Fe3O4@C Matrix with Tailorable Adsorption Capacities for Paracetamol and Acetylsalicylic Acid: Synthesis, Characterization, and Kinetic Modeling
Abstract
1. Introduction
2. Results and Discussion
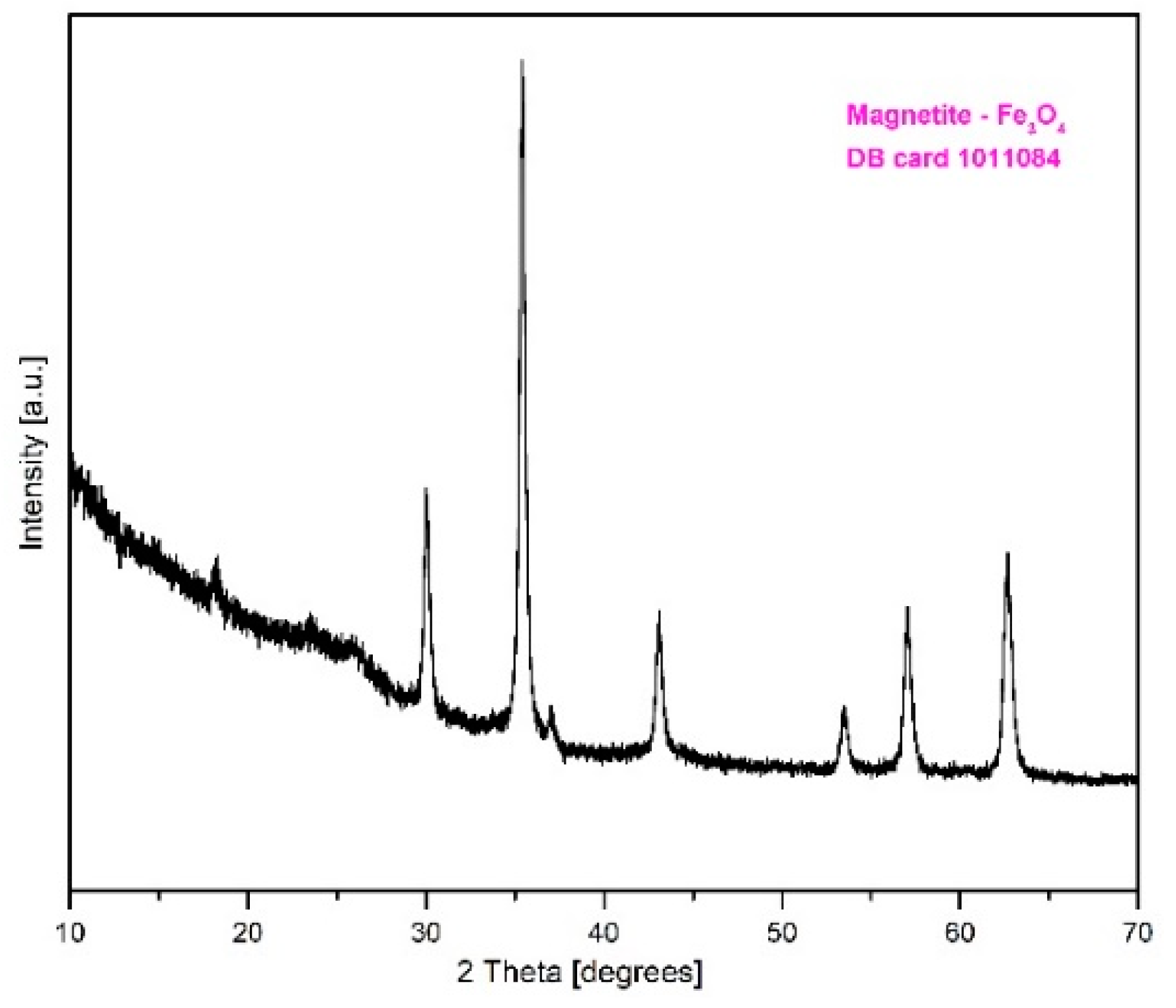
2.1. XRD Characterization of Fe3O4@C Matrix
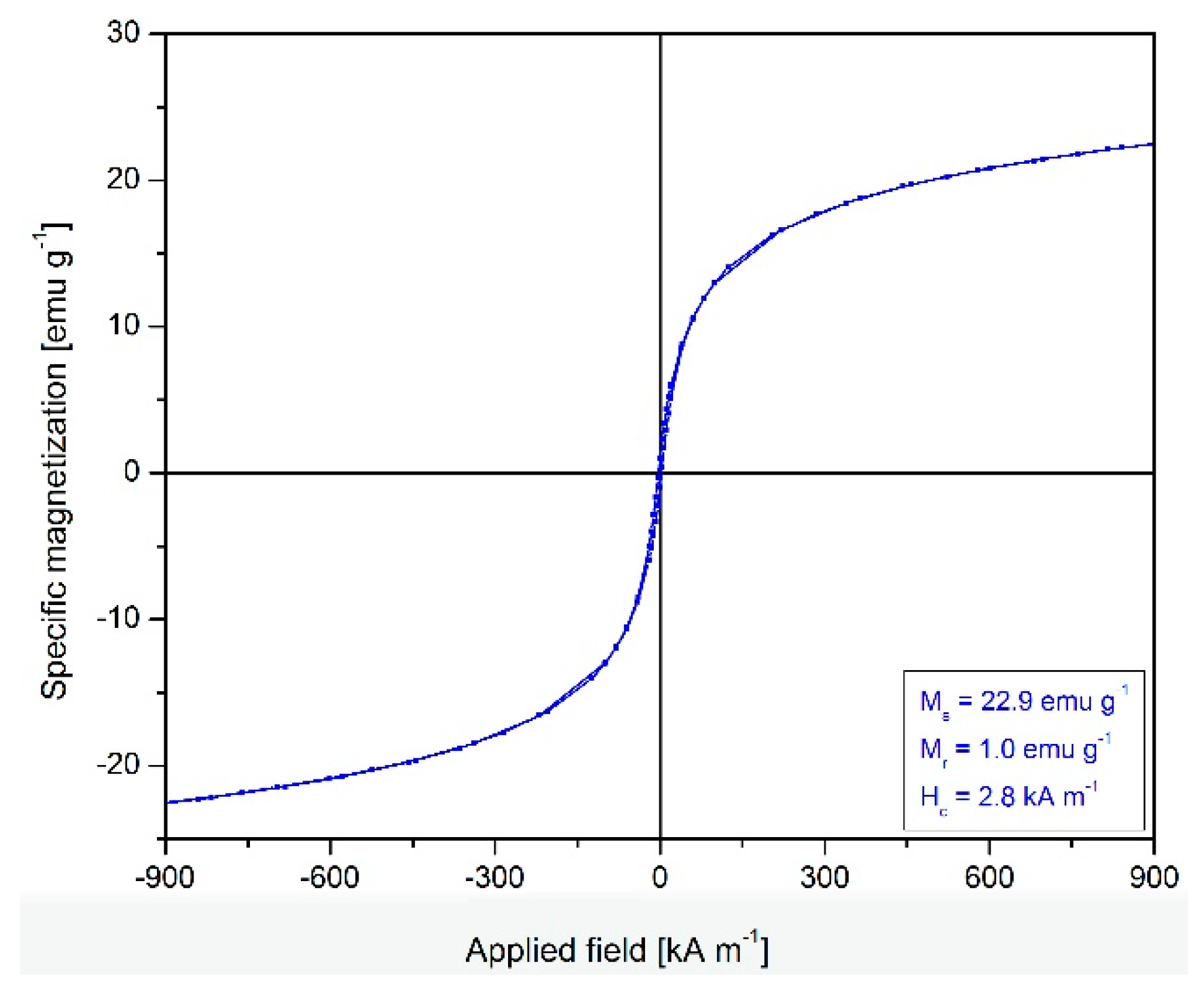
2.2. Magnetic Properties of Fe3O4@C Matrix
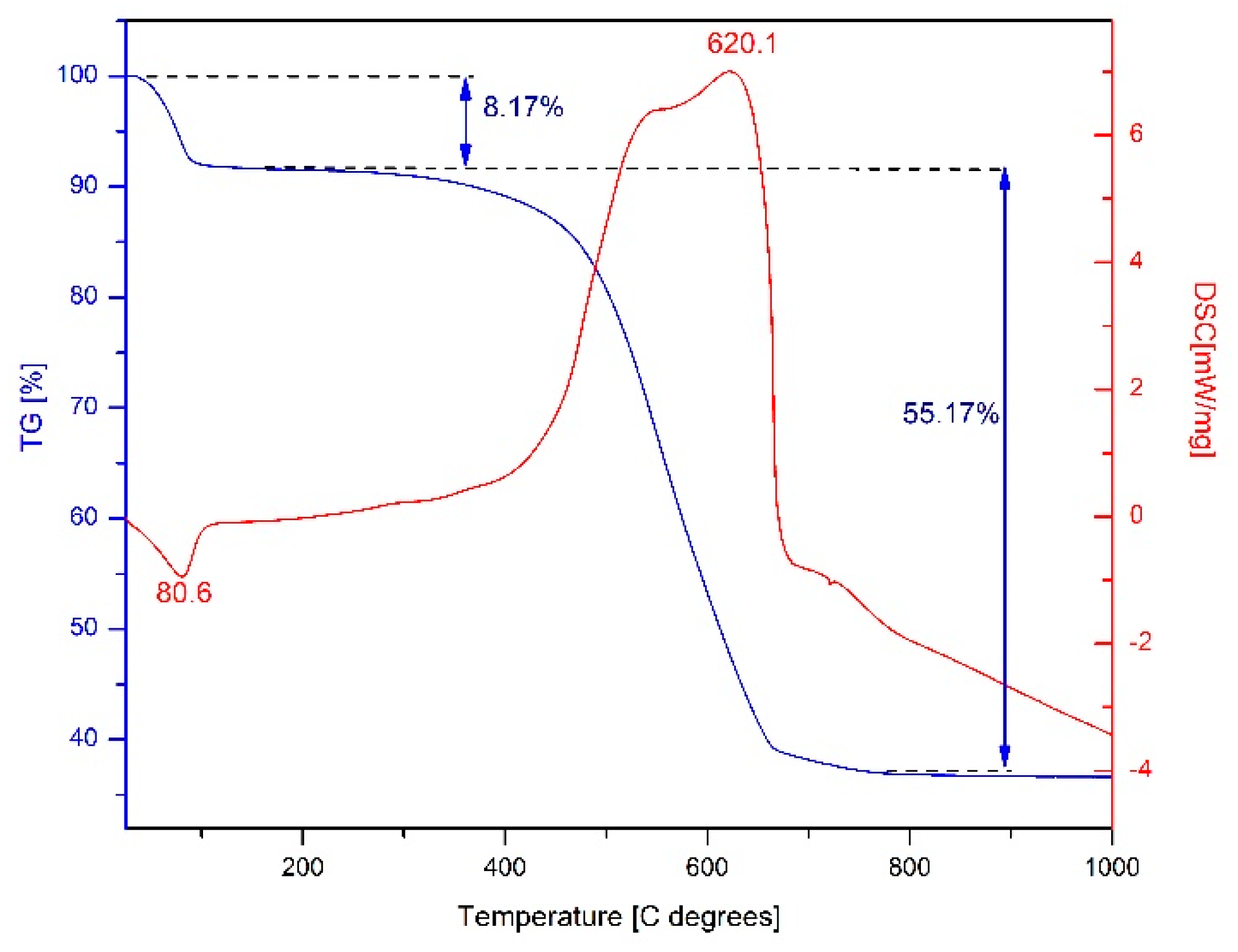
2.3. Thermal Analysis of Fe3O4@C Matrix
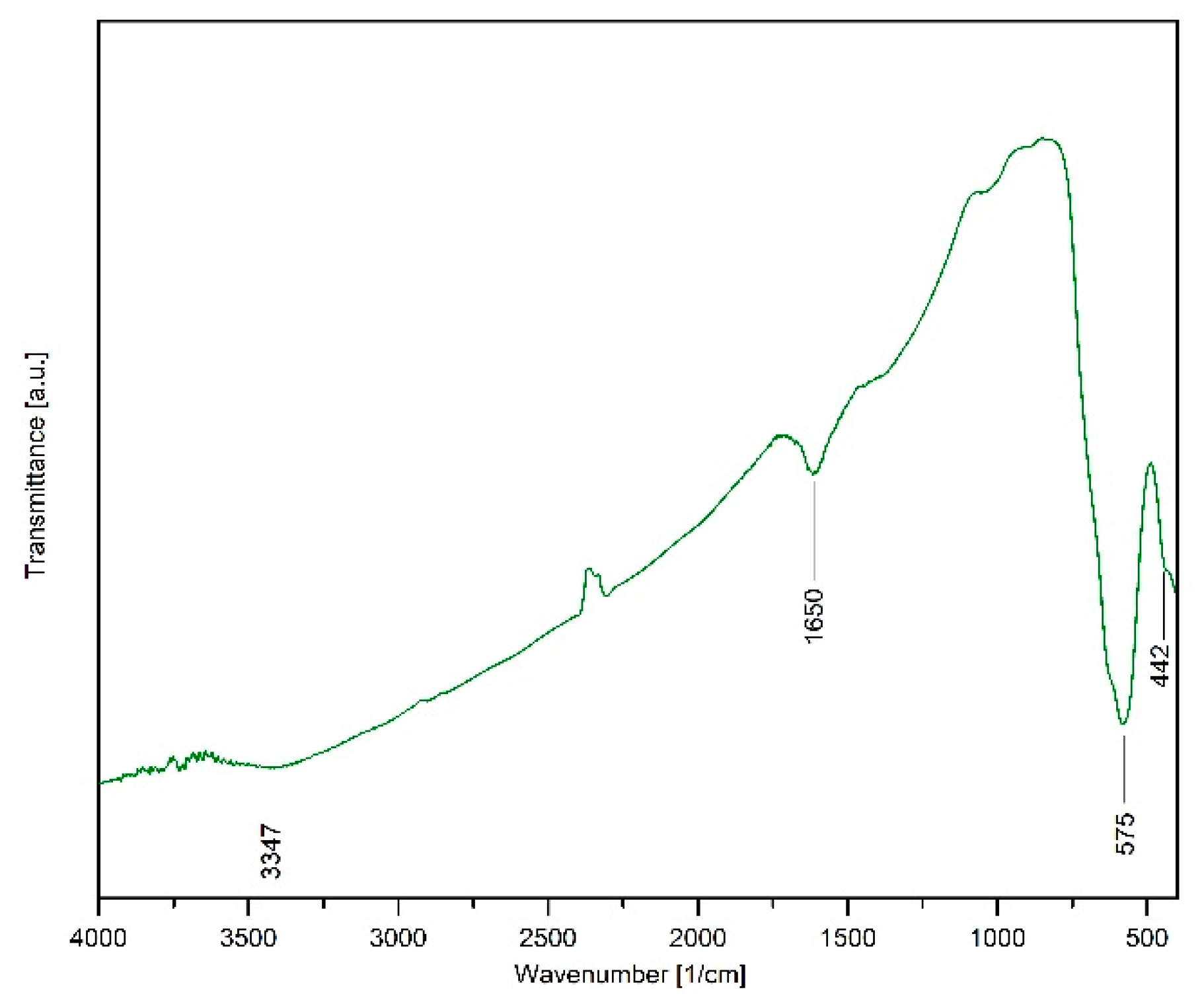
2.4. FTIR Investigations of Fe3O4@C Matrix
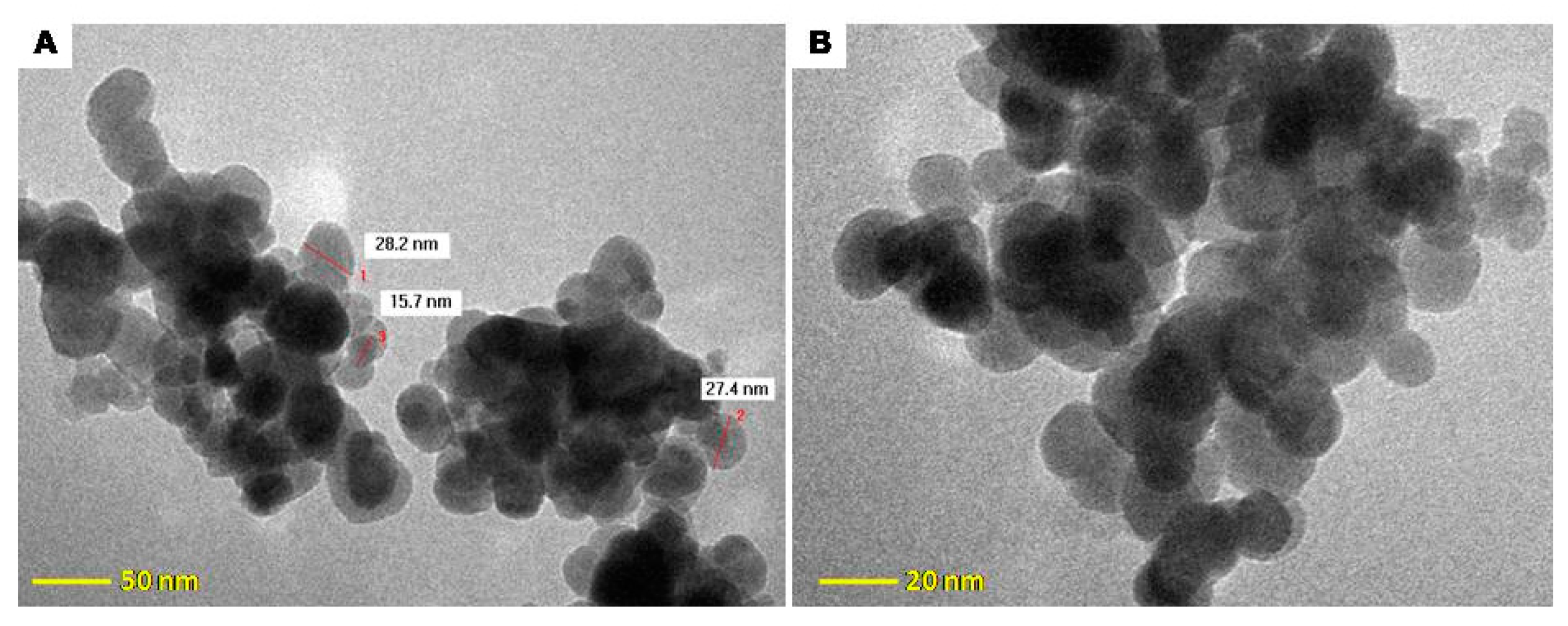
2.5. Electron Microscopy Characterization of Fe3O4@C Matrix
2.6. Paracetamol and Acetylsalicylic Acid Adsorption on the Fe3O4@C Matrix
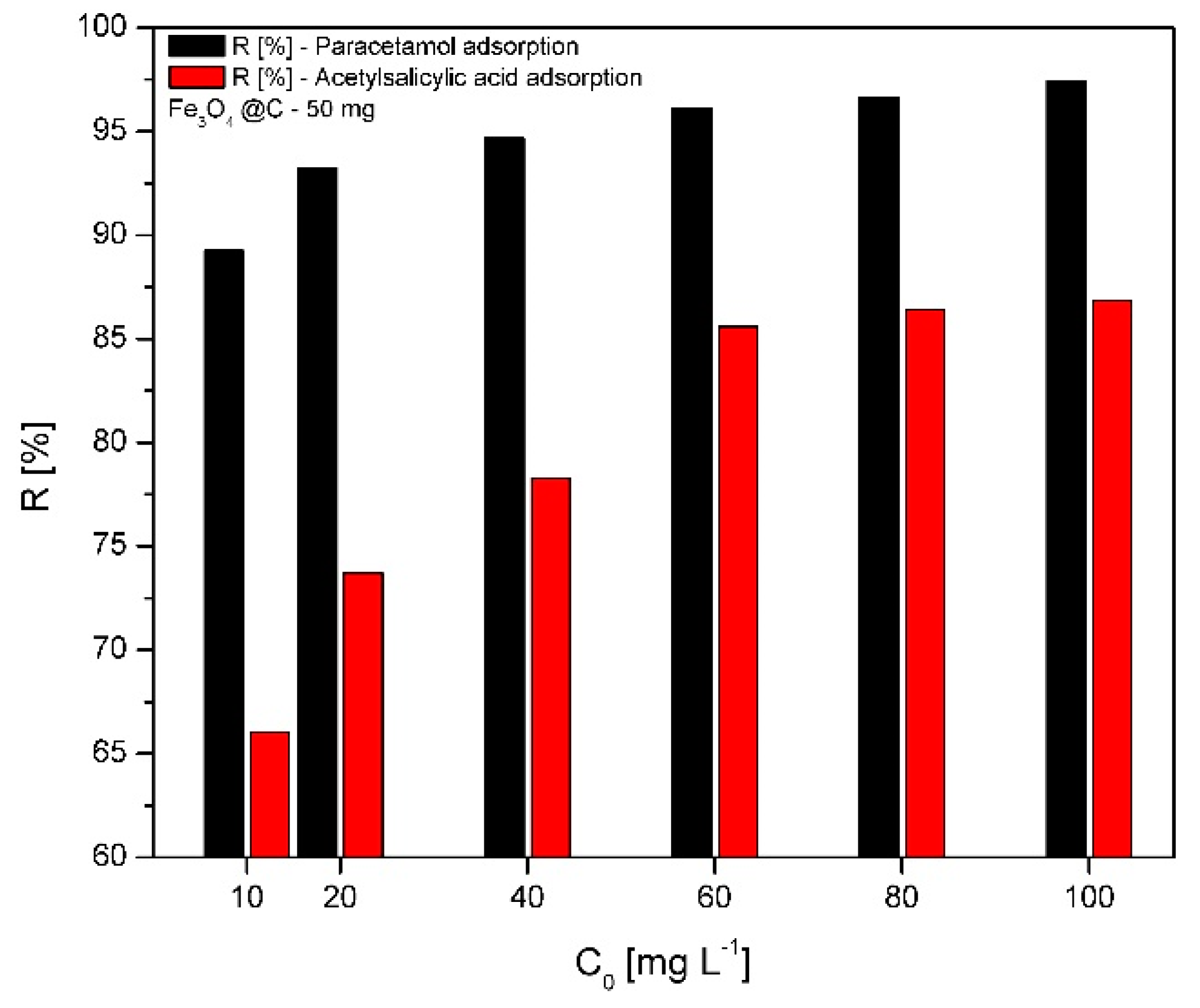
2.6.1. Effect of the Initial Concentration on the Removal Efficiency
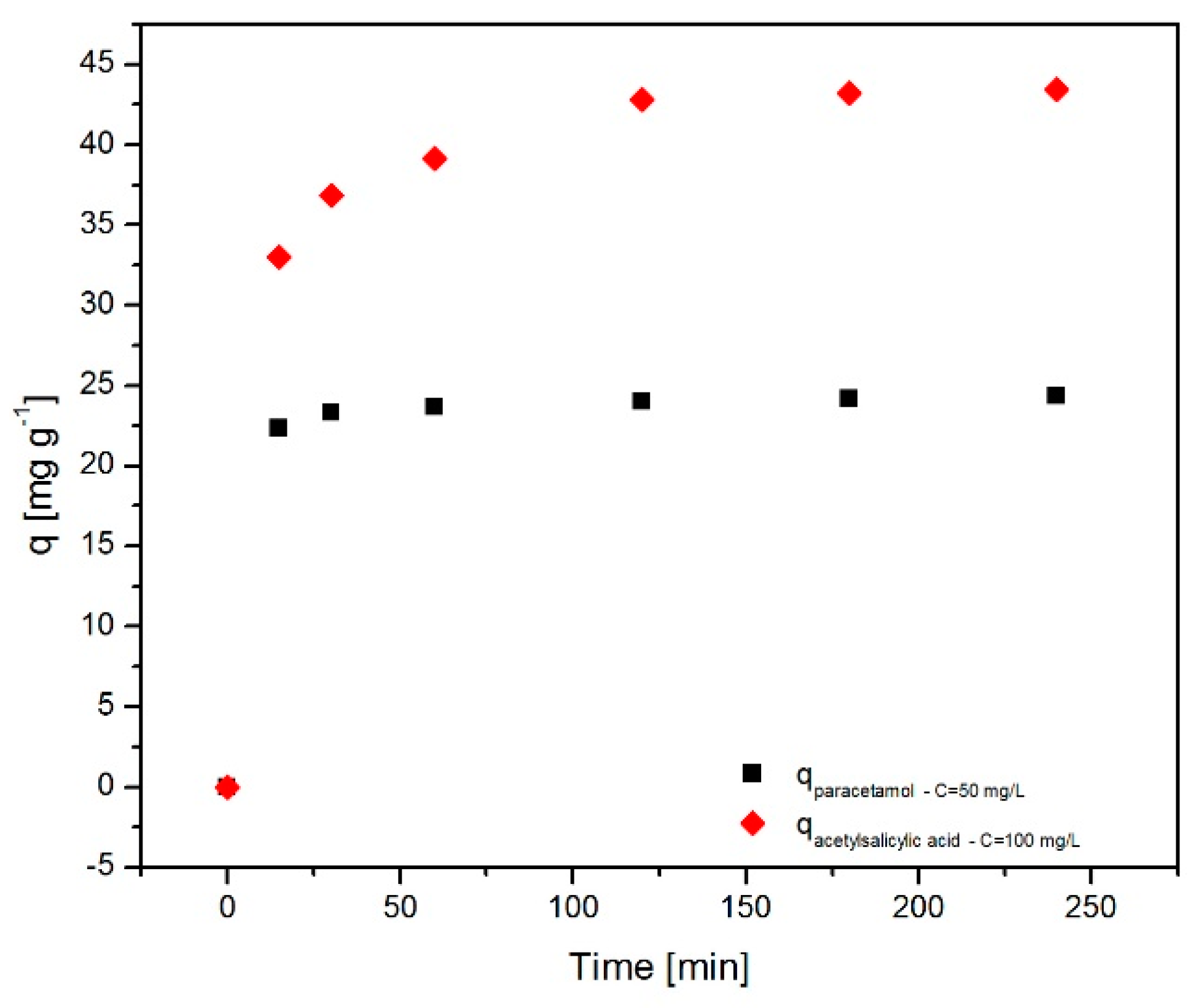
2.6.2. Effect of Contact Time
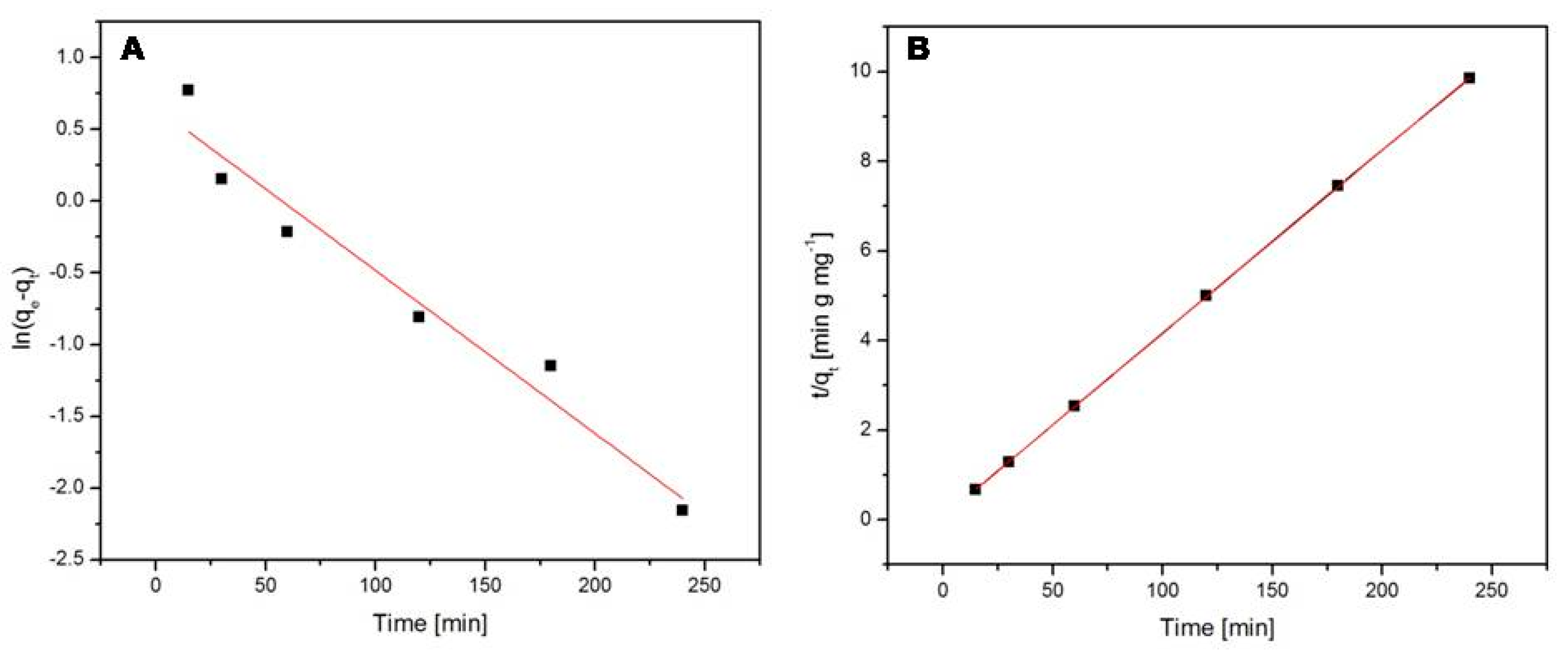
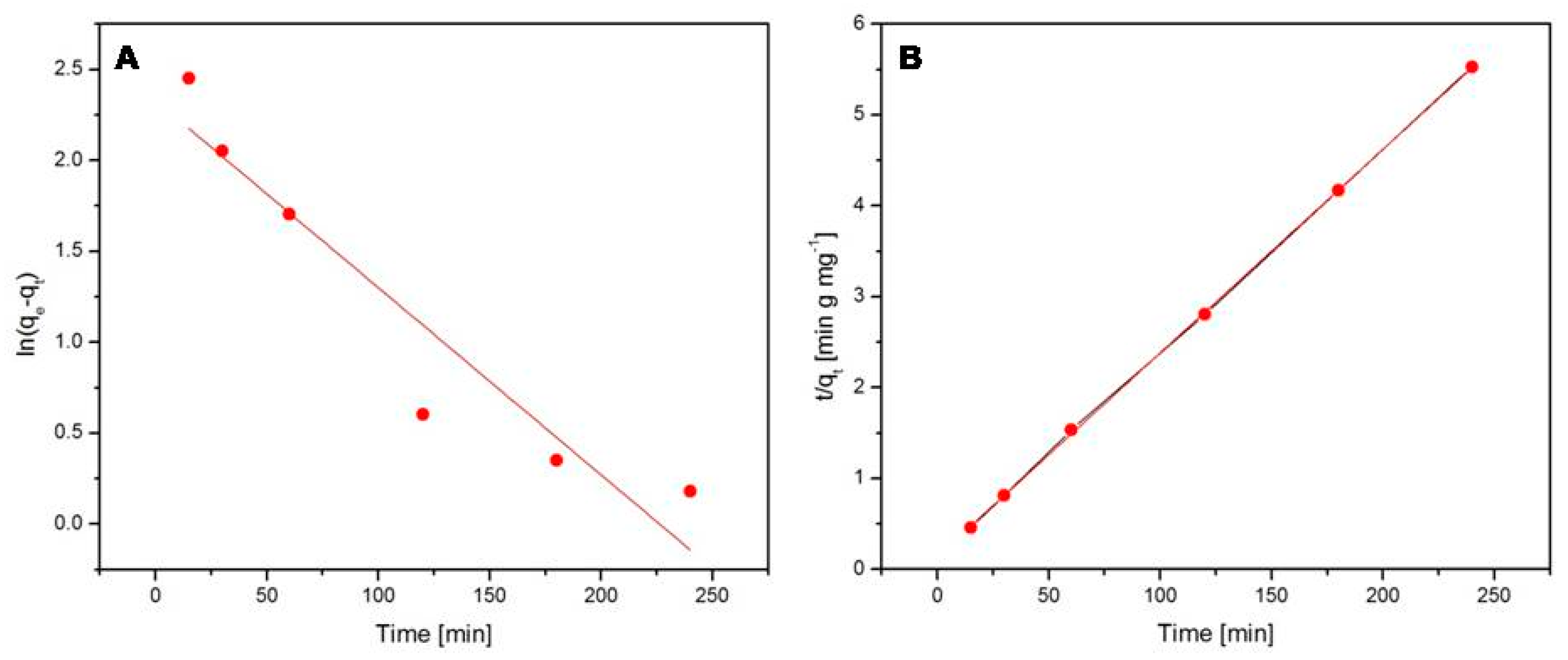
2.6.3. Kinetic Studies
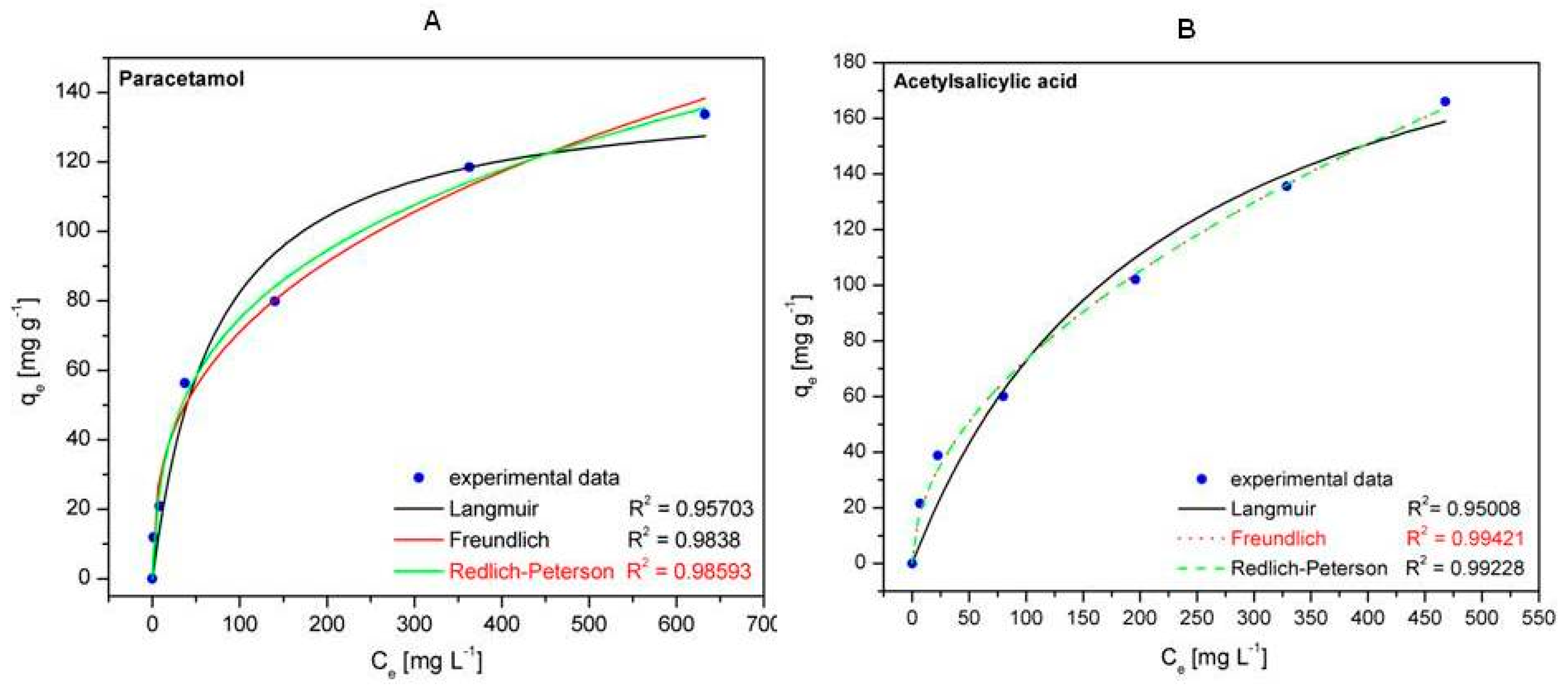
2.6.4. Adsorption Isotherms
3. Experimental Part
3.1. Preparation of Aqueous Solution Containing Drugs
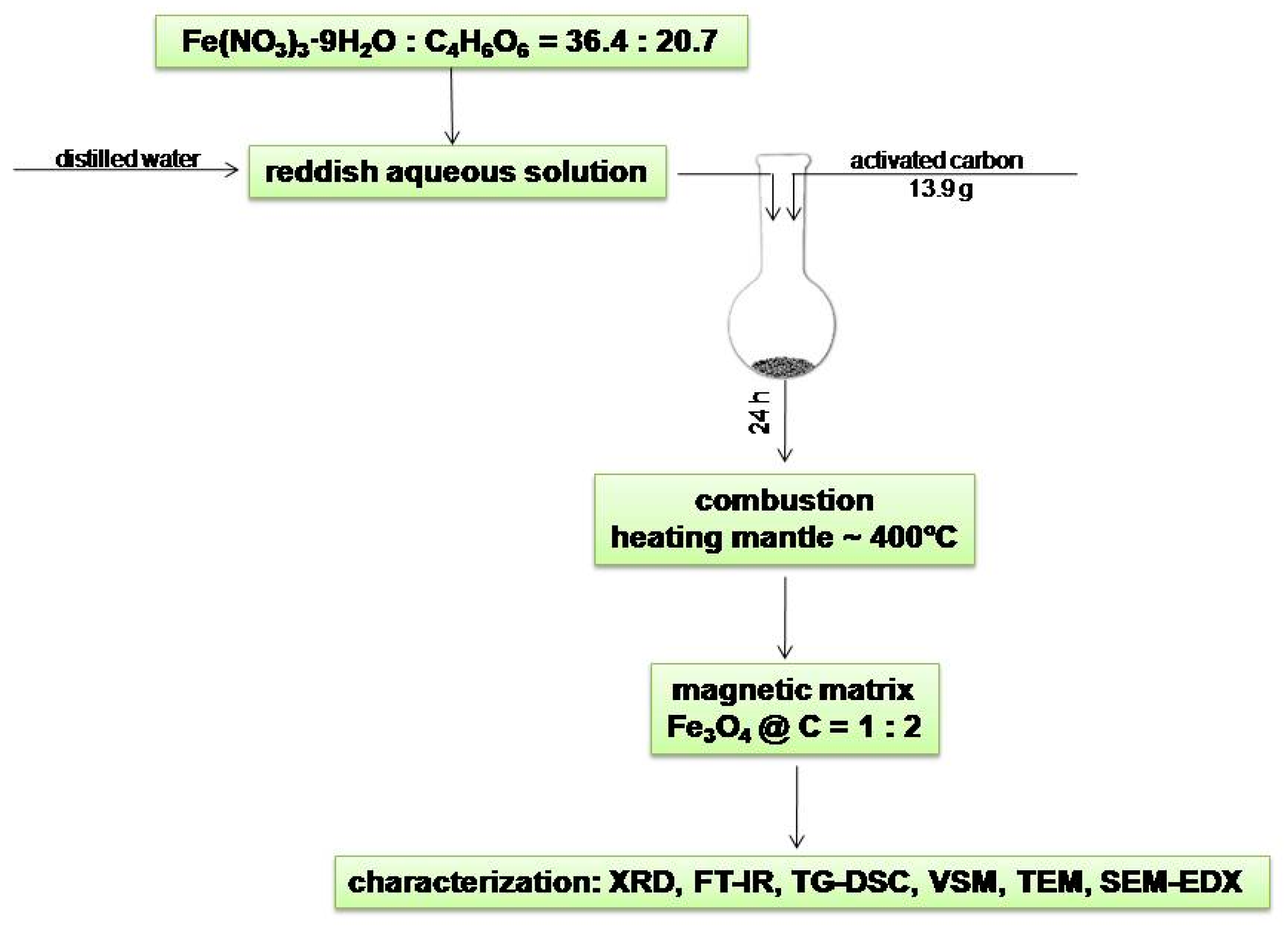
3.2. Fe3O4@C Matrix Preparation
3.3. Fe3O4@C Matrix Characterization
3.4. Paracetamol and Acetylsalicylic Acid Adsorption Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, W. The environmental impact of industrialization in East Asia and strategies toward sustainable development. Sustain. Sci. 2006, 1, 107–114. [Google Scholar] [CrossRef]
- Ilić, I.; Hafner, P. Environmental aspects of the process of globalization—Negative implications and crisis. Facta Univ. Ser. Econ. Organ. 2015, 12, 109–120. [Google Scholar]
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. Chapter 1. Soil and Pollution: An Introduction to the MainIssues, in Soil Pollution: From Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Huang, J.; Pan, X.; Guo, X.; Li, G. Impacts of air pollution wave on years of life lost: A crucial way to communicate the health risks of air pollution to the public. Environ. Int. 2018, 113, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Naidoo, S.; Olaniran, A.O. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int. J. Environ. Res. Public Health 2013, 11, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 1–67. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review on removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Saeid, F.; Bahador, N.; Abdalmajid, F.; Nezamaddin, M. Removal of Natural Organic Matter (NOM), Turbidity, and Color of Surface Water by Integration of Enhanced Coagulation Process and Direct Filtration. JAEHR 2017, 5, 108–113. [Google Scholar]
- Ozyonar, F.; Aksoy, S. Removal of salicylic acid from aqueous solutions using various electrodes and different connection modes by electrocoagulation. Int. J. Electrochem. Sci. 2016, 11, 3680–3696. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Purification of Contaminated Water with Reverse Osmosis: Effective Solution of Providing Clean Water for Human Needs in Developing Countries. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 75–89. [Google Scholar]
- Mohamed, E.F. Removal of Organic Compounds from Water by Adsorption and Photocatalytic Oxidation. Ph.D. Thesis, Universite de Toulouse, Toulouse, France, 20 May 2011. [Google Scholar]
- Mukoko, T.; Mupa, M.; Guyo, U.; Dziike, F. Preparation of Rice Hull Activated Carbon for the Removal of Selected Pharmaceutical Waste Compounds in Hospital Effluent. J. Environ. Anal. Toxicol. 2015, 7. [Google Scholar] [CrossRef]
- Draman, S.F.S.; Batra’azman, I.A.; Mohd, N. Removal of paracetamol from aqueous solution by dried cellulose and activated carbon. ARPN J. Eng. Appl. Sci. 2015, 10, 9544–9548. [Google Scholar]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of Solution pH on the Adsorption of Paracetamol on Chemically Modified Activated Carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, O.; Porcher, J.M.; Sanchez, W. Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: Review of evidence and need for knowledge. Chemosphere 2014, 115, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Adeniyi, I.F. The Impacts of Hospital Effluent Discharges on the Physico-chemical Water Quality of a Receiving Stream at Ile-Ife, Southwestern Nigeria. J. Sustain. Dev. 2012, 5, 82–92. [Google Scholar] [CrossRef]
- Fujii, H.; Managi, S. Wastewater Management Efficiency and Determinant Factors in the Chinese Industrial Sector from 2004 to 2014. Water 2017, 9, 586. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Nche, N.A.G.; Bopda, A.; Tchuifon, D.R.; Sadeu Ngakou, C.; Tiotsop Kuete, I.H.; Solomon Gabche, A. Removal of Paracetamol from Aqueous Solution by Adsorption onto Activated Carbon Prepared from Rice Husk. J. Chem. Pharm. Res. 2017, 9, 56–68. [Google Scholar]
- Ferreira, R.C.; Couto Junior, O.M.; Carvalho, K.Q.; Arroyo, P.A.; Barros, M.A.S.D. Effect of Solution pH on the Removal of Paracetamol by Activated Carbon of Dende Coconut Mesocarp. Chem. Biochem. Eng. Q. J. 2015, 29, 47–53. [Google Scholar] [CrossRef]
- Saucier, C.; Karthickeyan, P.; Ranjithkumar, V.; Lima, E.C.; dos Reis, G.S.; de Brum, I.A.S. Efficient removal of amoxicillin and paracetamol from aqueous solutions using magnetic activated carbon. Environ. Sci. Pollut. Res. 2017, 24, 5918–5932. [Google Scholar] [CrossRef]
- Wan, D.; Chen, F.; Geng, Q.; Lu, H.; Willcock, H.; Liu, Q.; Wang, F.; Zou, K.; Jin, M.; Pu, H.; et al. A multifunctional azobenzene-based polymeric adsorbent for effective water remediation. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Jacukowicz-Sobala, I.; Ociński, D.; Kociołek-Balawejder, E. Iron and aluminium oxides containing industrial wastes as adsorbents of heavy metals: Application possibilities and limitations. Waste Manag. Res. 2015, 33, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R. Advances in application of natural clay and its composites in removal of biological, organic, and inorganic contaminants from drinking water. Adv. Mater. Sci. Eng. 2011, 2011, 872531. [Google Scholar] [CrossRef]
- Ranđelović, M.S.; Zarubica, A.R.; Purenović, M.M. New Composite Materials in the Technology for Drinking Water Purification from Ionic and Colloidal Pollutants, Composites and Their Applications Ning Hu; IntechOpen: Rijeka, Croatia, 2012; pp. 273–300. [Google Scholar]
- Hu, P.; Zhang, Y.; Tong, K.; Wei, F.; An, Q.; Wang, X.; Chu, P.K.; Lv, F. Removal of organic pollutants from red water by magnetic-activated coke. Desalin. Water Treat. 2015, 54, 2710–2722. [Google Scholar] [CrossRef]
- Monteil, C.; Bar, N.; Bee, A.; Villemin, D. An efficient recyclable magnetic material for the selective removal of organic pollutants. Beilstein J. Nanotechnol. 2016, 7, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Caliskan Salihi, E. Adorption of Metamizole Sodium by Activated Carbon in Simulated Gastric and Intestinal Fluids. JOTCSA 2018, 5, 237–246. [Google Scholar]
- Jain, S.; Vyas, R.K.; Dalai, A.K. Adsorption of antiviral drug, acyclovir from aqueous solution on powdered activated charcoal: Kinetics, equilibrium, and thermodynamic studies. Desalin. Water Treat. 2014, 52, 4953–4968. [Google Scholar] [CrossRef]
- Iovino, P.; Canzano, S.; Capasso, S.; Erto, A.; Musmarra, D. A modeling analysis for the assessment of ibuprofen adsorption mechanism onto activated carbons. Chem. Eng. J. 2015, 277, 360–367. [Google Scholar] [CrossRef]
- Arevalo, P.; Isasi, J.; Caballero, A.C.; Marco, J.F.; Martin-Hernandez, F. Magnetic and structural studies of Fe3O4 nanoparticles synthesized via coprecipitation and dispersed in different surfactants. Ceram. Int. 2017, 43, 10333–10340. [Google Scholar] [CrossRef]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous synthesis of iron oxide (Fe3O4) nanoparticles via thermal decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef]
- Garcia, M.A.; Bouzas, V.; Costo, R.; Veintemillas, S.; Morales, P.; Garcia-Hernandez, M.; Alexandrescu, R.; Morjan, I.; Gasco, P. Magnetic Properties of Fe Oxide Nanoparticles Produced by Laser Pyrolysis for Biomedical Applications. AIP Conf. Proc. 2010, 1275, 26. [Google Scholar]
- Bena-Arfa, B.A.E.; Salvado, I.M.M.; Ferreira, J.M.F.; Pullar, R.C. Clove and cinnamon: Novel anti-oxidant fuels for preparing magnetic iron oxide particles by the sol-gel auto-ignition method. J. Alloys Compd. 2019, 786, 71–76. [Google Scholar] [CrossRef]
- Gyergyek, S.; Makovec, D.; Jagodic, M.; Drofenik, M.; Schenk, K.; Jordan, O.; Kovac, J.; Dražic, G.; Hofmann, H. Hydrothermal growth of iron oxide NPs with a uniform size distribution for magnetically induced hyperthermia: Structural, colloidal and magnetic properties. J. Alloys Compd. 2017, 694, 261–271. [Google Scholar] [CrossRef]
- Ianos, R.; Taculescu, A.; Pacurariu, C.; Lazau, I. Solution Combustion Synthesis and Characterization of Magnetite, Fe3O4, Nanopowders. J. Am. Ceram. Soc. 2012, 95, 2236–2240. [Google Scholar] [CrossRef]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: Magnetic and transport properties. J. Phys. D 2002, 35, R15. [Google Scholar] [CrossRef]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic Iron Oxide Nanoparticles with Variable Size and an Iron Oxidation State as Prospective Imaging Agents. Langmuir 2013, 29, 710–716. [Google Scholar] [CrossRef]
- Mishra, D.; Arora, R.; Lahiri, S.; Amritphale, S.S.; Chandra, N. Synthesis and characterization of iron oxide nanoparticles by solvothermal method. Prot. Met. Phys. Chem. Surf. 2014, 50, 628–631. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transforms infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Ercuta, A.; Chirita, M. Highly crystalline porous magnetite and vacancy-ordered maghemite microcrystals of rhombohedral habit. J. Cryst. Growth 2013, 380, 182–186. [Google Scholar] [CrossRef]
- Ianos̗, R.; Păcurariu, C.; Mihoc, G. Magnetite/carbon nanocomposites prepared by an innovative combustion synthesis technique—Excellent adsorbent materials. Ceram. Int. 2014, 40, 13649–13657. [Google Scholar] [CrossRef]
- Sze, M.F.F.; McKay, G. An adsorption diffusion model for removal of para-chlorophenol by activated carbon derived from bituminous coal. Environ. Pollut. 2010, 158, 1669–1674. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.V.F.N.; da Silva, A.R.; Pereira da Cunha, T.; dos Santos, R.T.L.; de Oliveira, J.P.; Pereira, E.V.; Licinio, M.V.V.J.; de Godoi Pereira, M.; dos Santos, A.V.; Ribeiro, J.N. Banana Peel for Acetylsalicylic Acid Retention. JEP 2016, 7, 1850–1859. [Google Scholar] [CrossRef]
- Mphahlele, K.Q.; Onyango, M.S.; Mhlanga, S.D. Adsorption of aspirin and paracetamol from aqueous solution using Fe/N-CNT/β-cyclodextrin nanocomopsites synthesized via a benign microwave assisted method. JECE 2015, 3, 2619–2630. [Google Scholar] [CrossRef]
- Beninati, S.; Semeraro, D.; Mastragostino, M. Adsorption of Paracetamol and Acetylsalicylic Acid onto Commercial Activated Carbons. Adsorpt. Sci. Technol. 2009, 26, 721–734. [Google Scholar] [CrossRef]
- Dutta, M.; Das, U.; Mondal, S.; Bhattachriya, S.; Khatun, R.; Bagal, R. Adsorption of acetaminophen by using tea waste derived activated carbon. IJES 2015, 6, 270–281. [Google Scholar]
- Mohd, N.; Sudirman, M.F.A.E.; Draman, S.F.S. Isotherm and Thermodynamic Study of Paracetamol Removal in Aqueous Solution by Activated Carbon. ARPN-JEAS 2015, 10, 9516–9520. [Google Scholar]
- Otero, M.; Grande, C.A.; Rodrigues, A.E. Adsorption of salicylic acid onto polymeric adsorbents and activated charcoal. React. Funct. Polym. 2004, 60, 203–213. [Google Scholar] [CrossRef]
- Tseng, R.L.; Wu, F.C.; Juang, R.S. Characteristics and applications of the Lagergren’s first-order equation for adsorption kinetics. J. Taiwan Inst. Chem. Eng. 2010, 41, 661–669. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. B 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Ho, Y.S. Selection of optimum sorption isotherm. Carbon 2004, 42, 2115–2116. [Google Scholar] [CrossRef]
- Czepirski, L.; Balys, M.R.; Komorowska-Czepirska, E. Some generalization of Langmuir adsorption isotherm. Internet J. Chem. 2000, 3, 14. [Google Scholar]
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Yadla, S.V.; Sridevi, V.; Lakshmi, M.V.V.C. A Review on Adsorption of Heavy Metals from Aqueous Solution. J. Chem. Biol. Phys. Sci. 2012, 2, 1585–1593. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
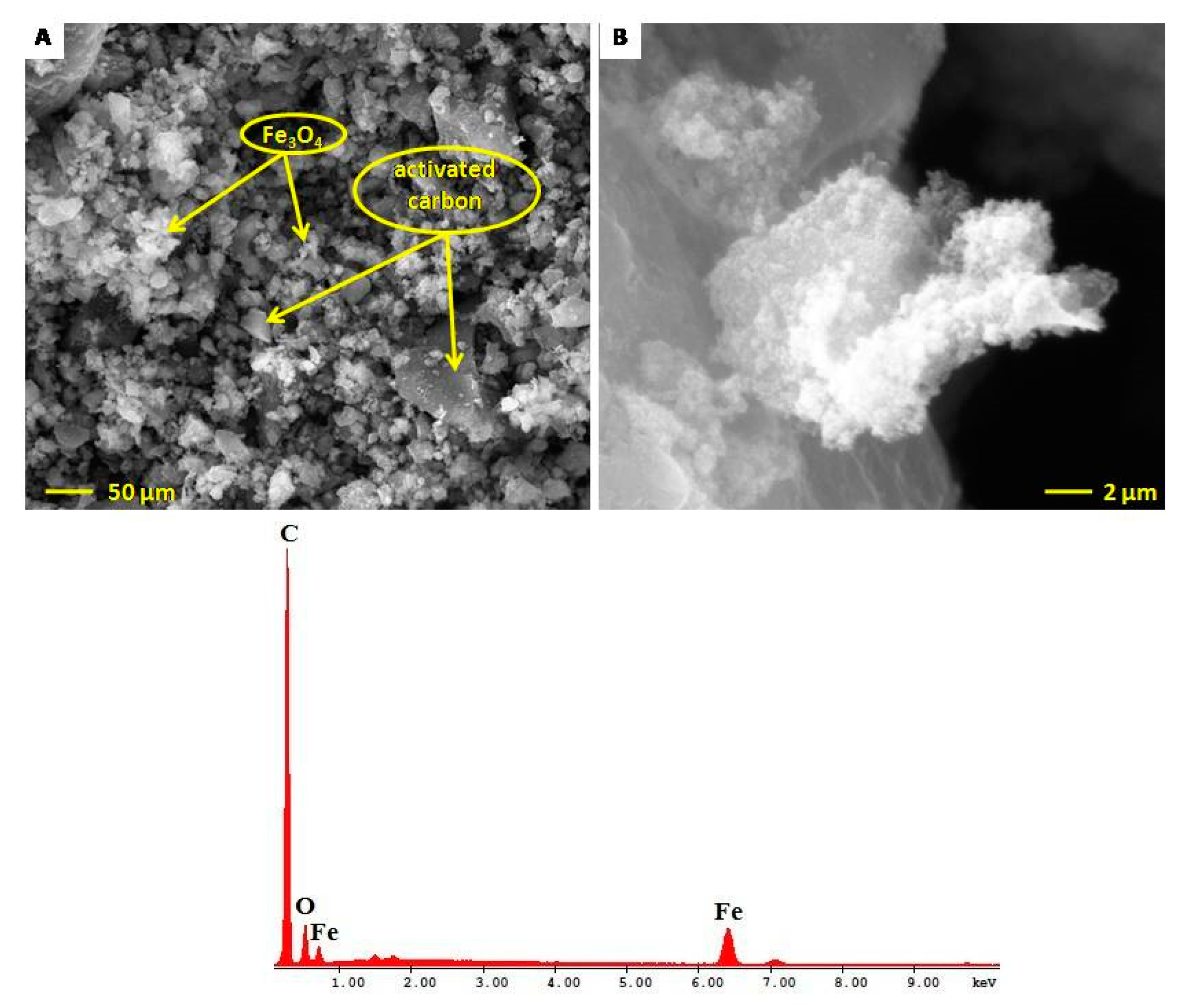
| Element | Wt% | At% | K-Ratio | Z | A | F |
|---|---|---|---|---|---|---|
| C k | 50.48 | 72.62 | 0.1729 | 1.0502 | 0.3261 | 1.0003 |
| O k | 15.66 | 16.91 | 0.0276 | 1.0342 | 0.1705 | 1.0010 |
| Fe k | 33.86 | 10.47 | 0.3140 | 0.8872 | 1.0453 | 1.0000 |
| Total | 100.00 | 100.00 |
| Pseudo-Second-Order | Pseudo-First-Order | ||||||
|---|---|---|---|---|---|---|---|
| Adsorbate | Conc. [mg/L] | k2·103 [g·mg−1·min−1] | R2 | qe [mg/g] | R2 | k1·103 [min−1] | |
| Experimental | Calculated | ||||||
| Paracetamol | 50 | 22.94 | 0.99996 | 24.36 | 24.47 | 0.94914 | 11.37 |
| Acetylsalicylic acid | 100 | 3.56 | 0.99977 | 43.43 | 44.62 | 0.88386 | 10.38 |
| Analgesic Drug | Isotherm Model | Parameter | |
|---|---|---|---|
| Paracetamol | Langmuir | KL [L mg−1] | 0.01384 |
| qm [mg g−1] | 142.011 | ||
| R2 | 0.95703 | ||
| χ2 | 107.22 | ||
| Freundlich | KF [mg1−(1/n)L1/ng−1] | 13.48712 | |
| n | 2.77126 | ||
| R2 | 0.9838 | ||
| χ2 | 40.41 | ||
| Redlich–Peterson | KRP [L g−1] | 8.94606 | |
| αRP [(L mg−1)β] | 0.41013 | ||
| β | 0.71298 | ||
| R2 | 0.98593 | ||
| χ2 | 35.10 | ||
| Acetylsalicylic acid | Langmuir | KL [L mg−1] | 0.00452 |
| qm [mg g−1] | 234.0139 | ||
| R2 | 0.95008 | ||
| χ2 | 161.44 | ||
| Freundlich | KF [mg1−(1/n)L1/ng−1] | 6.64178 | |
| n | 1.91846 | ||
| R2 | 0.99421 | ||
| χ2 | 18.73 | ||
| Redlich–Peterson | KRP [L g−1] | 479009.3512 | |
| αRP [(L mg−1)β] | 72115.6797 | ||
| β | 0.47876 | ||
| R2 | 0.99228 | ||
| χ2 | 24.97 |
| Adsorbent | Obtained/Acquisition Mode | Adsorbent Quantity [g L−1] | Adsorption Condition | Equilibrium Time | Maximum Adsorption Quantity [mg g−1] | References |
|---|---|---|---|---|---|---|
| Fe3O4@C matrix | combustion method | 2 | T = 25 °C pH = 3 | 1 h | 141.99 | this study |
| Reduced activated carbon | obtained from heated granulated carbon at 900°C | 4 | T = 25 °C pH = 7 | 48 h | 245.7 | [16] |
| Activated carbon | obtained by various biological precursors materials | 1 | T = 30 °C pH = 3 | 20 min | 99.42 | [52] |
| Activated carbon | purchased (Ever Gainfull Enterprise Sdn. Bhd) | 1 | T = 40 °C pH = 2 | 2 h | 92.17 | [53] |
| Fe/N-CNT/β-CD nanocomposite | microwave-assisted method | 1 | T = 25 °C pH = 7 | 30 min | 75.2 | [50] |
| N-CNT/β-CD nanocomposite | 41 |
| Adsorbent | Obtained/Acquisition Mode | Adsorbent Quantity [g L−1] | Adsorption Condition | Equilibrium Time | Maximum Adsorption Quantity [mg g−1] | References |
|---|---|---|---|---|---|---|
| Fe3O4@C matrix | combustion method | 2 | T = 25 °C pH = 3 | 2 h | 234.02 | this study |
| Banana peel bioadsorbent | obtained from the banana silver fruit | 6 | T = 25 °C pH = 7 | 15 min | 2.29 | [49] |
| Fe/N-CNT/β-CD nanocomposite | microwave-assisted method | 1 | T = 25 °C pH = 2 | 30 min | 101.0 | [50] |
| N-CNT/β-CD nanocomposite | 71.9 | |||||
| Activated carbon | purchased (Sigma-Aldrich) | 0.15 | T = 26 °C pH = 1.5 | 48 h | 236.0 | [51] |
| Sephabeads SP 206 Polymer | purchased | 0.84 | T = 20 °C pH = -- | 150 min | 45.2 | [54] |
| Sephabeads SP 207 Polymer | 0.72 | 81.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moacă, E.-A.; Mihali, C.-V.; Macaşoi, I.-G.; Racoviceanu, R.; Şoica, C.; Dehelean, C.-A.; Păcurariu, C.; Florescu, S. Fe3O4@C Matrix with Tailorable Adsorption Capacities for Paracetamol and Acetylsalicylic Acid: Synthesis, Characterization, and Kinetic Modeling. Molecules 2019, 24, 1727. https://doi.org/10.3390/molecules24091727
Moacă E-A, Mihali C-V, Macaşoi I-G, Racoviceanu R, Şoica C, Dehelean C-A, Păcurariu C, Florescu S. Fe3O4@C Matrix with Tailorable Adsorption Capacities for Paracetamol and Acetylsalicylic Acid: Synthesis, Characterization, and Kinetic Modeling. Molecules. 2019; 24(9):1727. https://doi.org/10.3390/molecules24091727
Chicago/Turabian StyleMoacă, Elena-Alina, Ciprian-Valentin Mihali, Ioana-Gabriela Macaşoi, Roxana Racoviceanu (Băbuţă), Codruţa Şoica, Cristina-Adriana Dehelean, Cornelia Păcurariu, and Sorin Florescu. 2019. "Fe3O4@C Matrix with Tailorable Adsorption Capacities for Paracetamol and Acetylsalicylic Acid: Synthesis, Characterization, and Kinetic Modeling" Molecules 24, no. 9: 1727. https://doi.org/10.3390/molecules24091727
APA StyleMoacă, E.-A., Mihali, C.-V., Macaşoi, I.-G., Racoviceanu, R., Şoica, C., Dehelean, C.-A., Păcurariu, C., & Florescu, S. (2019). Fe3O4@C Matrix with Tailorable Adsorption Capacities for Paracetamol and Acetylsalicylic Acid: Synthesis, Characterization, and Kinetic Modeling. Molecules, 24(9), 1727. https://doi.org/10.3390/molecules24091727





.jpg)




