Trimethoprim: An Old Antibacterial Drug as a Template to Search for New Targets. Synthesis, Biological Activity and Molecular Modeling Study of Novel Trimethoprim Analogs
Abstract
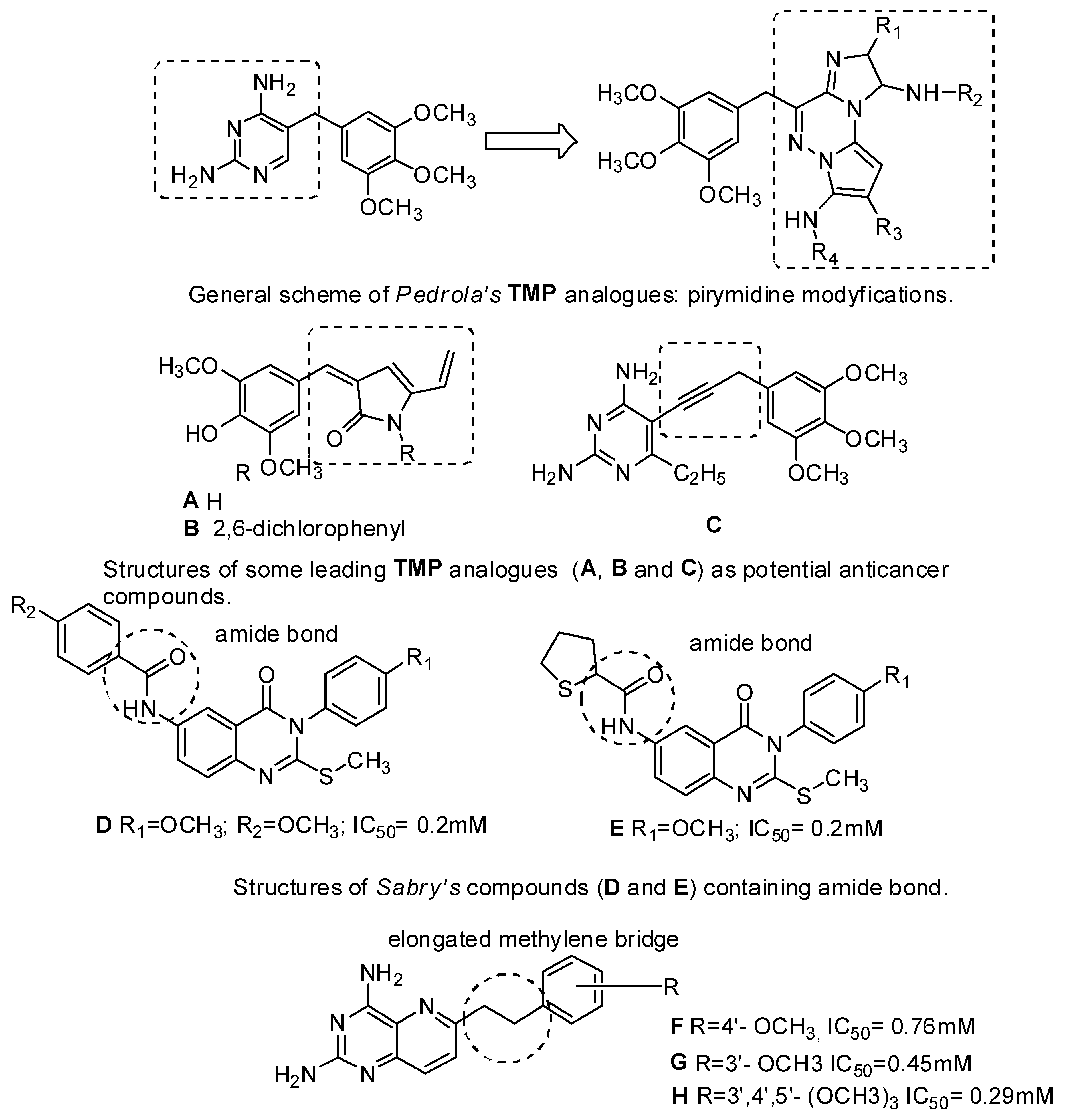
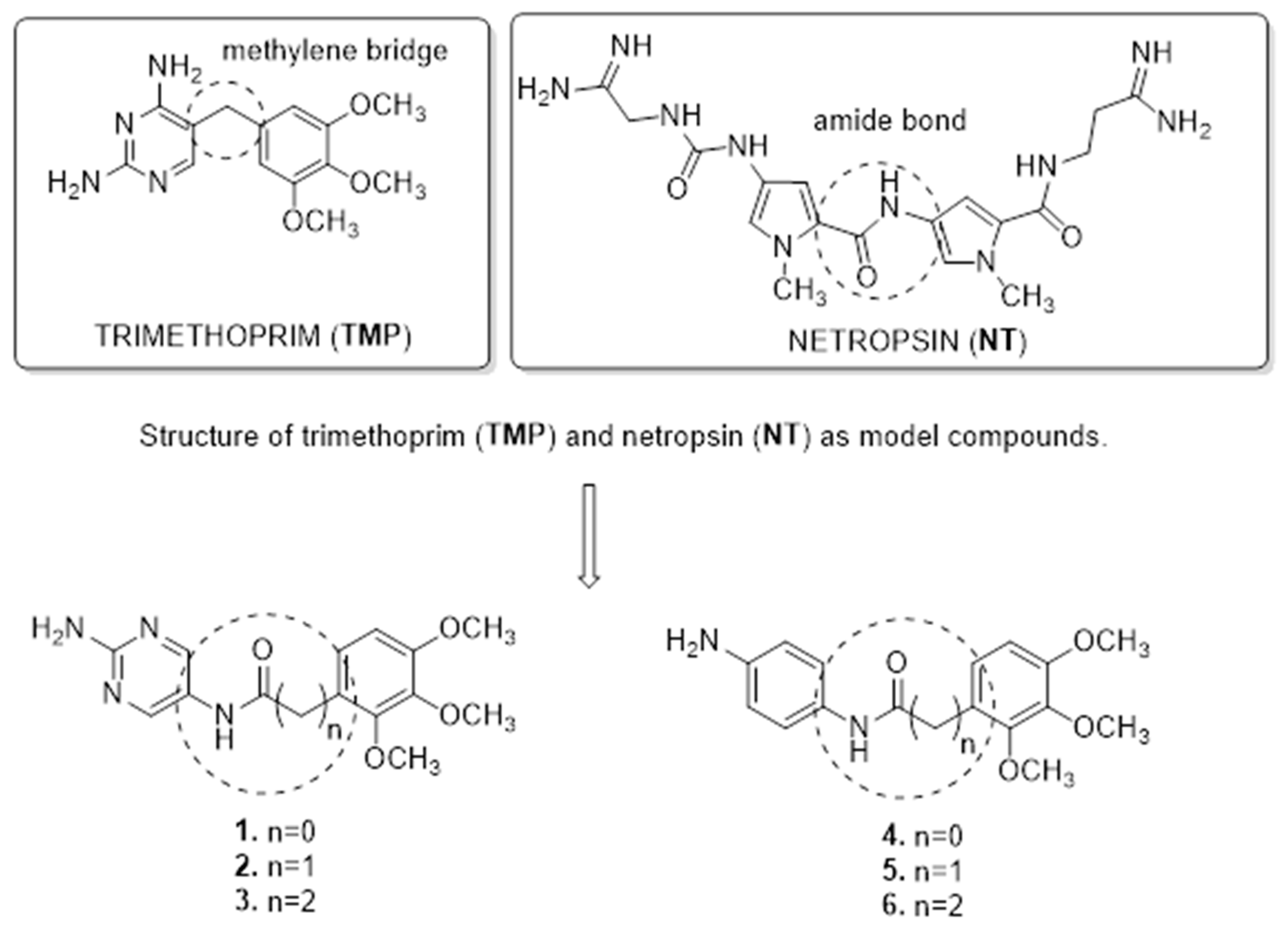
1. Introduction
2. Results and Discussion
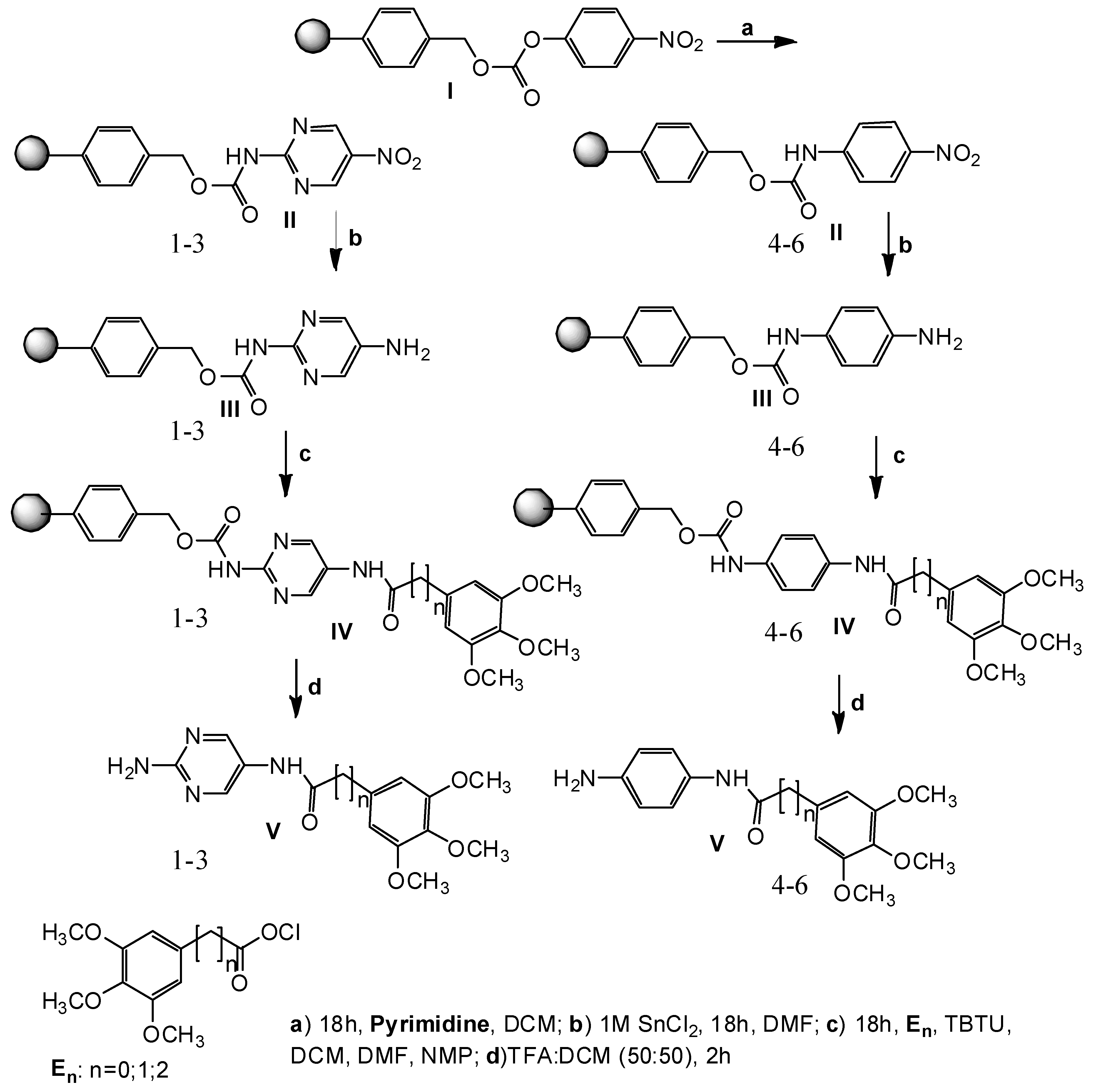
2.1. Preparation of TMP Analogs
2.2. Biological Assays
2.2.1. The Ethidium Bromide Assay—DNA-Binding Effects
2.2.2. Ethidium Displacement Assay—Determination of DNA-Binding Constants
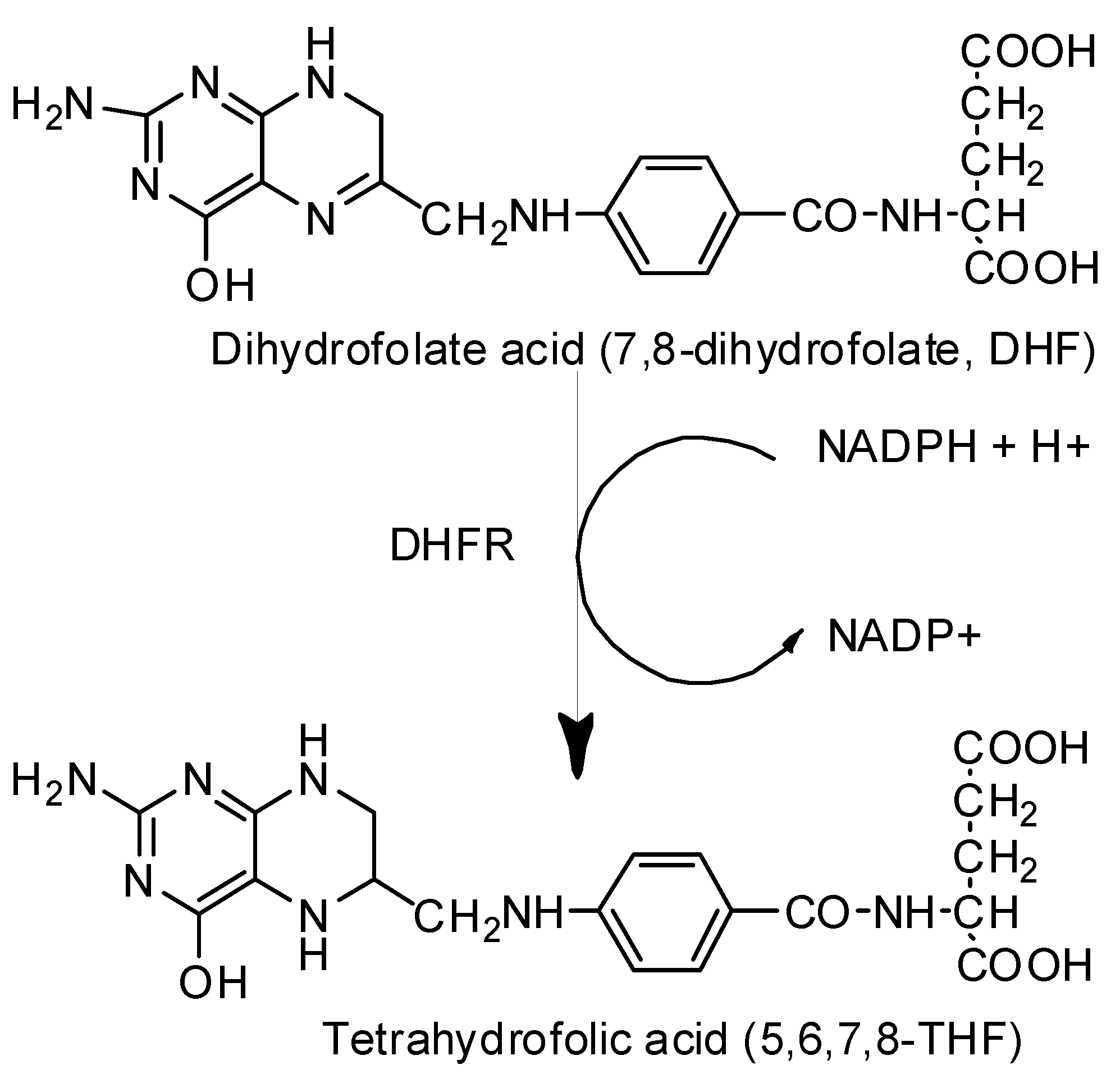
2.2.3. Dihydrofolate Reductase (DHFR) Inhibition
2.2.4. Molecular Docking
3. Material and Methods
3.1. General Information
3.2. General Procedure
3.3. The Ethidium Bromide Assay—DNA-Binding Effects
3.4. The Ethidium Displacement Bromide Assay—Determination of DNA-Binding Constants
3.5. Dihydrofolate Reductase (DHFR) Inhibition Assay
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Hawser, S.; Lociuro, S.; Islam, K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem. Pharmacol. 2006, 71, 941–948. [Google Scholar] [CrossRef]
- Chan, D.C.M.; Anderson, A.C. Towards species-specific antifolates. Curr. Med. Chem. 2006, 13, 377–398. [Google Scholar] [CrossRef]
- Champe, P.C.; Harvey, R.A. Lippincott’s Illustrated Reviews: Biochemistry, 2nd ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Foye, W.O.; Lemke, T.L.; Williams, D.A. Principles of medicinal chemistry, 4th ed.; Williams and Wilkins, Media: Philadelphia, PA, USA, 2005. [Google Scholar]
- Snapka, R.M.; Ge, S.; Trask, J.; Robertson, F. Unbalanced growth in mouse cells with amplified dhfr genes. Cell Prolif. 1997, 30, 385–399. [Google Scholar] [CrossRef]
- Wang, M.; Yanga, J.; Yuana, M. Synthesis and antiproliferative activity of a series of novel 6-substituted pyrido[3,2-d]pyrimidines as potential non-classical lipophilic antifolates targeting dihydrofolate reductase. Eur. J. Med. Chem. 2017, 128, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Blakley, R.L. Eukaryotic dihydrofolate reductase. Adv. Enzymol. Relat. Areas. Mol. Biol. 1995, 70, 23–102. [Google Scholar] [PubMed]
- Heaslet, H.; Harris, M.; Fahnoe, K.; Sarver, R.; Putz, H.; Chang, J.; Subramanyam, C.; Barreiro, G.; Miller, J.R. Structural comparison of chromosomal and exogenous dihydrofolate reductase from Staphylococcus aureus in complex with the potent inhibitor trimethoprim. Proteins 2009, 76, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Wang, Y.; Chang, Z.; Yang, Y.; Pu, J.; Sun, T.; Kaur, S.; Sacchettini, J.C.; Jung, H.; Wong, W.L.; et al. The identification of novel Mycobacterium tuberculosis DHFR inhibitors and the investigation of their binding preferences by using molecular modelling. Sci. Rep. 2015, 5, 15328. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Thapliyal, C.; Chaudhuri, P. Dihydrofolate reductase as a versatile drug target in healthcare. JPP 2016, 7, 247–257. [Google Scholar]
- Bhosle, A.; Chandra, N. Structural analysis of dihydrofolate reductases enables rationalization of antifolate binding affinities and suggests repurposing possibilities. FEBS J. 2016, 283, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Selassie, C.D.; Li, R.-L.; Poe, M.; Hansch, C. Optimization of hydrophobic and hydrophilic substituent interactions of 2,4-diamino-5-(substituted-benzyl)pyrimidines with dihydrofolate reductase. J. Med. Chem. 1991, 34, 46–54. [Google Scholar] [CrossRef]
- Nammalwar, B.; Bourne, C.R.; Wakeham, N.; Bourne, P.C.; Barrow, E.W.; Muddala, N.P.; Bunce, R.A.; Berlin, K.D.; Barrow, W.W. Modified 2,4-diaminopyrimidine-based dihydrofolate reductase inhibitors as potential drug scaffolds against Bacillus anthracis. Bioorg. Med. Chem. 2014, 23, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.G.; Rosowsky, A. Dicyclic and Tricyclic Diaminopyrimidine Derivatives as Potent Inhibitors of Cryptosporidium parvum Dihydrofolate Reductase: Structure-Activity and Structure-Selectivity Correlations. Antimicrob. Agents Chemother. 2001, 45, 3293–3303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Srinivasan, B.; Skolnick, J. Insights into the slow-onset tight-binding inhibition of Escherichia coli dihydrofolate reductase: Detailed mechanistic characterization of pyrrolo [3,2-f] quinazoline-1,3-diamine and its derivatives as novel tight-binding inhibitors. FEBS J. 2015, 282, 1922–1938. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.C.; Biggadike, K.; McKilligin, E.; Kinsman, O.S.; Queener, S.F.; Lane, A.; E Smith, J. 6,7-disubstituted 2,4-diaminopteridines: novel inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. Antimicrob. Agents Chemother. 1996, 40, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Tonddast-Navaei, S.; Skolnick, J. Ligand binding studies, preliminary structure-activity relationship and detailed mechanistic characterization of 1-phenyl-6,6-dimethyl-1,3,5-triazine-2,4-diamine derivatives as inhibitors of Escherichia coli dihydrofolate reductase. Eur. J. Med. Chem. 2015, 103, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef]
- Mc Carron, P.; Crowley, A.; O’Shea, D.; McCann, M.; Howe, O.; Hunt, M.; Devereux, M. Targeting the Folate Receptor: Improving Efficacy in Inorganic Medicinal Chemistry. Curr. Med. Chem. 2018, 25, 2675–2708. [Google Scholar] [CrossRef]
- Wróbel, A.; Drozdowska, D. Recent Design and Structure-Activity Relationship Studies on Modifications of DHFR Inhibitors as Anticancer Agents. Curr. Med. Chem. 2019, 26, 1. [Google Scholar] [CrossRef]
- Berman, E.M.; Werbel, L.M. The renewed potential for folate antagonists in contemporary cancer chemotherapy. J. Med. Chem. 1991, 34, 479–485. [Google Scholar] [CrossRef]
- Mullarkey, M.F.; Blumenstein, B.A.; Andrade, W.P.; Bailey, G.A.; Olason, I.; Wetzel, C.E. Methotrexate in the treatment of corticosteroid-dependent asthma. A double blind crossover study. N. Engl. J. Med. 1988, 318, 603–607. [Google Scholar] [CrossRef]
- Grivsky, E.M.; Lee, S.; Sigel, C.W.; Duch, D.S.; Nichol, C.A. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethyloxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J. Med. Chem. 1980, 23, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Bavetsias, V.; Jackman, A.L.; Marriott, J.H.; Kimbell, R.; Gibson, W.; Boyle, F.T.; Bisset, G.M. Folate based inhibitors of thymidylate synthase. J. Med. Chem. 1997, 40, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Bavetsias, V.; Marriott, J.H.; Melin, C.; Kimbell, R.; Matusiak, Z.S.; Boyle, F.T.; Jackman, A.L. Design and synthesis of cyclopenta[g]quinazoline-based antifolate as inhibitors of thymidylate synthase and potential antitumor agents. J. Med. Chem. 2000, 43, 1910–1926. [Google Scholar] [CrossRef] [PubMed]
- Werbel, L.M.; Degnan, M.J. Antimalarial drugs. 63. Synthesis and antimalarial and antitumor effects of 2-amino-4-(hydrazino and hydroxyamino)-6-[(aryl)thio]quinazolines. J. Med. Chem. 1987, 30, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.L.; Feng, Y.P.; Jiang, Y.Y.; Liu, S.Y.; Ding, G.Y.; Li, R.T. Synthesis and in vitro antitumor activity of 4(3H)-quinazolione derivatives with dithiocarbamate side chains. Bioorg. Med. Chem. Lett. 2005, 15, 1915–1917. [Google Scholar] [CrossRef] [PubMed]
- Wyss, P.C.; Gerber, P.; Hartman, P.G.; Hubschwerlen, C.; Locher, H.; Marty, H.P.; Stahl, M. Novel dihydrofolate reductase inhibitors. Structure-based versus diversity-based library design and high- throughput synthesis and screening. J. Med. Chem. 2003, 46, 2304–2312. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef]
- Drugs.com. Trimethoprim. Available online: https://www.drugs.com/pro/trimethoprim.html (accessed on 21 November 2019).
- Brogden, R.N.; Carmine, A.A.; Heel, R.C.; Speight, T.M.; Avery, G.S. Trimethoprim: A Review of itsantibacterial activity, pharmacokinetics and therapeutic use in urinary tract infections. Drugs 1982, 23, 405–430. [Google Scholar] [CrossRef]
- Sirotank, F.M.; Burchall, J.J.; Ensminger, W.B. Folate Antagonists as Therapeutic Agents; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Wróbel, A.; Arciszewska, K.; Maliszewski, D.; Drozdowska, D. Trimethoprim and other non-classical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors. J. Antibiot. 2019, 73, 5–27. [Google Scholar] [CrossRef]
- Pedrola, M.; Jorba, M.; Jardas, E.; Jardi, F.; Ghashghaei, O.; Viñas, M.; Lavilla, R. Multicomponent Reactions Upon the Known Drug Trimethoprim as a Source of Novel Antimicrobial Agents. Front. Chem. 2019, 7, 475. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, M.; Sachdeva, S. Mechanism inspired the development of rationally designed dihydrofolate reductase inhibitors as anticancer agents. J. Med. Chem. 2012, 55, 6381–6390. [Google Scholar] [CrossRef] [PubMed]
- Algul, O.; Paulsen, J.L.; Anderson, A.C. 2,4-Diamino-5-(2′-aryl-propargyl)pyrimidine derivatives as new nonclassical antifolates for human dihydrofolate reductase inhibition. J. Mol. Graph. Model. 2011, 29, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Viegas-Junior, C.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Fonseca, L.M. The benefits of the multi-target approach in drug design and discovery. Bioorg. Med. Chem. 2006, 14, 896–897. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Meegan, M.J. Designed multiple ligands for cancer therapy. Curr. Med. Chem. 2011, 18, 4722–4737. [Google Scholar]
- Finlay, A.C.; Hochstein, F.A.; Sobin, B.A. Netropsin, a new antibiotic producedby a Streptomyces. JACS 1951, 73, 341–343. [Google Scholar] [CrossRef]
- Berman, H.M.; Neidle, S.; Zimmer, C. Netropsin, a DNA-binding oligopeptidestructural and binding studies. Biochim. Biophys. Acta 1979, 561, 124–131. [Google Scholar] [CrossRef]
- Arcamone, F.; Lazzari, E.; Menozzi, M.; Soranzo, C.; A Verini, M. Synthesis, DNA binding and antiviral activity of distamycin analogues containing different heterocyclic moieties. Anti-Cancer Drug Des. 1986, 1, 124–131. [Google Scholar]
- Ong, C.W.; Yang, P.S. Minor-Groove Binding Agents: Rational Design of Carboxamide Bond Isosteres. Curr. Top. Med. Chem. 2015, 15, 1359–1371. [Google Scholar] [CrossRef]
- Sabry, M.A.; Ewida, H.A.; Hassan, G.S.; Ghaly, M.A.; El-Subbagh, H.I. Synthesis, antitumor testing and molecular modeling study of some new 6-substituted amido, azo or thioureido-quinazolin-4(3H)-ones. Bioorg. Chem. 2019, 88, 102923. [Google Scholar] [CrossRef]
- Szerszenowicz, J.; Drozdowska, D. Semi-Automatic Synthesis, Antiproliferative Activity and DNA-Binding Properties of New Netropsin and bis-Netropsin Analogues. Molecules 2014, 19, 11300–11315. [Google Scholar] [CrossRef]
- Drozdowska, D.; Rusak, M.; Miltyk, W.; Markowska, A.; Samczuki, P. Antiproliferative effects on breast cancer cells and some interactions of new distamycin analogues with dna, endonucleases and dna topoisomerases. Acta Pol. Pharm.-Drug Res. 2016, 73, 47–53. [Google Scholar]
- Morgan, A.R.; Lee, J.S.; Pulleyblank, D.E.; Murray, N.L.; Evans, D.H. Review: Ethidium fluorescence assays. Part 1. Physicochemical studies. Nucleic Acids Res. 1979, 7, 547. [Google Scholar] [CrossRef] [PubMed]
- Debart, F.; Periguad, C.; Gosselin, G.; Mrani, D.; Rayner, B.; Le Ber, P.; Auclair, C.; Balzarini, J.; De Clercq, E.; Paoletti, C. Synthesis, DNA binding, and biological evaluation of synthetic precursors and novel analogues of netropsin. J. Med. Chem. 1989, 32, 1074. [Google Scholar] [CrossRef] [PubMed]
- Lown, J.W. Newer approaches to the study of the mechanisms of action of antitumor antibiotics. Acc. Chem. Res. 1982, 15, 381. [Google Scholar] [CrossRef]
- Sigma Aldrich. Technical Bulletin. Available online: https://www.sigmaaldrich.com/content/dam/sigmaaldrich/docs/Sigma/Bulletin/cs0340bul.pdf (accessed on 21 November 2019).
- Francesconi, V.; Giovannini, L.; Santucci, M.; Cichero, E.; Costi, M.P.; Naesens, L.; Giordanetto, F.; Tonelli, M. Synthesis, biological evaluation and molecular modeling of novel azaspiro dihydrotriazines as influenza virus inhibitors targeting the host factor dihydrofolate reductase (DHFR). Eur. J. Med. Chem. 2018, 155, 229–243. [Google Scholar] [CrossRef]
- Rana, R.M.; Rampogu, S.; Zeb, A.; Son, M.; Park, C.; Lee, G.; Yoon, S.; Baek, A.; Parameswaran, S.; Park, S.J.; et al. In Silico Study Probes Potential Inhibitors of Human Dihydrofolate Reductase for Cancer Therapeutics. J. Clin. Med. 2019, 8, 233. [Google Scholar] [CrossRef]
- Drozdowska, D. New solid phase synthesis of distamycin analogues. Molecules 2011, 16, 3066–3076. [Google Scholar]
- Pućkowska, A.; Drozdowska, D.; Midura-Nowaczek, K. Carbocyclic analogues of lexitropsin--DNA affinity and endonuclease inhibition. Acta Pol. Pharm. 2007, 64, 115. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Cody, V.; Luft, J.; Pangborn, W. Understanding the Role of Leu22 Variants in Methotrexate Resistance: Comparison of Wild-type and Leu22Arg Variant Mouse and Human Dihydrofolate Reductase Ternary Crystal Complexes with Methotrexate and NADPH. Acta Crystallogr. Sect. D Biol. Crystallogr. 2005, 61, 147–155. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: No available. |
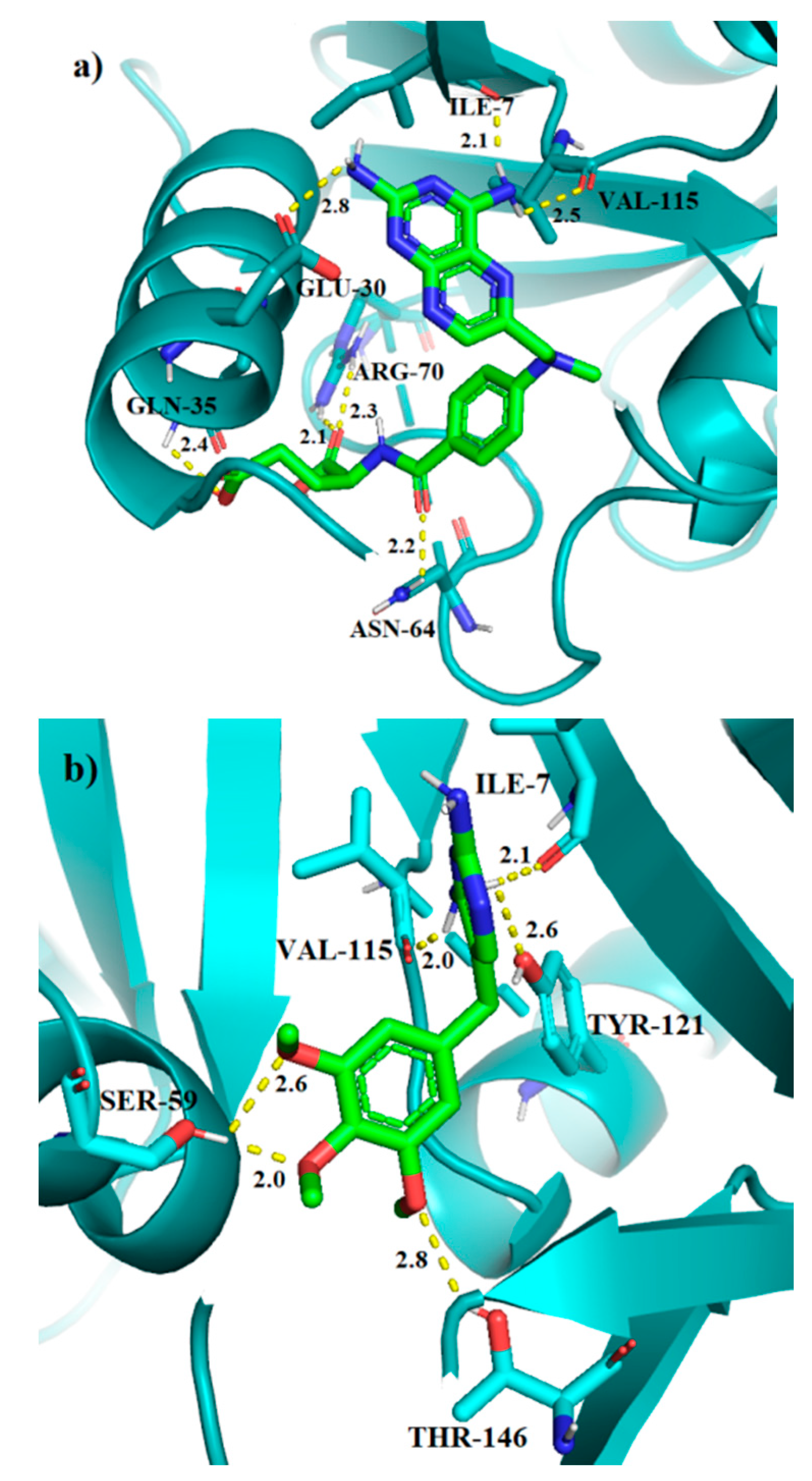
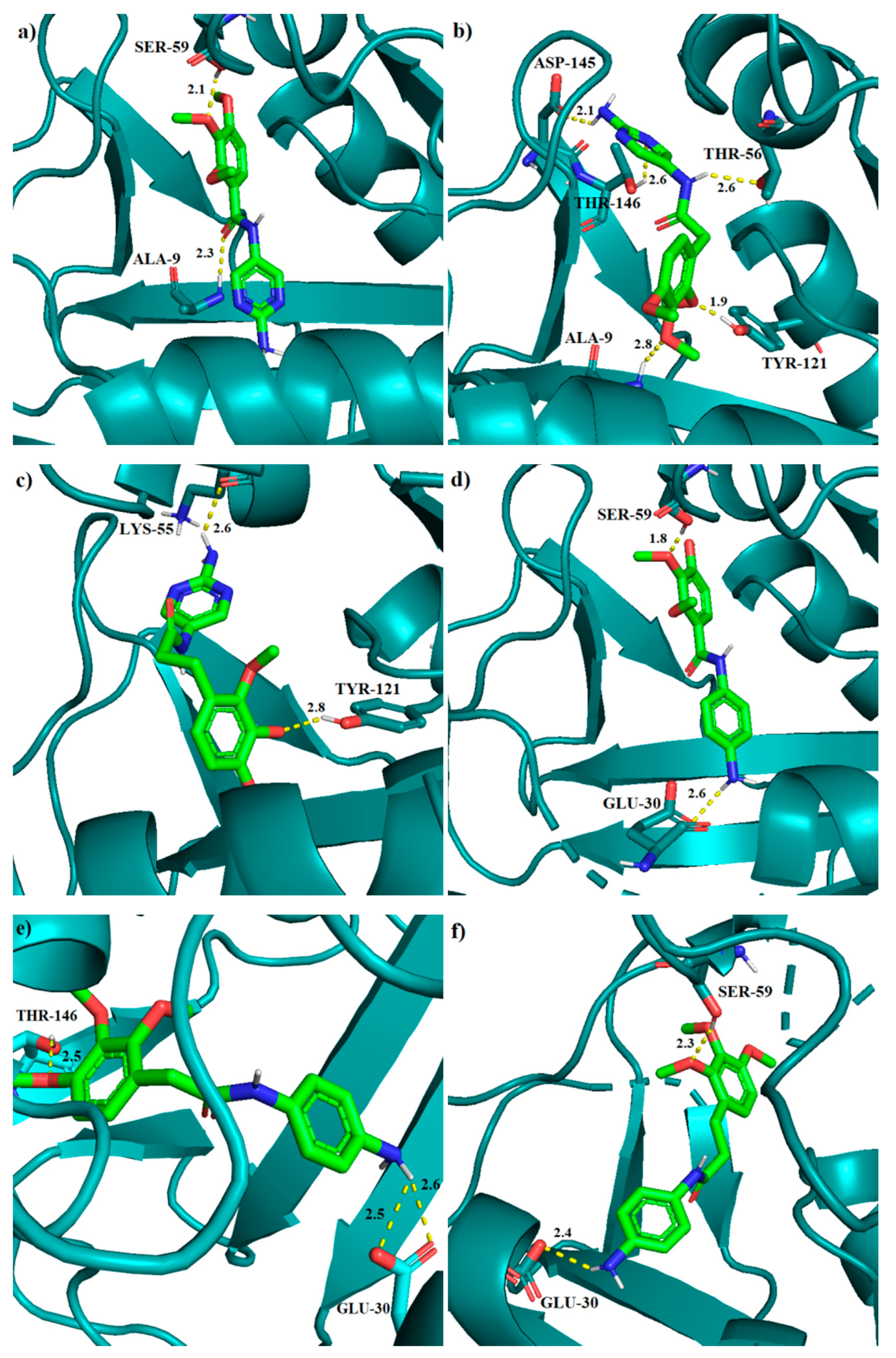
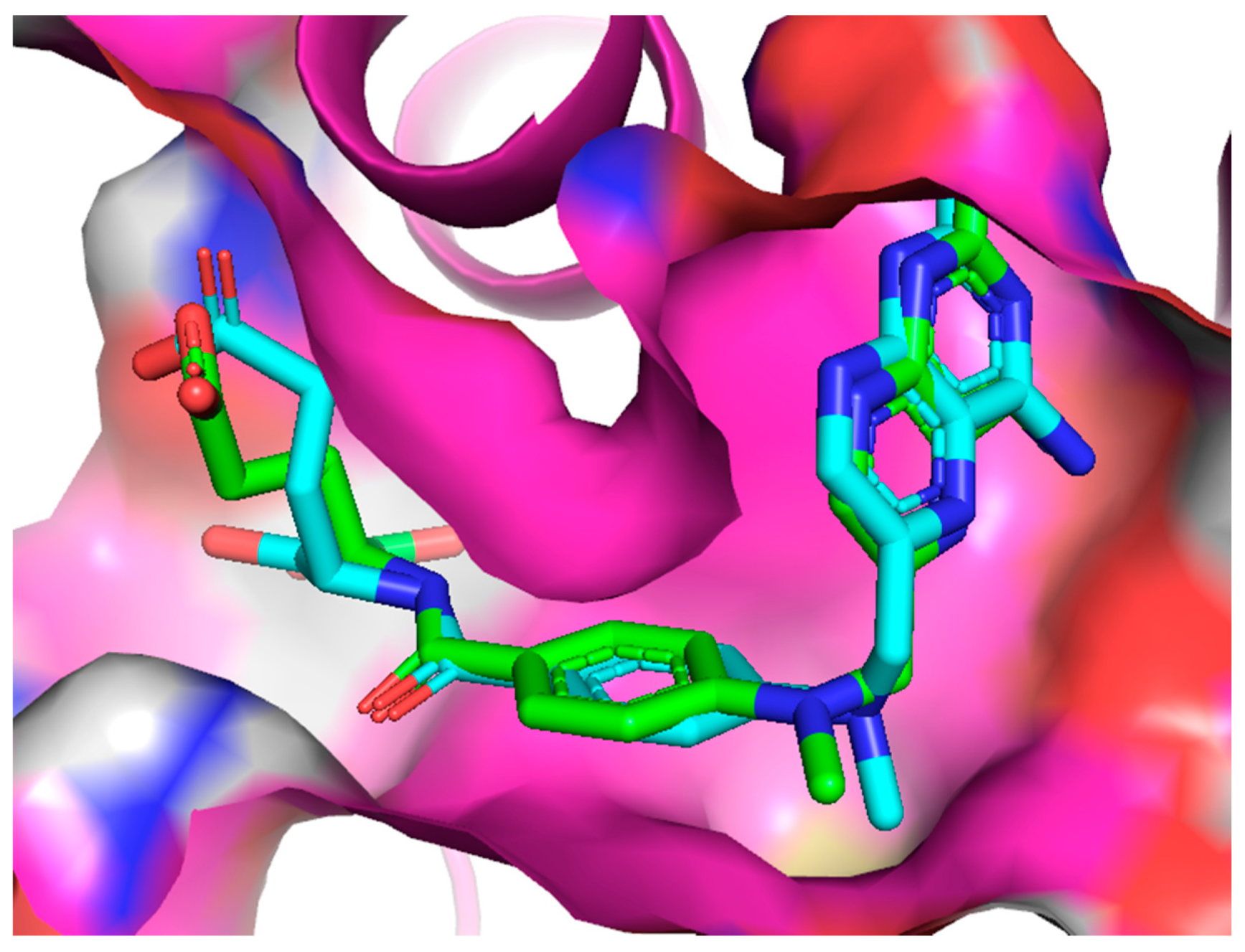
| No. | Decrease of Fluorescence [%] | Kapp × 105 M−1 | DHFR Affinity kcal/mol | DHFR IC50 [µM] | |||
|---|---|---|---|---|---|---|---|
| Calf Thymus DNA | T4 DNA | Poly (dA-dT)2 | Poly (dG-dC)2 | ||||
| EtBr | 100 | 100 | 100 | 95 b* | 99 | - | n.d. * |
| NT | 74 | 8.7 | 8.3 | 875 | 2.5 | −9.6 | n.d. |
| TMP | 100 | n.d. | n.d. | n.d. | n.d. | −7.5 | 55.26 |
| MTX | 100 | n.d. | n.d. | n.d. | n.d. | −9.5 | 0.08 |
| 1 | 71.43 | 2.4 | 2.9 | 11.7 | 1.3 | −7.7 | 21.78 |
| 2 | 45.18 | 4.4 | 1.1 | 3.9 | 0.8 | −8.3 | 0.99 |
| 3 | 69.92 | 3.7 | 7.8 | 3.6 | 0.5 | −8.1 | 0.72 |
| 4 | 80.43 | 5.9 | 3.9 | 7.9 | 1.6 | −8.0 | 1.02 |
| 5 | 69.17 | 5.2 | 6.8 | 14 | 3.0 | −7.9 | 15.94 |
| 6 | 71.43 | 4.6 | 0.9 | 4.2 | 1.1 | −7.9 | 15.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, A.; Maliszewski, D.; Baradyn, M.; Drozdowska, D. Trimethoprim: An Old Antibacterial Drug as a Template to Search for New Targets. Synthesis, Biological Activity and Molecular Modeling Study of Novel Trimethoprim Analogs. Molecules 2020, 25, 116. https://doi.org/10.3390/molecules25010116
Wróbel A, Maliszewski D, Baradyn M, Drozdowska D. Trimethoprim: An Old Antibacterial Drug as a Template to Search for New Targets. Synthesis, Biological Activity and Molecular Modeling Study of Novel Trimethoprim Analogs. Molecules. 2020; 25(1):116. https://doi.org/10.3390/molecules25010116
Chicago/Turabian StyleWróbel, Agnieszka, Dawid Maliszewski, Maciej Baradyn, and Danuta Drozdowska. 2020. "Trimethoprim: An Old Antibacterial Drug as a Template to Search for New Targets. Synthesis, Biological Activity and Molecular Modeling Study of Novel Trimethoprim Analogs" Molecules 25, no. 1: 116. https://doi.org/10.3390/molecules25010116
APA StyleWróbel, A., Maliszewski, D., Baradyn, M., & Drozdowska, D. (2020). Trimethoprim: An Old Antibacterial Drug as a Template to Search for New Targets. Synthesis, Biological Activity and Molecular Modeling Study of Novel Trimethoprim Analogs. Molecules, 25(1), 116. https://doi.org/10.3390/molecules25010116





