A Strategy for Selecting “Q-Markers” of Chinese Medical Preparation via Components Transfer Process Analysis with Application to the Quality Control of Shengmai Injection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of SMI by HPLC–QTOF-MSE
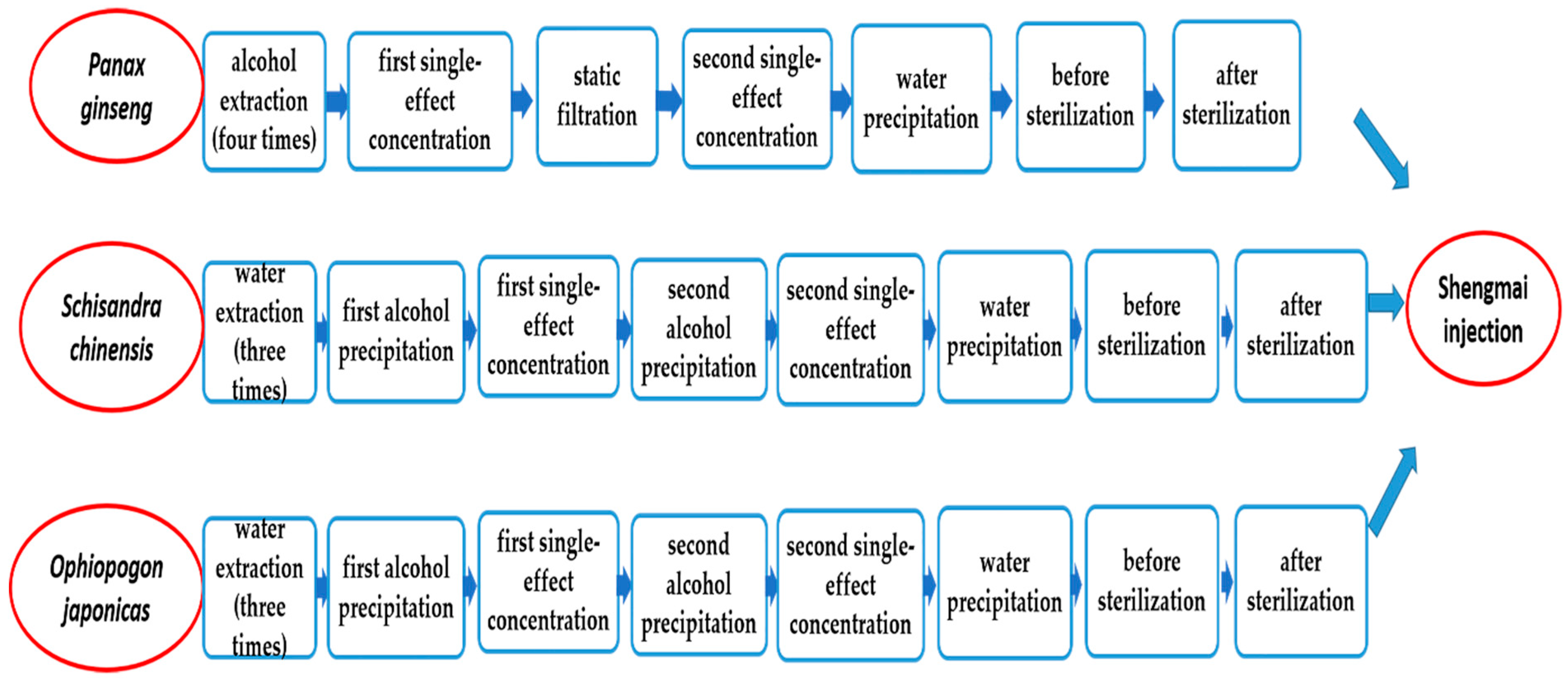
2.2. The Study of the Components Transfer Process
2.3. Quantitative Analysis of the Major Constituents in SMI by UPLC-DAD and HPLC-ELSD
2.4. Selection of Q-Markers
3. Methods
3.1. Materials and Reagents
3.2. Sample Preparation
3.2.1. Preparation of Sample Solutions for UPLC-QTOF-MSE Analysis
3.2.2. Preparation of Sample Solutions for the Study of Components Transfer Process
3.2.3. Preparation of Sample Solutions for Quantification
3.3. UPLC-QTOF-MSE Analysis
3.3.1. UPLC-QTOF-MSE Conditions
3.3.2. Establishment of Chemical Composition Database of SMI
3.4. HPLC-PDA and HPLC-ELSD Analysis
3.5. Quantitative Analysis of Representative Compounds in SMI by UPLC-DAD and HPLC-ELSD
3.5.1. UPLC-DAD and HPLC-ELSD Conditions
3.5.2. Method Validation
Calibration Curves
Precision, Stability, Repeatability, and Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.X.; Guo, D.A.; Liu, L. Quality transitivity and traceability system of herbal medicine products based on quality markers. Phytomedicine 2018, 44, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, H.B.; Fan, S.S.; Zhang, Y.D.; Yang, Z.; Fan, S.M.; Zhuang, P.W.; Zhang, Y.J. Quality markers based on biological activity: A new strategy for the quality control of traditional Chinese medicine. Phytomedicine 2018, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Zhang, Y.B.; Wu, W.Y.; Huang, L.Q.; Guo, D.A.; Liu, C.X. Approaches to establish Q-markers for the quality standards of traditional Chinese medicines. Acta Pharmacol. Sin. 2017, 7, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Cheng, Y.Y.; Guo, D.A.; Zhang, T.J.; Li, Y.Z.; Hou, W.B.; Huang, L.Q.; Xu, H.Y. A new concept on quality marker for quality assessment and process control of chinese medicines. Chin. Herb. Med. 2017, 9, 3–13. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Xiao, M.F.; Liao, Q.; Liu, W.L.; Deng, K.W.; Zhou, Y.Q.; Tang, Y.; He, F.Y.; Yang, Y.T. Application of TQSM polypharmacokinetics and its similarity approach to ascertain Q-marker by analyses of transitivity in vivo of five candidates in Buyanghuanwu injection. Phytomedicine 2018, 45, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Liu, J.; Zhang, D.Q.; Du, X.; Han, L.F.; Lv, C.X.; Li, Y.F.; Wang, R.H.; Wang, B.H.; Huang, Y.H. Nuciferine and paeoniflorin can be quality markers of tangzhiqing tablet, a chinese traditional patent medicine, based on the qualitative, quantitative and dose-exposure-response analysis. Phytomedicine 2018, 44, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Z.; Yang, J.; Wang, Y.F. Discrimination and identification of Q-markers based on ‘spider-web’ mode for quality control of traditional chinese medicine. Phytomedicine 2018, 44, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Wang, J.S.; Kong, L.Y. Protective effects of shengmai san and its three fractions on cerebral ischemia-reperfusion injury. Chin. J. Nat. Med. 2013, 11, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, W.X.; Yang, F.W.; Wang, Y.; Yue, S.Q.; Wang, T.; Wang, X.Y. Shengmai injection reduces apoptosis and enhances angiogenesis after myocardial ischaemia and reperfusion injury in rats. Biomed. Pharmacother. 2018, 104, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.J.; Zhuang, P.W.; Wang, Y.; Zhang, J.B.; Lu, Z.Q.; Wang, Y.; Zhang, M.X.; Wu, J.; Chen, Z.; Sun, M.; et al. Protection of shengmai recipe on improving cardiac function and attenuating kidney injury in pressure overload rats. Chin. Herb Med. 2014, 6, 290–296. [Google Scholar] [CrossRef]
- Gao, L.L.; Li, T.T.; Li, L.; Li, Z.; Nie, L.; Zhou, H.Y.; Sun, Z.Y.; Ye, S.Y.; Liu, R.C.; Zang, H.C. Rapid determination of lumbrokinase potency in the earthworm extract intermediate by near-infrared spectroscopy. Chemom. Intell. Lab. Syst. 2019, 185, 59–64. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae sargassum duplicatum: The structure and anticancer activity in vitro. Carbohydr. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae alaria marginata and a. Angusta. Carbohydr. Polym. 2016, 153, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, T.B.; Ren, X.C.; Zhang, Z.F.; Huang, W.X.; Liu, L.; Luo, P.; Zhou, H. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion 2016, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cui, X.; Jin, H.H.; Hong, L.; Liu, X.; Li, X.; Zhang, Q.G.; Liu, L.P. Ginsenoside re prevents angiotensin ii-induced gap-junction remodeling by activation of pparγ in isolated beating rat atria. Life Sci. 2017, 190, 36–45. [Google Scholar] [CrossRef]
- Zhang, N.N.; An, X.B.; Lang, P.P.; Wang, F.; Xie, Y.P. Ginsenoside Rd contributes the attenuation of cardiac hypertrophy in vivo and in vitro. Biomed. Pharmacother. 2019, 109, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.S.; Huang, X.N.; Dai, Z.K.; Yang, G.Z.; Zhou, Q.X.; Shi, J.S.; Wu, Q. Inhibitory effect of ginsenoside Rb1 on cardiac hypertrophy induced by monocrotaline in rat. J. Ethnopharmacol. 2007, 111, 567–572. [Google Scholar] [CrossRef]
- Wan, C.K.; Tse, A.K.; Yu, Z.L.; Zhu, G.Y.; Wang, H.; Fong, D.W.F. Inhibition of cytochrome P450 3A4 activity by schisandrol A and gomisin A isolated from Fructus Schisandrae chinensis. Phytomedicine 2010, 17, 702–705. [Google Scholar] [CrossRef]
- Brymora, A.; Flisinski, M.; Johnson, R.J.; Goszka, G.; Stefanska, A.; Manitius, J. Low-fructose diet lowers blood pressure and inflammation in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 608–612. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |













| Compounds | Regression Equation | R2 | Linear Range (mg/mL) | Precision (RSD%) | Repeatability (RSD%) | Stability (RSD%) | Average Recovery (%) | Recovery (RSD%) | LOQs (mg/mL) | LODs (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Y = 3.306 × 106X − 4240.81 | 0.9999 | 0.00990–0.317 | 0.57 | 0.83 | 0.27 | 101.42 | 1.738 | 1.22 × 10−3 | 4.07 × 10−4 |
| Re | Y = 2.976 × 106X + 2341.61 | 0.9997 | 0.00952–0.305 | 0.53 | 1.36 | 0.21 | 97.46 | 2.050 | 1.16 × 10−3 | 3.87 × 10−4 |
| Rb1 | Y = 2.619 × 106X − 1101.56 | 0.9998 | 0.0195–0.625 | 0.46 | 0.60 | 0.16 | 100.33 | 1.933 | 1.35 × 10−3 | 4.48 × 10−4 |
| Rd | Y = 2.751 × 106X + 5171.96 | 0.9992 | 0.00584–0.187 | 0.44 | 1.50 | 0.26 | 100.48 | 2.062 | 1.30 × 10−3 | 4.33 × 10−4 |
| SolA | Y = 5.523 × 107X + 36309.46 | 0.9998 | 0.00347–0.111 | 0.45 | 0.63 | 0.43 | 100.89 | 2.089 | 5.52 × 10−6 | 1.84 × 10−6 |
| SolB | Y = 5.505 × 107X + 13971.00 | 0.9995 | 0.00179–0.0573 | 0.43 | 2.65 | 1.63 | 96.50 | 1.041 | 7.36 × 10−6 | 2.45 × 10−6 |
| Fru | lgY = 1.3943lgX + 5.21081 | R2 = 0.9994 | 0.74988–11.998 | 0.25 | 0.18 | 0.30 | 97.18 | 2.06 | 0.119 | 0.0595 |
| NO. | Contents (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rb1 | Rd | SolA | SolB | Fru | |
| Lot.1 | 0.135 | 0.101 | 0.197 | 0.052 | 0.015 | 0.002 | 14.36 |
| Lot.2 | 0.149 | 0.111 | 0.212 | 0.056 | 0.016 | 0.002 | 13.30 |
| Lot.3 | 0.137 | 0.102 | 0.199 | 0.052 | 0.015 | 0.002 | 17.47 |
| Lot.4 | 0.137 | 0.102 | 0.197 | 0.052 | 0.015 | 0.002 | 16.83 |
| Lot.5 | 0.153 | 0.117 | 0.232 | 0.061 | 0.016 | 0.002 | 15.15 |
| Lot.6 | 0.14 | 0.103 | 0.196 | 0.053 | 0.02 | 0.003 | 16.68 |
| Lot.7 | 0.143 | 0.106 | 0.182 | 0.046 | 0.016 | 0.002 | 19.47 |
| Lot.8 | 0.137 | 0.103 | 0.195 | 0.053 | 0.015 | 0.002 | 17.39 |
| Lot.9 | 0.149 | 0.111 | 0.21 | 0.051 | 0.016 | 0.002 | 22.02 |
| Lot.10 | 0.136 | 0.102 | 0.193 | 0.048 | 0.016 | 0.002 | 17.61 |
| Average | 0.142 | 0.106 | 0.201 | 0.052 | 0.016 | 0.002 | 17.03 |
| RSD% | 4.60 | 5.10 | 6.80 | 7.80 | 9.32 | 15.06 | 14.68 |
| Compounds | P1 | P2 | P3 | IMI | Final Rv | |||
|---|---|---|---|---|---|---|---|---|
| Rv | Sv | Rv | Sv | Rv | Sv | |||
| Rg1 | 3 | 0.7 | 1 | 0.9 | 2 | 0.8 | 19.20 | 1 |
| Re | 4 | 0.6 | 2 | 0.8 | 1 | 0.9 | 11.54 | 3 |
| Rb1 | 2 | 0.8 | 3 | 0.7 | 4 | 0.6 | 14.70 | 2 |
| Rd | 5 | 0.5 | 4 | 0.6 | 3 | 0.7 | 10.80 | 4 |
| SolA | 6 | 0.4 | 5 | 0.5 | 6 | 0.4 | 9.76 | 5 |
| SolB | 7 | 0.3 | 7 | 0.3 | 7 | 0.3 | 0.12 | 7 |
| Fru | 1 | 0.9 | 6 | 0.4 | 5 | 0.5 | 4.08 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Liu, H.; Miao, P.; Wang, H.; Yu, H.; Wang, C.; Li, Z. A Strategy for Selecting “Q-Markers” of Chinese Medical Preparation via Components Transfer Process Analysis with Application to the Quality Control of Shengmai Injection. Molecules 2019, 24, 1811. https://doi.org/10.3390/molecules24091811
Zhao C, Liu H, Miao P, Wang H, Yu H, Wang C, Li Z. A Strategy for Selecting “Q-Markers” of Chinese Medical Preparation via Components Transfer Process Analysis with Application to the Quality Control of Shengmai Injection. Molecules. 2019; 24(9):1811. https://doi.org/10.3390/molecules24091811
Chicago/Turabian StyleZhao, Chunxia, Huan Liu, Peiqi Miao, Houen Wang, Heshui Yu, Chunhua Wang, and Zheng Li. 2019. "A Strategy for Selecting “Q-Markers” of Chinese Medical Preparation via Components Transfer Process Analysis with Application to the Quality Control of Shengmai Injection" Molecules 24, no. 9: 1811. https://doi.org/10.3390/molecules24091811
APA StyleZhao, C., Liu, H., Miao, P., Wang, H., Yu, H., Wang, C., & Li, Z. (2019). A Strategy for Selecting “Q-Markers” of Chinese Medical Preparation via Components Transfer Process Analysis with Application to the Quality Control of Shengmai Injection. Molecules, 24(9), 1811. https://doi.org/10.3390/molecules24091811







