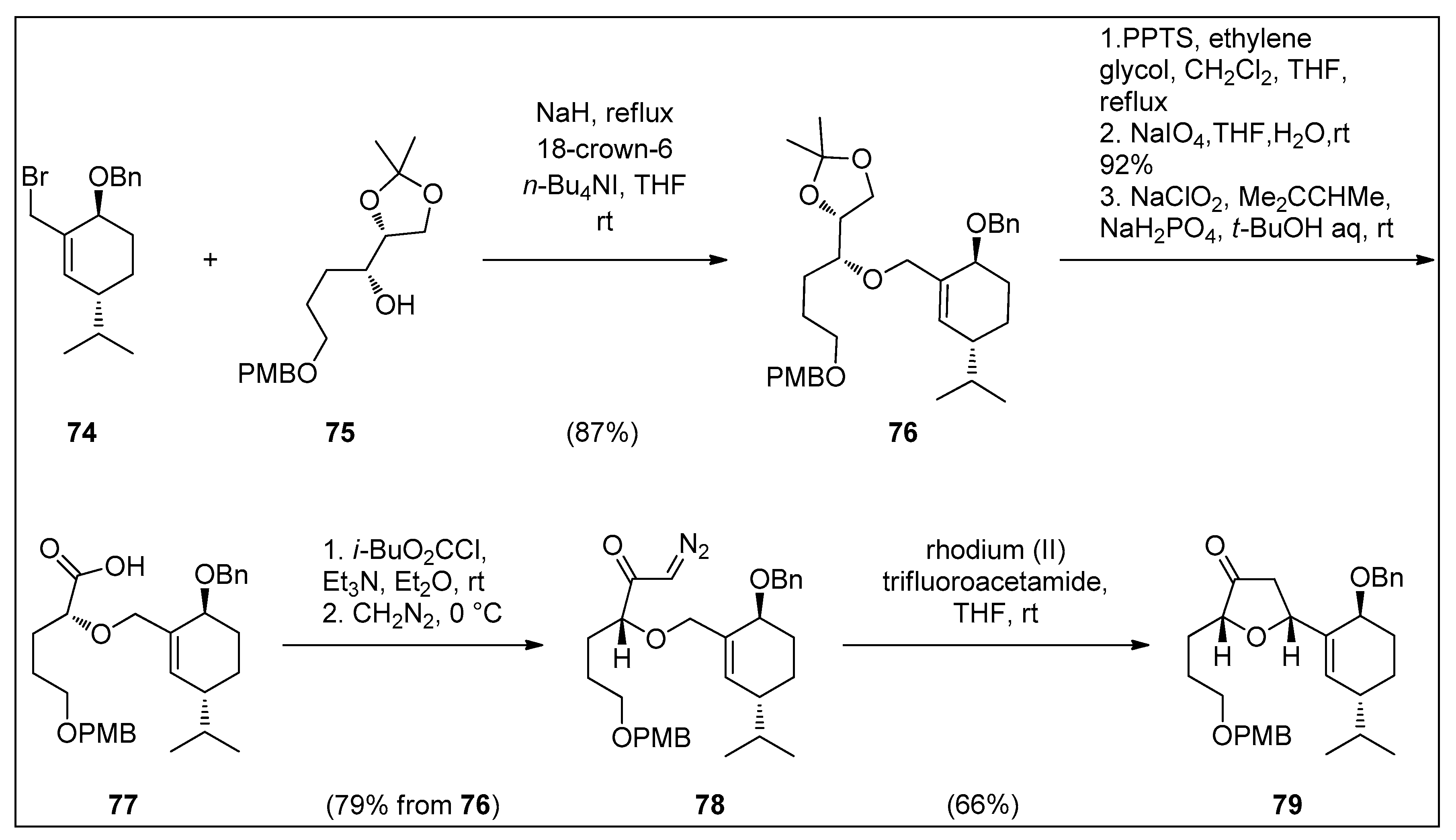
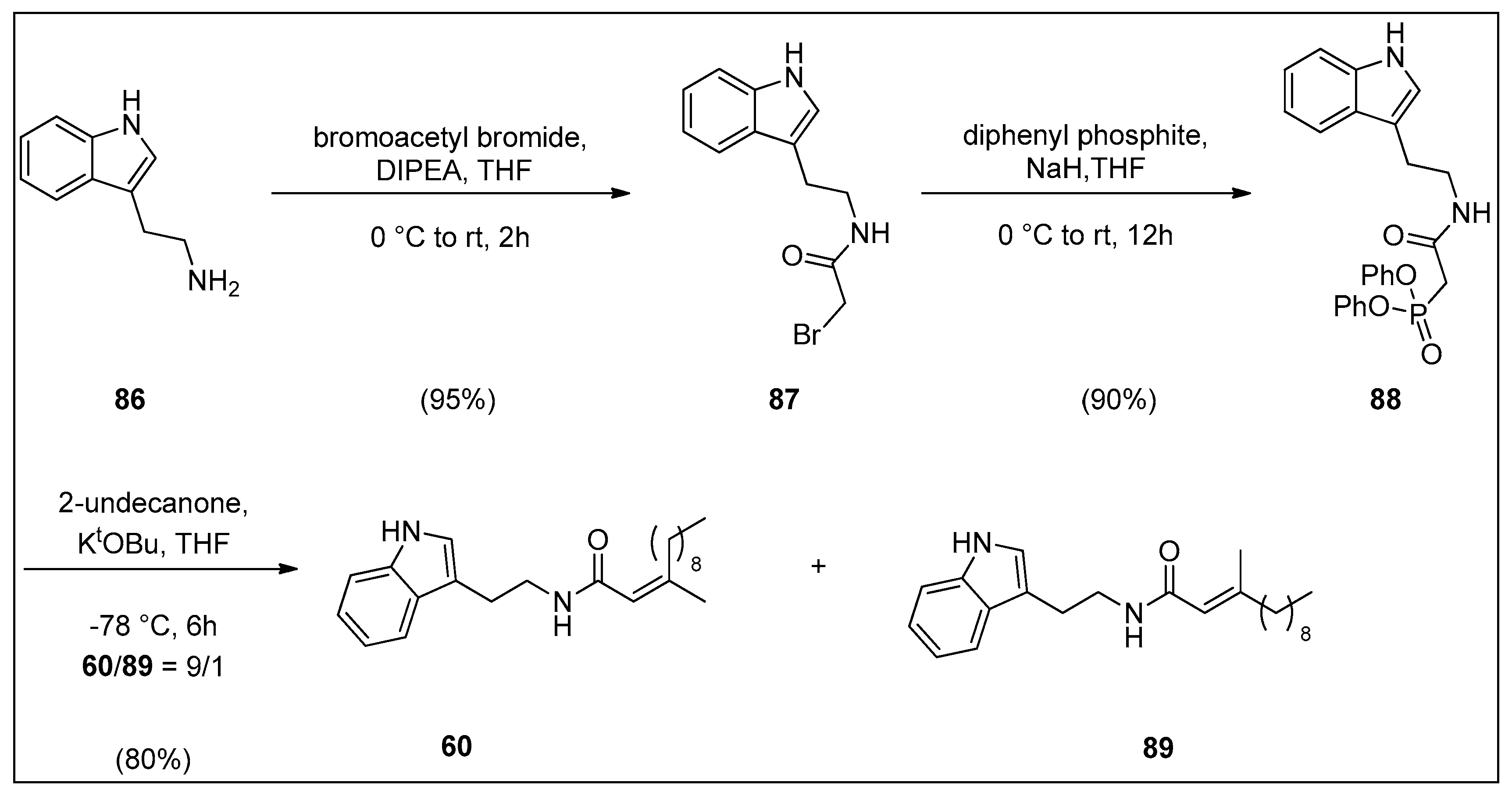
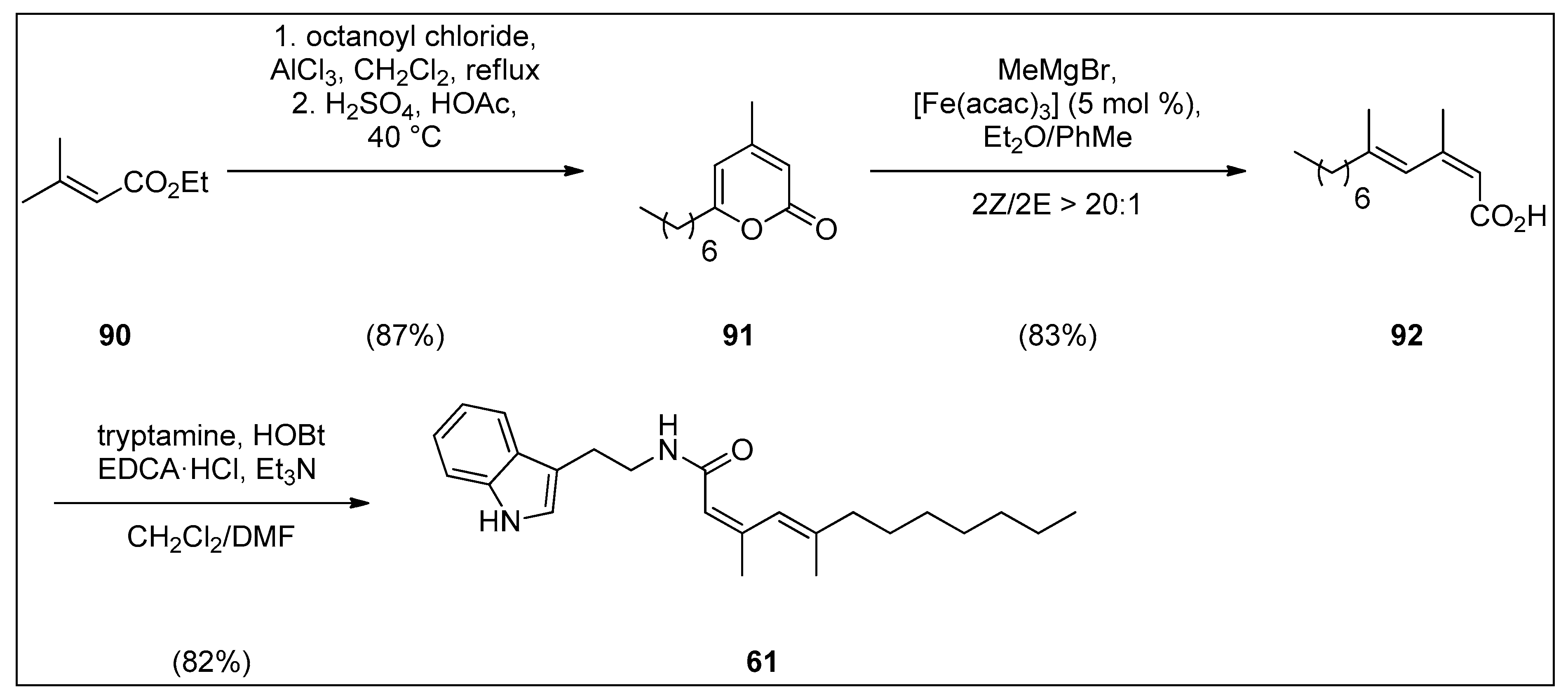
4.2. Synthesis of Granulatamide Alkaloids
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| A2780 | human ovarian carcinoma cell line |
| A498 | human renal epithelial cancer cell line |
| A549 | human lung carcinoma cell line |
| ASL | acetylsalicylate of lysine |
| COX-2 | cyclooxygenase-2 |
| DDQ | 2,3-dichloro-5,6-dicyano-1,4-benzoquinone |
| (DHQD)2PHAL | hydroquinidine 1,4-phthalazinediyl diether |
| DIPEA | N,N-diisopropylethylamine |
| DMAP | 4-dimethylaminopyridine |
| DMF | dimethylformamide |
| Du145 | human prostate cancer cell line |
| EDTA | ethylenediaminetetraacetic acid |
| H460 | human large cell lung cancer cell line |
| HCT-115 | human colorectal adenocarcinoma cell line |
| HCT-116 | human colon cancer cell line |
| HeLa | human cervical cancer cell line |
| HepG2 | human hepatocellular carcinoma cell line |
| Hfacac | hexafluoroacetylacetonat |
| HGC-27 | human gastric cancer cell line |
| HK-2 | human papillomavirus 16 (HPV-16) transformed renal cell line |
| HL-60 | human leukemia cell line |
| HT29 | human colon adenocarcinoma cell line |
| HTLV-1 | human T-lymphotropic virus 1 |
| IC50 | half maximal inhibitory concentration |
| IGROV | human ovarian cancer cell line |
| iNOS | inducible nitric oxide synthase |
| K562 | human myelogenous leukemia cell line |
| KB | keratin-forming tumor cell line HeLa |
| KHMDS | potassium bis(trimethylsilyl)amide |
| KtOBu | potassium t-butoxide |
| L1210 | murine lymphocytic leukemia cell line |
| LDA | lithium diisopropylamide |
| LNcaP | androgen-sensitive human prostate adenocarcinoma cell line |
| LPS | lipopolysaccharide |
| MCF-7 | human breast adenocarcinoma cell line |
| MES-SA | human uterine carcinoma cell line |
| MIC50 | minimum inhibitory concentration required to inhibit the growth of 50% or organisms |
| NO | nitric oxide |
| NSCLC-N6 | human non-small cell lung cancer cell line |
| P-388 | menogaril-resistant leukemia cell line |
| PPTS | pyridinium p-toluensulfonate |
| SGC-7901 | human gastric cancer cell line |
| SK-BR3 | human breast cancer cell line |
| SK-MEL-2 | human melanoma cell line derived from metastasis on skin of thigh |
| SK-OV-3 | human ovarian carcinoma cell line |
| THF | tetrahydrofuran |
| TMSCl | trimethylsilyl chloride |
| Vero | epithelial cell line derived from kidney of Cercopithecus aethiops |
| XF498 | human glioblastoma cell line |
References
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Lefranc, F.; Koutsaviti, A.; Ioannou, E.; Kornienko, A.; Roussis, V.; Kiss, R.; Newman, D. Algae metabolites: From in vitro growth inhibitory effects to promising anticancer activity. Nat. Prod. Rep. 2019, 36, 810–841. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New Drugs from the Sea: Pro-Apoptotic Activity of Sponges and Algae Derived Compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef]
- Simmons, T.L.; Andrianasolo, E.; Mcphail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs Minireview Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar] [PubMed]
- Dyshlovoy, S.A.; Honecker, F. Marine compounds and cancer: 2017 updates. Mar. Drugs 2018, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H. Bioactive Compounds from Marine Gorgonian Corals. Stud. Nat. Prod. Chem. 2012, 38, 325–351. [Google Scholar]
- Sánchez, J.A.; Dueñas, L.F.; Rowley, S.J.; Gonzalez-Zapata, F.L.; Vergara, D.C.; Montaño-Salazar, S.M.; Calixto-Botía, I.; Gómez, C.E.; Abeytia, R.; Colin, P.L.; et al. Gorgonian Corals. In Mesophotic Coral Ecosystems; Loya, Y., Puglise, K.A., Bridge, T.C.L., Eds.; Springer: Cham, Switzerland, 2019; pp. 729–747. [Google Scholar]
- Angiolillo, M.; Canese, S. Deep Gorgonians and Corals of the Mediterranean Sea. In Corals in a Changing World; Duque Beltran, C., Tello Camacho, E., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 29–49. [Google Scholar]
- Bayer, T.; Arif, C.; Ferrier-Pagès, C.; Zoccola, D.; Aranda, M.; Voolstra, C.R. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Mar. Ecol. Prog. Ser. 2013, 479, 75–84. [Google Scholar] [CrossRef]
- Raimundo, I.; Silva, S.G.; Costa, R.; Keller-Costa, T. Bioactive Secondary Metabolites from Octocoral-Associated Microbes—New Chances for Blue Growth. Mar. Drugs 2018, 16, 485. [Google Scholar] [CrossRef]
- Kodzius, R.; Gojobori, T. Marine metagenomics as a source for bioprospecting. Mar. Genomics 2015, 24, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.; Tchigvintsev, A.; Tran, H.; Chernikova, T.N.; Golyshina, O.V.; Yakimov, M.M.; Golyshin, P.N.; Yakunin, A.F. Metagenomics as a tool for enzyme discovery: Hydrolytic enzymes from marine-related metagenomes. In Advances in Experimental Medicine and Biology; Krogan, N.J., Babu, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 883, pp. 1–20. [Google Scholar]
- Ngara, T.R.; Zhang, H. Recent Advances in Function-based Metagenomic Screening. Genom. Proteom. Bioinforma. 2018, 16, 405–415. [Google Scholar] [CrossRef]
- Sini, M.; Kipson, S.; Linares, C.; Koutsoubas, D.; Garrabou, J. The yellow gorgonian Eunicella cavolini: Demography and disturbance levels across the Mediterranean Sea. PLoS ONE 2015, 10, e0126253. [Google Scholar] [CrossRef] [PubMed]
- WoRMS—World Register of Marine Species. Available online: http://marinespecies.org/index.php (accessed on 27 October 2019).
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Lajili, S.; Deghrigue, M.; Abdelhamid, A.; Bjelogrlić, S.; Muller, C.D.; D’auria, M.V.; Bouraoui, A. Anticancer activity of diterpenes and steroids from Eunicella singularis against two- and threedimensional breast cancer cell models. J. Coast. Life Med. 2017, 5, 531–539. [Google Scholar] [CrossRef]
- Ortega, M.J.; Zubia, E.; He, H.-Y.; Salva, J. New Eunicellin-Based Diterpenoids from the Gorgonian Eunicella verrucosa. Tetrahedron 1993, 49, 7823–7828. [Google Scholar] [CrossRef]
- De Rosa, S.; Cimino, G.; De Giulio, A.; Milone, A.; Crispino, A.; Iodice, C. A New Bioactive Eunicellin-Type Diterpene from the Gorgonian Eunicella cavolini. Nat. Prod. Lett. 1995, 7, 259–265. [Google Scholar] [CrossRef]
- Kakonikos, C.; Vagias, C.; Roussis, V.; Roussakis, C.; Kornprobst, J.M. New eunicellin type diterpenoids from the gorgonian coral Eunicella labiata. Nat. Prod. Lett. 1999, 13, 89–95. [Google Scholar] [CrossRef]
- Welford, A.J.; Collins, I. The 2,11-cyclized cembranoids: Cladiellins, Asbestinins, and Briarellins (Period 1998-2010). J. Nat. Prod. 2011, 74, 2318–2328. [Google Scholar] [CrossRef]
- Ortega, M.J.; Zubía, E.; Salva, J. A New Cladiellane Diterpenoid from Eunicella labiata. J. Nat. Prod. 1997, 60, 485–487. [Google Scholar] [CrossRef]
- Kennard, O.; Watson, D.G.; di Sanseverino, L.R.; Tursch, B.; Bosmans, R.; Djerassi, C. Chemical studies of marine invertebrates. IV. Terpenoids LXII. Eunicellin, a diterpenoid of the gorgonian. X-ray diffraction analysis of Eunicellin dibromide. Tetrahedron Lett. 1968, 9, 2879–2884. [Google Scholar] [CrossRef]
- Roussis, V.; Fenical, W.; Kornprobst, J.M.; Vagias, C.; Miralles, J. Labiatamides A, B, and other eunicellan diterpenoids from the Senegalese gorgonian Eunicella labiata. Tetrahedron 1996, 52, 2735–2742. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Zibrowius, H.; Laurent, D.; Pietra, F. A novel type of a second epoxy bridge in eunicellane diterpenes: Isolation and characterization of massileunicellins A - C from the gorgonian Eunicella cavolinii. Helv. Chim. Acta 1999, 82, 1681–1689. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Zibrowius, H.; Pietra, F. Configuration, conformation, and reactivity of highly functionalized eunicellane diterpenes isolated from the gorgonians Eunicella cavolinii and Eunicella singularis from Marseille. Helv. Chim. Acta 2000, 83, 1561–1575. [Google Scholar] [CrossRef]
- Deghrigue, M.; Dellai, A.; Bouraoui, A. In vitro antiproliferative and antioxidant activities of the organic extract and its semi-purified fractions from the Mediterranean gorgonian Eunicella singularis. Int. J. Pharm. Pharm. Sci. 2013, 5, 432–439. [Google Scholar]
- Deghrigue, M.; Festa, C.; Ghribi, L.; D’Auria, M.V.; De Marino, S.; Ben Jannet, H.; Bouraoui, A. Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis. Asian Pac. J. Trop. Med. 2015, 8, 606–611. [Google Scholar] [CrossRef]
- Deghrigue, M.; Dellai, A.; Akremi, N.; Le Morvan, V.; Robert, J.; Bouraoui, A. Evaluation of antiproliferative and antioxidant activities of the organic extract and its polar fractions from the Mediterranean gorgonian Eunicella singularis. Environ. Toxicol. Pharmacol. 2013, 36, 339–346. [Google Scholar] [CrossRef]
- Deghrigue, M.; Festa, C.; Ghribi, L.; D’auria, M.V.; de Marino, S.; Ben Jannet, H.; Ben Said, R.; Bouraoui, A. Pharmacological evaluation of the semi-purified fractions from the soft coral Eunicella singularis and isolation of pure compounds. Daru 2014, 22, 64. [Google Scholar] [CrossRef]
- Ioannou, E.; Abdel-Razik, A.F.; Zervou, M.; Christofidis, D.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. 5alpha,8alpha-Epidioxysterols from the gorgonian Eunicella cavolini and the ascidian Trididemnum inarmatum: isolation and evaluation of their antiproliferative activity. Steroids 2009, 74, 73–80. [Google Scholar] [CrossRef]
- Ioannou, E.; Abdel-Razik, A.F.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. 9,11-Secosterols with antiproliferative activity from the gorgonian Eunicella cavolini. Bioorg. Med. Chem. 2009, 17, 4537–4541. [Google Scholar] [CrossRef]
- Cimino, G.; Desiderio, B.; De Stefano, S.; Sodano, G. Chemistry of mediterranean gorgonians. II. Pregna 4,20-dien-11α-ol-3-one acetate, a novel steroid from the gorgonian Eunicella cavolini. Experientia 1979, 35, 298–299. [Google Scholar] [CrossRef]
- Kashman, Y.; Green, D.; Garcia, C.; Garcia Arevalos, D. Verrucoside, A new cytotoxic pregnane glycoside from a gorgonian eunicella verrucosa. J. Nat. Prod. 1991, 54, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Abdel-Razik, A.F.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. Pregnanes with antiproliferative activity from the gorgonian Eunicella cavolini. Tetrahedron 2008, 64, 11797–11801. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.L.; Zhong, B.L.; Liao, X.J.; Xu, S.H. 9,11-Secosteroids with cytotoxic activity from the South China Sea gorgonian coral Subergorgia suberosa. Steroids 2015, 98, 100–106. [Google Scholar] [CrossRef]
- Chen, W.T.; Liu, H.L.; Yao, L.G.; Guo, Y.W. 9,11-Secosteroids and polyhydroxylated steroids from two South China Sea soft corals Sarcophyton trocheliophorum and Sinularia flexibilis. Steroids 2014, 92, 56–61. [Google Scholar] [CrossRef]
- Bu, M.; Yang, B.; Hu, L. Natural Bioactive Sterol 5α,8α-endoperoxides as Drug Lead Compounds. Med. Chem. 2014, 4, 709–716. [Google Scholar] [CrossRef]
- Bu, M.; Yang, B.; Hu, L. Natural Endoperoxides as Drug Lead Compounds. Curr. Med. Chem. 2016, 23, 383–405. [Google Scholar] [CrossRef]
- Pereira, R.B.; Pereira, D.M.; Jiménez, C.; Rodríguez, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentaõ, P. Anti-inflammatory effects of 5α,8α-epidioxycholest-6-en-3β-ol, a steroidal endoperoxide isolated from aplysia depilans, based on bioguided fractionation and NMR analysis. Mar. Drugs 2019, 17, 330. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Ma, W.; Fang, W.; Chen, Z.; Yang, B.; Liu, Y. Bioactivities of six sterols isolated from marine invertebrates. Pharm. Biol. 2014, 52, 187–190. [Google Scholar] [CrossRef]
- Zhong, C.; Jiang, C.; Xia, X.; Mu, T.; Wei, L.; Lou, Y.; Zhang, X.; Zhao, Y.; Bi, X. Antihepatic Fibrosis Effect of Active Components Isolated from Green Asparagus (Asparagus officinalis L.) Involves the Inactivation of Hepatic Stellate Cells. J. Agric. Food Chem. 2015, 63, 6027–6034. [Google Scholar] [CrossRef]
- Hua, L.; Li, Y.; Wang, F.; Lu, D.F.; Gao, K. Biologically active steroids from the aerial parts of Vernonia anthelmintica Willd. Fitoterapia 2012, 83, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Yang, K.; Wu, C.; Gao, S.; Wang, P.; Chen, L.; Li, H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia 2018, 125, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Shao, C.L.; Mei, W.L.; Huang, H.; Wang, C.Y.; Dai, H.F. Chemical constituents of gorgonian Verrucella umbraculum from the South China Sea. Biochem. Syst. Ecol. 2010, 38, 1085–1087. [Google Scholar] [CrossRef]
- Tian, X.R.; Tang, H.F.; Li, Y.S.; Lin, H.W.; Chen, X.L.; Ma, N.; Yao, M.N.; Zhang, P.H. New cytotoxic oxygenated sterols from the marine bryozoan Cryptosula pallasiana. Mar. Drugs 2011, 9, 162–183. [Google Scholar] [CrossRef]
- Bensemhoun, J.; Bombarda, I.; Aknin, M.; Faure, R.; Gaydou, E.M. 5α,8α-Epidioxysterols from the tunicate Didemnum salary. Biochem. Syst. Ecol. 2008, 36, 942–944. [Google Scholar] [CrossRef]
- Samorì, C.; Costantini, F.; Galletti, P.; Tagliavini, E.; Abbiati, M. Inter- and Intraspecific Variability of Nitrogenated Compounds in Gorgonian Corals via Application of a Fast One-Step Analytical Protocol. Chem. Biodivers. 2018, 15, e1700449. [Google Scholar] [CrossRef]
- Reyes, F.; Martín, R.; Fernández, R. Granulatamides A and B, Cytotoxic Tryptamine Derivatives from the Soft Coral Eunicella granulata. J. Nat. Prod. 2006, 69, 668–670. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, S.; De Stefano, S. Antiviral agents from a gorgonian, Eunicella cavolini. Experientia 1984, 40, 339–340. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Marwood, J.F.; Wells, R.J. 2-phenyiethylamides of a novel lipid acid; Atrial stimulants from the soft coral sinularia flexibilis. Aust. J. Chem. 1980, 33, 1799–1803. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Ravi, B.N.; Lance Sanders, R.; Wells, J.R. Spermidine derivatives and 9,11-secosteroids from a soft coral (Sinularia sp.). Aust. J. Chem. 1982, 35, 69–75. [Google Scholar] [CrossRef]
- Ortega, M.J.; Zubía, E.; Salva, J. Structure and absolute configuration of palmonine F, a new eunicellin-based diterpene from the gorgonian Eunicella verrucosa. J. Nat. Prod. 1994, 57, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Byon, J.C.H.; Kusari, A.B.; Kusari, J. Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol. Cell. Biochem. 1998, 182, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bentires-Alj, M.; Neel, B.G. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 2007, 67, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Di Leva, F.S.; D’Amore, C.; Festa, C.; De Marino, S.; Renga, B.; D’Auria, M.V.; Novellino, E.; Limongelli, V.; D’Souza, L.; et al. Marine and semi-synthetic hydroxysteroids as new scaffolds for pregnane X receptor modulation. Mar. Drugs 2014, 12, 3091–3115. [Google Scholar] [CrossRef]
- Carazo, A.; Mladěnka, P.; Pávek, P. Marine Ligands of the Pregnane X Receptor (PXR): An Overview. Mar. Drugs 2019, 17, 554. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Bifulco, G.; D’Auria, M.V.; Zampella, A. Marine sponge steroids as nuclear receptor ligands. Trends Pharmacol. Sci. 2012, 33, 591–601. [Google Scholar] [CrossRef]
- Kongkathip, B.; Hasakunpaisarn, A.; Boonananwong, S.; Kongkathip, N. Synthesis of cytotoxic novel 9,11-secosterol analogs: Structure/activity studies. Steroids 2010, 75, 834–847. [Google Scholar] [CrossRef]
- Luo, X.; Li, F.; Shinde, P.B.; Hong, J.; Lee, C.O.; Im, K.S.; Jung, J.H. 26,27-cyclosterols and other polyoxygenated sterols from a marine sponge Topsentia sp. J. Nat. Prod. 2006, 69, 1760–1768. [Google Scholar] [CrossRef]
- Mun, B.; Wang, W.; Kim, H.; Hahn, D.; Yang, I.; Won, D.H.; Kim, E.H.; Lee, J.; Han, C.; Kim, H.; et al. Cytotoxic 5α,8α-epidioxy sterols from the marine sponge Monanchora sp. Arch. Pharm. Res. 2015, 38, 18–25. [Google Scholar] [CrossRef]
- Wu, S.B.; Bao, Q.Y.; Wang, W.X.; Zhao, Y.; Xia, G.; Zhao, Z.; Zeng, H.; Hu, J.F. Cytotoxic triterpenoids and steroids from the bark of Melia azedarach. Planta Med. 2011, 77, 922–928. [Google Scholar] [CrossRef]
- Cao, S.; Brodie, P.; Miller, J.S.; Birkinshaw, C.; Rakotondrafara, A.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Antiproliferative compounds of Helmiopsis sphaerocarpa from the Madagascar rainforest. Nat. Prod. Res. 2009, 23, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Raslan, A.E.; Radwan, M.M.; Ahmed, S.A.; Nafady, A.M.; Zaki, M.A.; Wanas, A.S.; Abou-Karam, M.; Shier, T.W.; Hassanean, H.A.; ElSohly, M.A. Monanchoramides A–D, ceramides from the marine sponge Monanchora clathrata with cytotoxic activity. Phytochem. Lett. 2018, 23, 83–89. [Google Scholar] [CrossRef]
- Tian, X.R.; Gao, Y.Q.; Tian, X.L.; Li, J.; Tang, H.F.; Li, Y.S.; Lin, H.W.; Ma, Z.Q. New cytotoxic secondary metabolites from marine bryozoan Cryptosula pallasiana. Mar. Drugs 2017, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, Y.T.; Chang, C.I.; Ho, C.T.; Pan, B.S. Apoptotic-inducing epidioxysterols identified in hard clam (Meretrix lusoria). Food Chem. 2007, 102, 788–795. [Google Scholar] [CrossRef]
- Yang, M.; Liang, L.F.; Li, H.; Tang, W.; Guo, Y.W. A new 5α,8α-epidioxysterol with immunosuppressive activity from the South China Sea soft coral Sinularia sp. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Huynh, T.-H.; Chen, P.-C.; Yang, S.-N.; Lin, F.-Y.; Su, T.-P.; Chen, L.-Y.; Peng, B.-R.; Hu, C.-C.; Chen, Y.-Y.; Wen, Z.-H.; et al. New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp. Mar. Drugs 2019, 17, 530. [Google Scholar] [CrossRef]
- Gauvin, A.; Smadja, J.; Aknin, M.; Faure, R.; Gaydou, E.M. Isolation of bioactive 5α,8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can. J. Chem. 2000, 78, 986–992. [Google Scholar] [CrossRef]
- Liang, L.F.; Wang, X.J.; Zhang, H.Y.; Liu, H.L.; Li, J.; Lan, L.F.; Zhang, W.; Guo, Y.W. Bioactive polyhydroxylated steroids from the Hainan soft coral Sinularia depressa Tixier-Durivault. Bioorganic Med. Chem. Lett. 2013, 23, 1334–1337. [Google Scholar] [CrossRef]
- Huang, R.M.; Chen, Y.N.; Zeng, Z.; Gao, C.H.; Su, X.; Peng, Y. Marine nucleosides: Structure, bioactivity, synthesis and biosynthesis. Mar. Drugs 2014, 12, 5817–5838. [Google Scholar] [CrossRef]
- Calado, R.; Leal, M.C.; Gaspar, H.; Santos, S.; Marques, A.; Nunes, M.L.; Vieira, H. How to Succeed in Marketing Marine Natural Products for Nutraceutical, Pharmaceutical and Cosmeceutical Markets. In Grand Challenges in Marine Biotechnology; Springer: Berlin, Germany, 2018; pp. 317–403. [Google Scholar]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- Turicchia, E.; Abbiati, M.; Sweet, M.; Ponti, M. Mass mortality hits gorgonian forests at Montecristo Island. Dis. Aquat. Organ. 2018, 131, 79–85. [Google Scholar] [CrossRef] [PubMed]
- de la Linde-Rubio, A.; Tierno de Figueroa, J.M.; López-Rodríguez, M.J.; Sánchez-Tocino, L. Mass mortality of Eunicella singularis (Anthozoa: Octocorallia) in the Chafarinas Islands (north Africa, western Mediterranean Sea). Rev. Biol. Mar. Oceanogr. 2018, 53, 285–290. [Google Scholar] [CrossRef]
- Ezzat, L.; Merle, P.L.; Furla, P.; Buttler, A.; Ferrier-Pagès, C. The Response of the Mediterranean Gorgonian Eunicella singularis to Thermal Stress Is Independent of Its Nutritional Regime. PLoS ONE 2013, 8, e64370. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Aceto, S.; Saggiomo, M.; Mangoni, O.; De Vico, G. Gorgonian disease outbreak in the Gulf of Naples: Pathology reveals cyanobacterial infection linked to elevated sea temperatures. Dis. Aquat. Organ. 2014, 111, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.; Cebrian, E.; Kipson, S.; Garrabou, J. Does thermal history influence the tolerance of temperate gorgonians to future warming? Mar. Environ. Res. 2013, 89, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Calado, R.; Sheridan, C.; Alimonti, A.; Osinga, R. Coral aquaculture to support drug discovery. Trends Biotechnol. 2013, 31, 555–561. [Google Scholar] [CrossRef]
- Jansen, D.J.; Shenvi, R.A. Synthesis of medicinally relevant terpenes: reducing the cost and time of drug discovery. Futur. Med Chem 2014, 6, 1127–1148. [Google Scholar] [CrossRef]
- Mandrekar, V.K.; Gawas, U.B.; Majik, M.S. Brominated Molecules From Marine Algae and Their Pharmacological Importance. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 61, pp. 461–490. [Google Scholar]
- Clark, J.S.; Baxter, C.A.; Castro, J.L. Synthesis of the tricyclic core of the marine natural product labiatin A. Synthesis (Stuttg.) 2005, 19, 3398–3404. [Google Scholar] [CrossRef]
- Clark, J.S.; Vignard, D.; Parkin, A. Synthesis of the tricyclic core of labiatin A and Australin A. Org. Lett. 2011, 13, 3980–3983. [Google Scholar] [CrossRef]
- Chai, Y.; Vicic, D.A.; McIntosh, M.C. Cycloaldol approach to the isobenzofuran core of eunicellin diterpenes. Org. Lett. 2003, 5, 1039–1042. [Google Scholar] [CrossRef]
- Chai, Y.; Mou, Z.; McIntosh, M.C. Studies directed toward the synthesis of the massileunicellins. Part 2. Tetrahedron Lett. 2010, 51, 2393–2395. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.F.; White, J.M.; Holmes, A.B. Development of the claisen rearrangement/organocatalytic diels-alder approach for the synthesis of eunicellins. Aust. J. Chem. 2014, 67, 1189–1194. [Google Scholar] [CrossRef]
- Ellis, J.M.; Crimmins, M.T. Strategies for the total synthesis of C2-C11 cyclized cembranoids. Chem. Rev. 2008, 108, 5278–5298. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, D.W.; Overman, L.E. Enantioselective Total Synthesis of (−)-7-Deacetoxyalcyonin Acetate. First Synthesis of a Eunicellin Diterpene. J. Am. Chem. Soc. 1995, 117, 10391–10392. [Google Scholar] [CrossRef]
- Chai, Y.; McIntosh, M.C. Studies directed toward the synthesis of the massileunicellins. Tetrahedron Lett. 2004, 45, 3269–3272. [Google Scholar] [CrossRef][Green Version]
- Clark, J.S.; Hayes, S.T.; Wilson, C.; Gobbi, L. A concise total synthesis of (±)-vigulariol. Angew. Chemie Int. Ed. 2007, 46, 437–440. [Google Scholar] [CrossRef]
- Clark, J.S.; Berger, R.; Hayes, S.T.; Thomas, L.H.; Morrison, A.J.; Gobbi, L. Enantioselective total syntheses of three cladiellins (Eunicellins): A general approach to the entire family of natural products. Angew. Chemie Int. Ed. 2010, 49, 9867–9870. [Google Scholar] [CrossRef]
- Pakhare, D.; Kusurkar, R. Application of Horner-Wadsworth-Emmons olefination for the synthesis of granulatamide A, its: E isomer and other amides of tryptamine. New J. Chem. 2016, 40, 5428–5431. [Google Scholar] [CrossRef]
- Legros, J.; Figadère, B. Iron-promoted C-C bond formation in the total synthesis of natural products and drugs. Nat. Prod. Rep. 2015, 32, 1541–1555. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

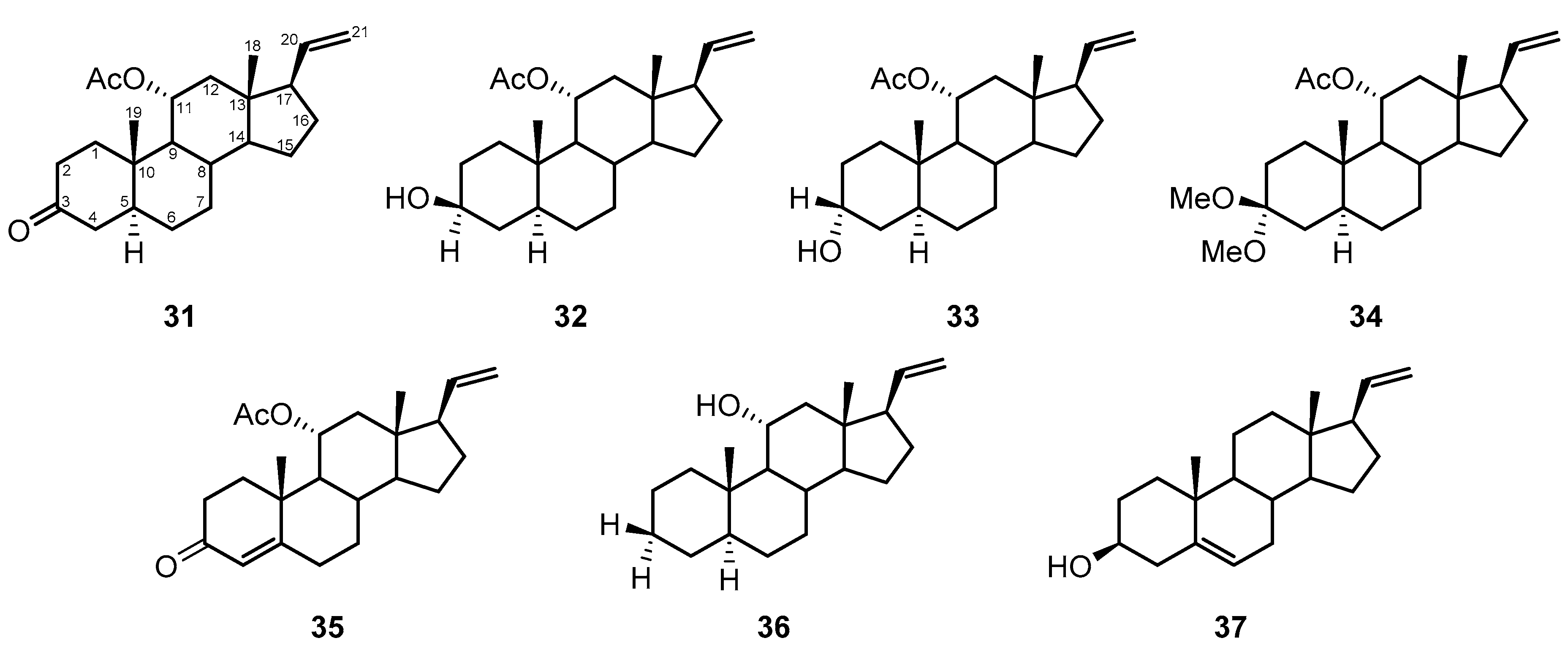
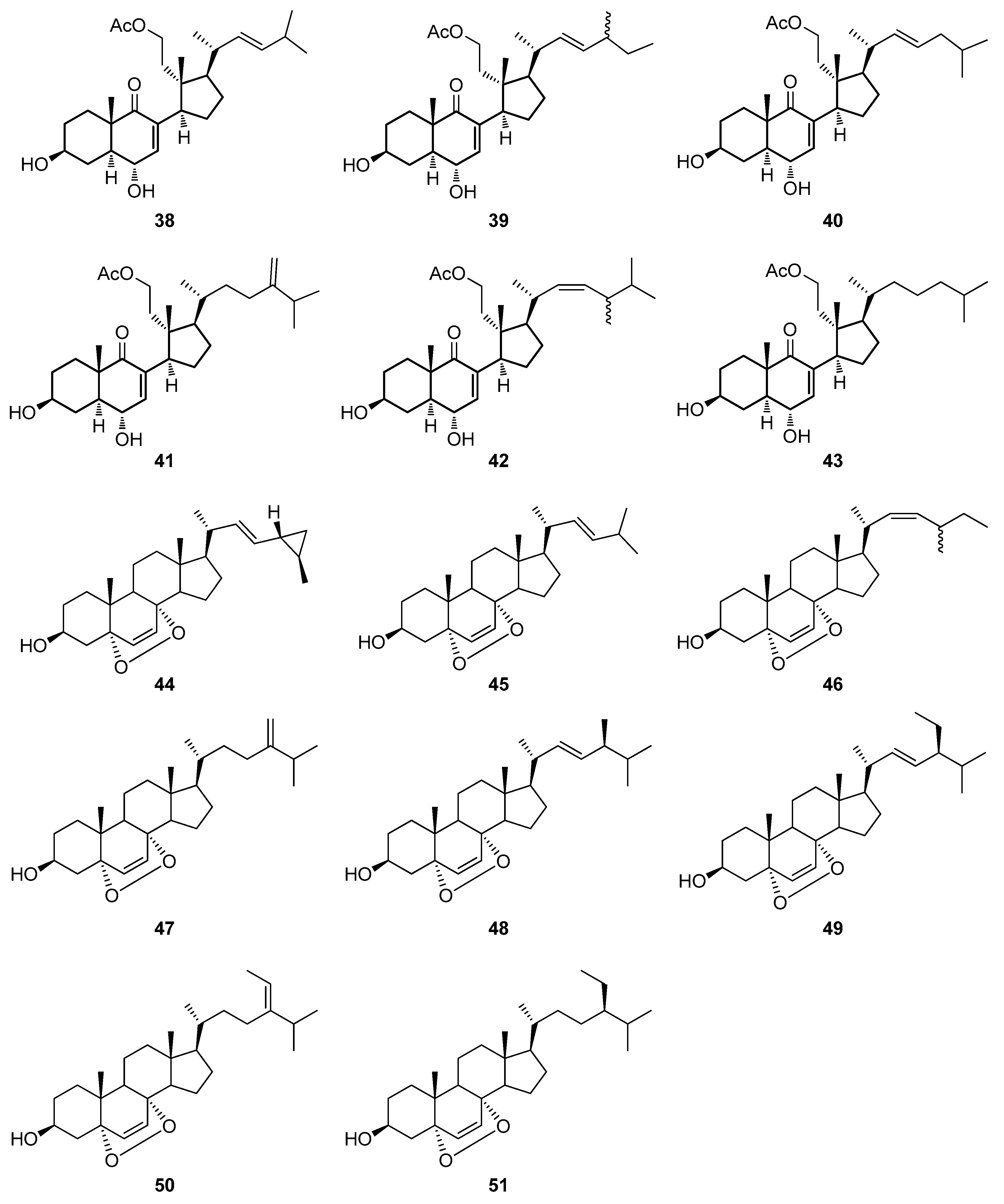
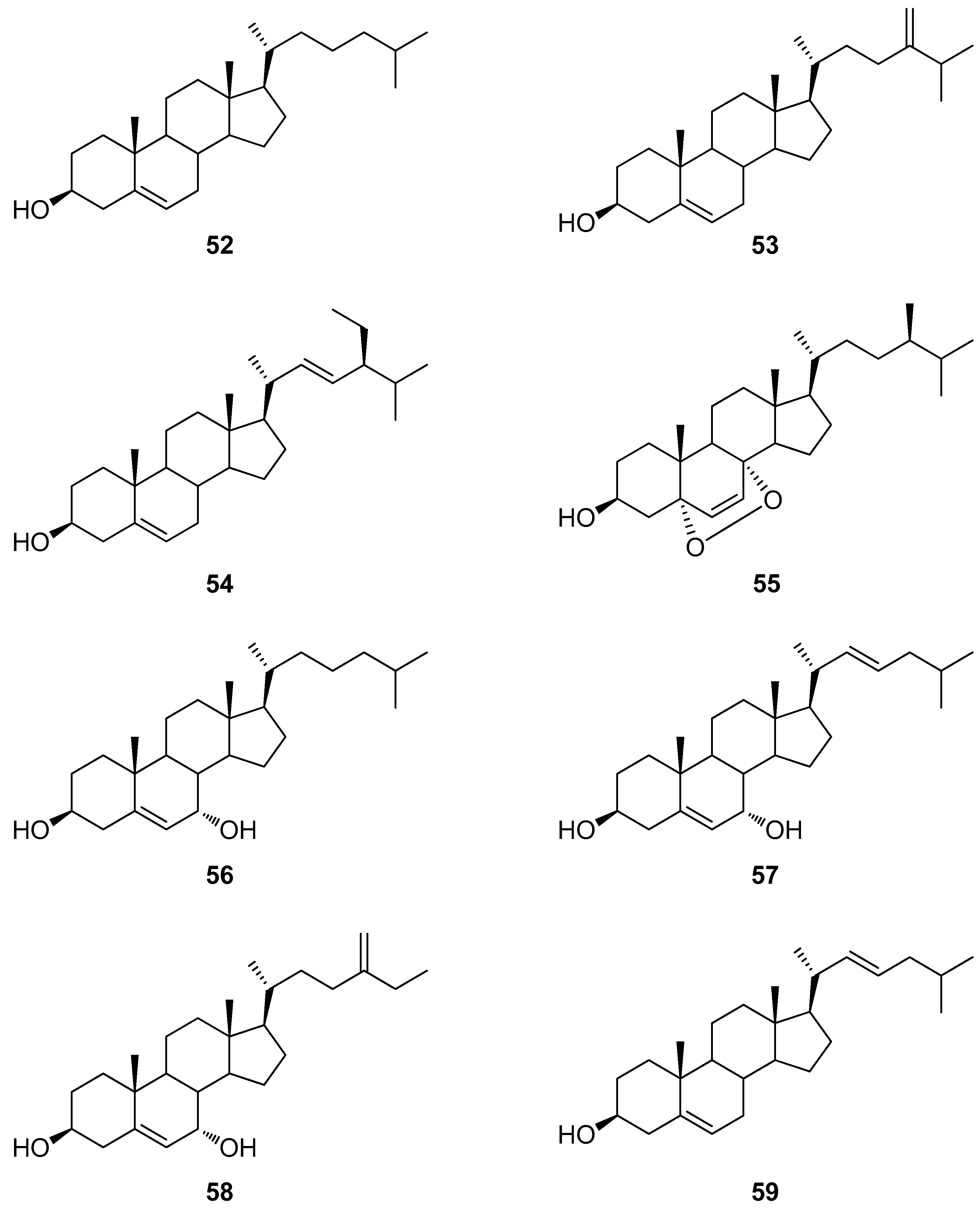
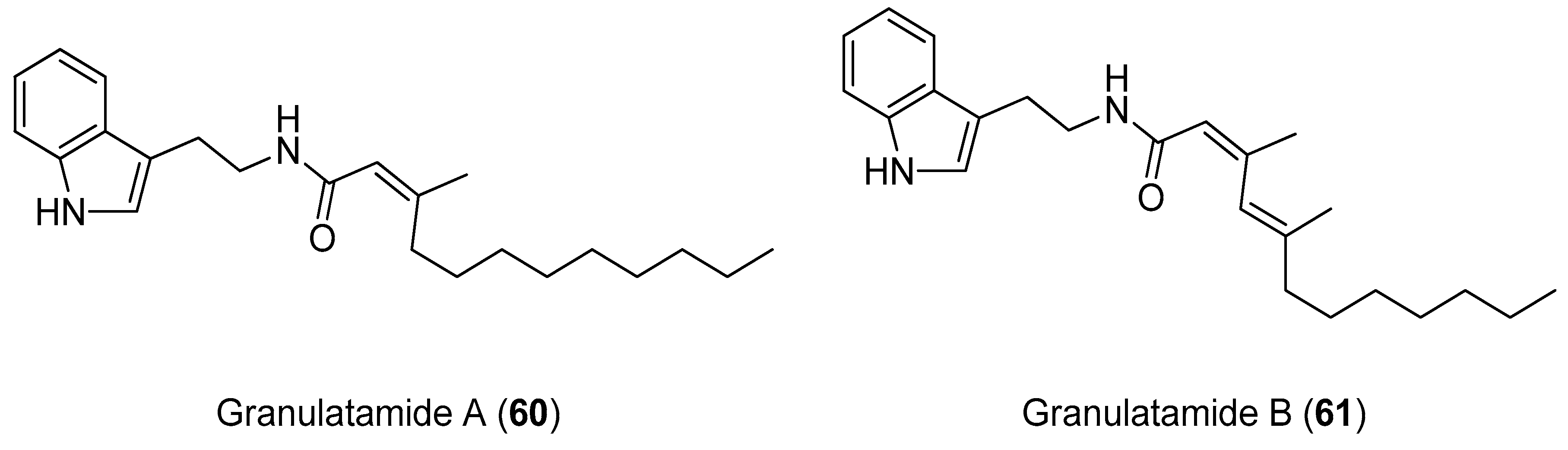
| Compound | Name | Source | Tumor Cell Line | IC50/(µg/mL) | Reference |
|---|---|---|---|---|---|
| 3 | Palmonine B | E. verrucosa | P-388 MEL28 | 5 5 | [55] |
| 5 | Palmonine D | E. verrucosa E. singularis | MCF-7 | 49 | [19] |
| 7 | Palmonine F | E. verrucosa E. singularis | MCF-7 | 13 | [19] |
| 12 | Labiatin B | E. labiata | HCT-116 | 0.85 | [26] |
| 13 | Labiatin C | E. labiata | NSCLC-N6 | 7.7 | [22] |
| 15 | Labiatin E | E. labiata | NSCLC-N6 | 35 | [22] |
| Compound | Class | Source | Tumor Cell Line | IC50 * | Reference |
|---|---|---|---|---|---|
| 38 | Secosterol | E. cavolini | MCF-7 HeLa | 7.6 a 28.1 a | [34] [38] |
| 39 | Secosterol | E. cavolini | MCF-7 | 7.4 a | [34] |
| 43 | Secosterol | E. cavolini S. suberosa | HeLa | 23.3 a | [38] |
| 45 | Epidioxysterol | E. cavolini Topsentia sp. | A549 SK-OV-3 SK-MEL-2 HCT15 | 30.0 b 19.4 b 25.9 b 21.4 b | [62] |
| 47 | Epidioxysterol | E. cavolini Monanchora sp. | A-498 | 23 a | [63] |
| 48 | Epidioxysterol | E. cavolini E. singularis M. azedarach H. sphaerocarpa | A549 H460 HGC27 A2780 | 10.9 b 10.9 b 11.6 b 16 b | [64,65] |
| 59 | Sterol | E. singularis | MCF-7 | 30 b | [19] |
| Compound | IC50/µM | ||||||
|---|---|---|---|---|---|---|---|
| Du-145 | LN-caP | SK-BR3 | HT29 | IGROV | A549 | K-562 | |
| 60 | 1.7 | 4.7 | 2.7 | 2.2 | 6.7 | 6.7 | 6.8 |
| 61 | 7.7 | 3.5 | 6.0 | >10 | 8.2 | 8.9 | 4.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulja, D.; Kolympadi Markovic, M.; Ambrožić, G.; Laclef, S.; Pavelić, S.K.; Marković, D. Secondary Metabolites from Gorgonian Corals of the Genus Eunicella: Structural Characterizations, Biological Activities, and Synthetic Approaches. Molecules 2020, 25, 129. https://doi.org/10.3390/molecules25010129
Matulja D, Kolympadi Markovic M, Ambrožić G, Laclef S, Pavelić SK, Marković D. Secondary Metabolites from Gorgonian Corals of the Genus Eunicella: Structural Characterizations, Biological Activities, and Synthetic Approaches. Molecules. 2020; 25(1):129. https://doi.org/10.3390/molecules25010129
Chicago/Turabian StyleMatulja, Dario, Maria Kolympadi Markovic, Gabriela Ambrožić, Sylvain Laclef, Sandra Kraljević Pavelić, and Dean Marković. 2020. "Secondary Metabolites from Gorgonian Corals of the Genus Eunicella: Structural Characterizations, Biological Activities, and Synthetic Approaches" Molecules 25, no. 1: 129. https://doi.org/10.3390/molecules25010129
APA StyleMatulja, D., Kolympadi Markovic, M., Ambrožić, G., Laclef, S., Pavelić, S. K., & Marković, D. (2020). Secondary Metabolites from Gorgonian Corals of the Genus Eunicella: Structural Characterizations, Biological Activities, and Synthetic Approaches. Molecules, 25(1), 129. https://doi.org/10.3390/molecules25010129







