Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia
Abstract
:1. Introduction
2. Results
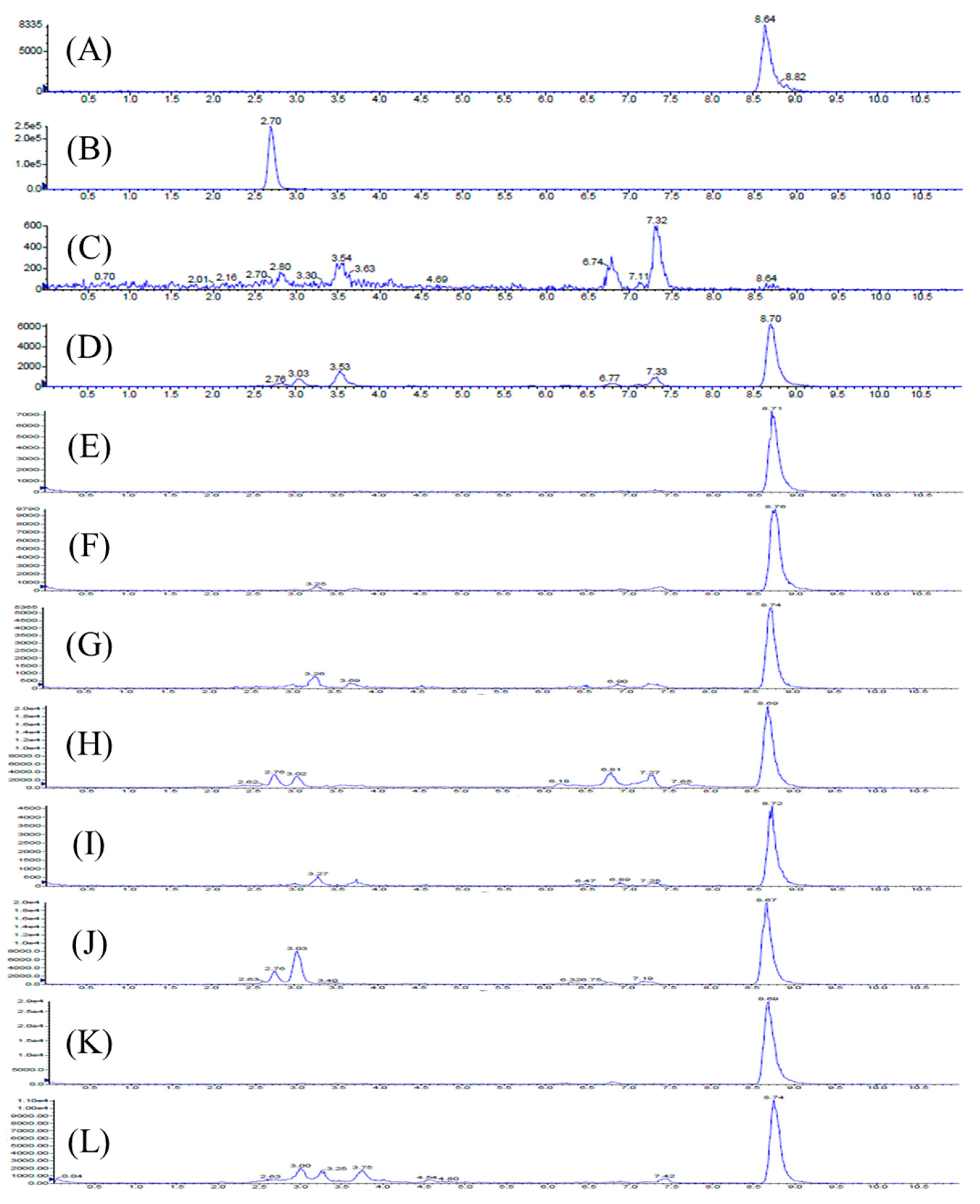
2.1. Method Validation
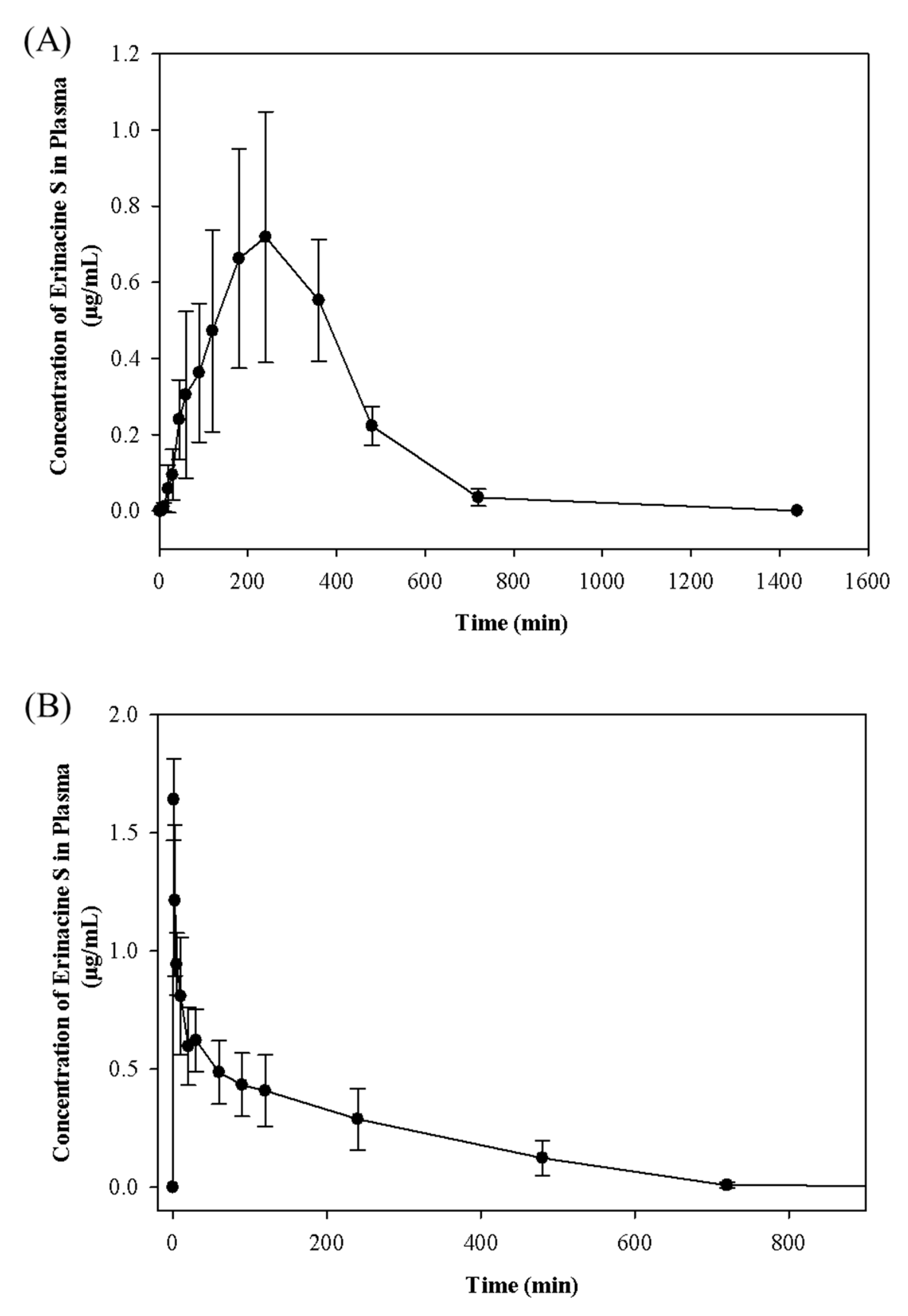
2.2. Pharmacokinetic Study
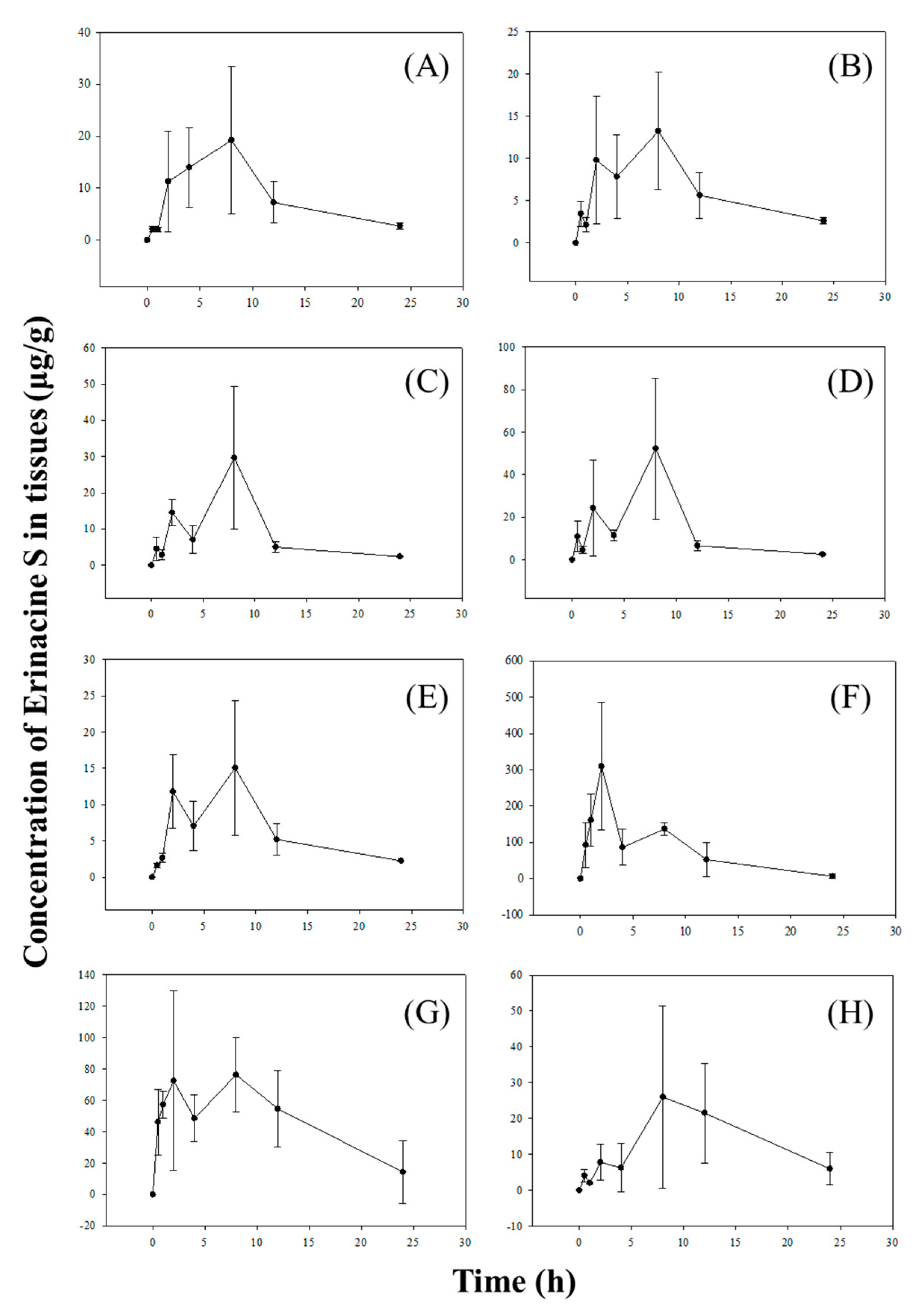
2.3. Tissue Distribution Study
2.4. Excretion Study
3. Discussion
4. Materials and Methods
4.1. Preparation of H. erinaceus Mycelia
4.2. Preparation of the H. erinaceus Mycelia Extract and Erinacine S
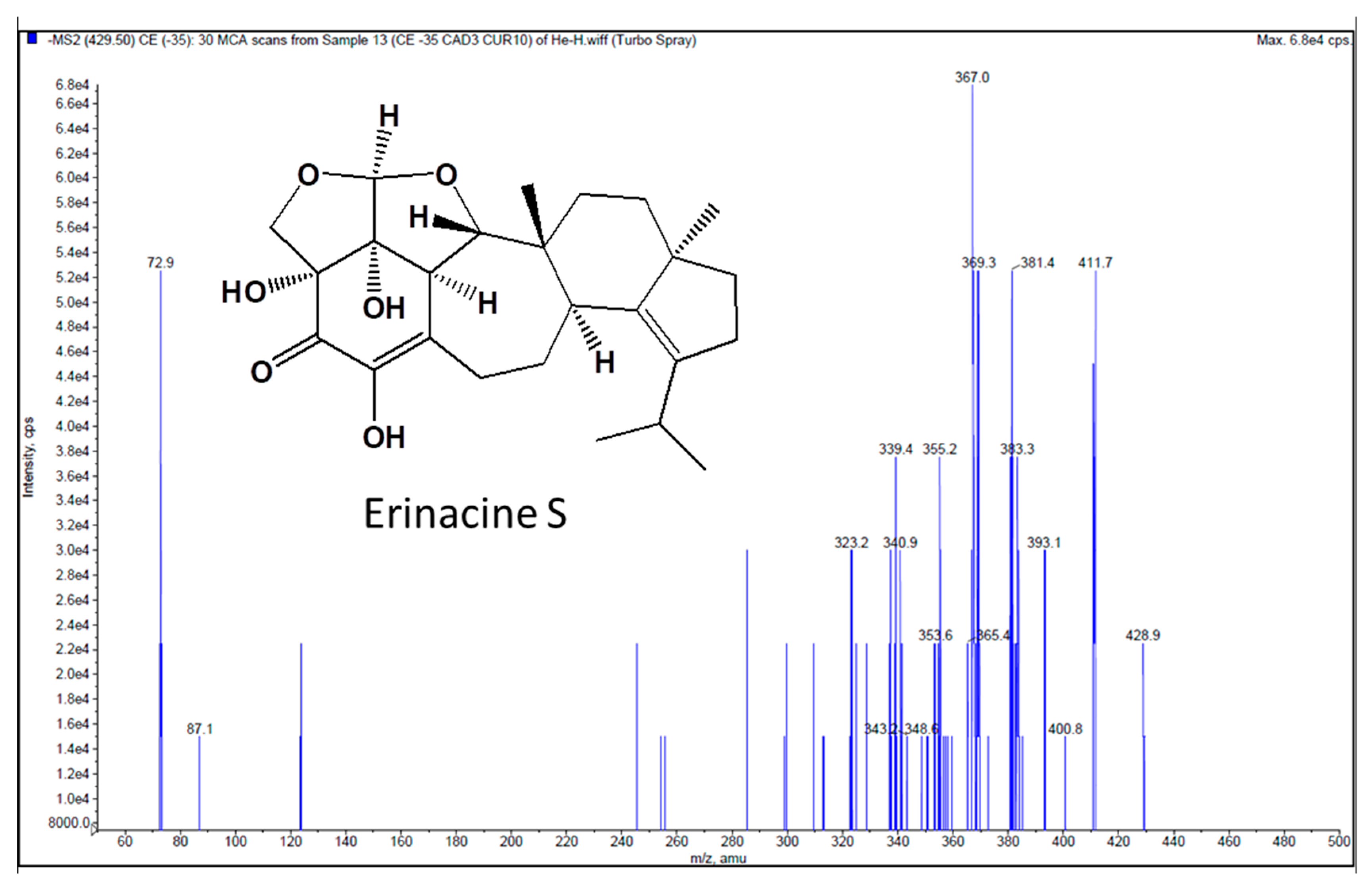
4.3. Bioanalysis of Erinacine S
4.4. Method Validation
4.5. Animal Study
4.6. Pharmacokinetic Study
4.7. Distribution Study
4.8. Excretion Study
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complement. Integr. Med. 2013, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, W.; Kim, E.J.; Shim, S.H.; Kang, H.K.; Kim, Y.H. Isolation and identification of aromatic compounds in lion’s mane mushroom and their anticancer activities. Food Chem. 2015, 170, 336–342. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, J.; Meng, Y.; Kang, Y.; Han, Z.; Tai, G.; Zhou, Y.; Cheng, H. Immunomodulatory effects of Hericium erinaceus derived polysaccharides are mediated by intestinal immunology. Food Funct. 2017, 8, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Guo, Z.; Xie, F.; Zhao, A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013, 13, 253. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.H.; Jang, H.D.; Ma, J.Y.; Kim, Y.H. Antioxidant and anti-osteoporotic activities of aromatic compounds and sterols from Hericium erinaceum. Molecules 2017, 22, 108. [Google Scholar] [CrossRef]
- Shen, T.; Morlock, G.; Zorn, H. Production of cyathane type secondary metabolites by submerged cultures of Hericium erinaceus and evaluation of their antibacterial activity by direct bioautography. Fungal Biol. Biotechnol. 2015, 2, 8. [Google Scholar] [CrossRef]
- Abdulla, M.A.; Fard, A.A.; Sabaratnam, V.; Wong, K.H.; Kuppusamy, U.R.; Abdullah, N.; Ismail, S. Potential activity of aqueous extract of culinary-medicinal lion’s mane mushroom, Hericium erinaceus (bull.: Fr.) pers. (aphyllophoromycetideae) in accelerating wound healing in rats. Int. J. Med. Mushrooms 2011, 13, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Li, I.-C.; Lee, L.-Y.; Tzeng, T.-T.; Chen, W.-P.; Chen, Y.-P.; Shiao, Y.-J.; Chen, C.-C. Neurohealth properties of Hericium erinaceus mycelia enriched with erinacines. Behav. Neurol. 2018, 2018, 580263. [Google Scholar] [CrossRef] [PubMed]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Lorio, C.; De Lua, F.; et al. Hericium erinaceus improves recognition memory and induces hippocapal and cerebella neurogenesis in frail mice during aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef]
- Rossi, P.; Cesaroni, V.; Brandalise, F.; Occhinegro, A.; Ratto, D.; Perrucci, F.; Lanaia, V.; Girometta, C.; Orrù, G.; Savino, E. Dietary supplementation of lion’s mane medicinal mushroom, Hericium erinaceus (agaricomycetes), and spatial memory in wild-type mice. Int. J. Med. Mushrooms 2018, 20, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Gulielminetti, M.L.; Savino, E.; et al. Dietary supplementation of Hericium erinaceus increases mossy fiber-CA3 hippocampal neurotransmission and recognition memory in wild-type mice. Evid. Based Complement. Altern. Med. 2017, 2017, 3864340. [Google Scholar] [CrossRef]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine a against ischemia-injury-induced neuronal cell death via the inhibition of inos/p38 mapk and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef]
- Tzeng, T.T.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Lu, C.K.; Shen, C.C.; Huang, F.C.Y.; Chen, C.C.; Shiao, Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates alzheimer’s disease-related pathologies in appswe/ps1de9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar]
- Kuo, H.C.; Lu, C.C.; Shen, C.H.; Tung, S.Y.; Hsieh, M.C.; Lee, K.C.; Lee, L.Y.; Chen, C.C.; Teng, C.C.; Huang, W.S.; et al. Hericium erinaceus mycelium and its isolated erinacine a protection from mptp-induced neurotoxicity through the er stress, triggering an apoptosis cascade. J. Transl. Med. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Chyau, C.-C.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Liu, J.-L.; Lin, W.-H.; Mong, M.-C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating bdnf/pi3k/akt/gsk-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Tzeng, T.-T.; Chen, C.-C.; Ni, C.-L.; Lee, L.-Y.; Chen, W.-P.; Shiao, Y.-J.; Shen, C.-C. Erinacine S, a rare sesterterpene from the mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]
- Tzeng, T.T.; Chen, C.C.; Chen, C.C.; Tsay, H.J.; Lee, L.Y.; Chen, W.P.; Shen, C.C.; Shiao, Y.J. The cyanthin diterpenoid and sesterterpene constituents of Hericium erinaceus mycelium ameliorate alzheimer’s disease-related pathologies in app/ps1 transgenic mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Lesko, L.J.; Rowland, M.; Peck, C.C.; Blaschke, T.F. Optimizing the science of drug development: Opportunities for better candidate selection and accelerated evaluation in humans. Eur. J. Pharm. Sci. 2000, 10, iv–xiv. [Google Scholar] [CrossRef]
- Causon, R. Validation of chromatographic methods in biomedical analysis viewpoint and discussion. J. Chormatogr. B Biomed. Sci. Appl. 1997, 689, 175–180. [Google Scholar] [CrossRef]
- Li, I.C.; Chen, Y.L.; Chen, W.P.; Lee, L.Y.; Tsai, Y.T.; Chen, C.C.; Chen, C.S. Genotoxicity profile of erinacine A-enriched Hericium erinaceus mycelium. Toxicol. Rep. 2014, 1, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Chen, Y.L.; Lee, L.Y.; Chen, W.P.; Tsai, Y.T.; Chen, C.C.; Chen, C.S. Evaluation of the toxicological safety of erinacine A-enriched Hericium erinaceus in a 28-day oral feeding study in sprague-dawley rats. Food Chem. Toxicol. 2014, 70, 61–67. [Google Scholar] [CrossRef]
- Li, I.C.; Chen, W.P.; Chen, Y.P.; Lee, L.Y.; Tsai, Y.T.; Chen, C.C. Acute and developmental toxicity assessment of erincine A-enriched Hericium erinaceus mycelia in sprague-dawley rats. Drug Chem. Toxicol. 2018, 41, 459–464. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals/Section 4: Health Effects Test No. 420: Acute Oral Toxicity—Fixed Dose Procedure; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Tian, X.; Xu, Z.; Chen, M.; Hu, P.; Liu, F.; Sun, Z.; Liu, H.; Guo, X.; Li, Z.; Huang, C. Simultaneous determination of eight bioactive compounds by lc-ms/ms and its application to the pharmacokinetics, liver first-pass effect, liver and brain distribution of orally administrated gouteng-baitouweng (gb) in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1084, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Okusanya, O.; Forrest, A.; DiFrancesco, R.; Bilic, S.; Rosenkranz, S.; Para, M.F.; Adams, E.; Yarasheski, K.E.; Reichman, R.C.; Morse, G.D. Compartmental pharmacokinetic analysis of oral amprenavir with secondary peaks. Antimicrob. Chemother. 2007, 51, 1822–1826. [Google Scholar] [CrossRef]
- Metsugi, Y.; Miyaji, Y.; Ogawara, K.; Higaki, K.; Kimura, T. Appearance of double peaks in plasma concentration-time profile after oral administration depends on gastric emptying profile and weight function. Pharm. Res. 2008, 25, 886–895. [Google Scholar] [CrossRef]
- Davies, N.M.; Takemoto, J.K.; Brocks, D.R.; Yanez, J.A. Multiple peaking phenomena in pharmacokinetic disposition. Clin. Pharmacokinet. 2010, 49, 351–377. [Google Scholar] [CrossRef]
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol. Nutr. Food Res. 2012, 56, 466–474. [Google Scholar] [CrossRef]
- Deng, C.; Gao, C.; Tian, X.; Chao, B.; Wang, F.; Zhang, Y.; Zou, J.; Liu, D. Pharmacokinetics, tissue distribution and excretion of luteolin and its major metabolites in rats: Metabolites predominate in blood, tissues and are mainly excreted via bile. J. Funct. Foods 2017, 35, 332–340. [Google Scholar] [CrossRef]
- Liu, N.; Abell, T. Gastroparesis updates on pathogenesis and management. Gut Liver 2017, 11, 579–589. [Google Scholar] [CrossRef]
- Leeson, P. Drug discovery: Chemical beauty contest. Nature 2012, 481, 455–456. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood–brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Hung, W.-L.; Chang, W.-S.; Lu, W.-C.; Wei, G.-J.; Wang, Y.; Ho, C.-T.; Hwang, L.S. Pharmacokinetics, bioavailability, tissue distribution and excretion of tangeretin in rat. J. Food Drug Anal. 2018, 26, 849–857. [Google Scholar] [CrossRef]
- Van Amsterdam, P.; Companjen, A.; Brudny-Kloeppel, M.; Golob, M.; Luedtke, S.; Timmerman, P. The European bioanalysis forum community’s evaluation, interpretation and implementation of the European medicines agency guideline on bioanalytical method validation. Bioanalysis 2013, 5, 645–659. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Chen, C.-C.; Lin, T.-W.; Tsai, P.-C.; Kuo, C.-F. Absolute bioavailability, tissue distribution, and excretion of 2,4,5-trimethoxybenzaldehyde in rats. J. Funct. Foods 2017, 35, 90–96. [Google Scholar] [CrossRef]
- Cen, M.; Liang, H.; Xiong, X.; Zeng, J.; Cheng, X.; Wang, S. Development and validation of a HPLC method for determination of isochlorogenic acid a in rat plasma and application to pharmacokinetic study. J. Chromatogr. Sci. 2017, 55, 1037–1042. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Intra-Day | Inter-Day | |||||
|---|---|---|---|---|---|---|
| Theoretical Conc. | Observed Conc. | Precision | Accuracy | Observed Conc. | Precision | Accuracy |
| (ng/mL) | (ng/mL) | (% CV) | (% bias) | (ng/mL) | (% CV) | (% bias) |
| 5 | 5.69 ± 0.47 | 8.29 | 13.83 | 5.70 ± 0.44 | 7.69 | 14.04 |
| 10 | 8.71 ± 0.88 | 10.14 | −12.94 | 9.13 ± 0.63 | 6.88 | −8.69 |
| 20 | 20.62 ± 1.62 | 7.86 | 3.12 | 19.36 ± 0.43 | 2.23 | −3.18 |
| 50 | 49.96 ± 1.55 | 3.1 | −0.08 | 45.31 ± 1.22 | 2.69 | −9.38 |
| 100 | 111.40 ± 1.32 | 1.18 | 11.4 | 97.91 ± 5.09 | 5.2 | −2.09 |
| 200 | 198.56 ± 5.22 | 2.63 | −0.72 | 206.33 ± 2.54 | 1.23 | 3.17 |
| 500 | 499.83 ± 9.19 | 1.84 | −0.03 | 499.65 ± 6.62 | 1.33 | −0.07 |
| Theoretical Conc. (ng/mL) | |||
|---|---|---|---|
| 50 | 200 | 500 | |
| Plasma | 99.29 ± 4.70 | 97.63 ± 4.66 | 100.40 ± 2.37 |
| Brain | 102.53 ± 4.47 | 98.12 ± 2.69 | 95.98 ± 4.57 |
| Heart | 99.54 ± 4.09 | 83.04 ± 5.96 | 91.73 ± 5.96 |
| Liver | 92.18 ± 9.60 | 80.46 ± 3.97 | 91.66 ± 10.15 |
| Lung | 100.40 ± 11.72 | 94.44 ±9.56 | 98.48 ± 6.25 |
| Kidney | 101.69 ± 4.14 | 98.48 ± 5.35 | 87.94 ± 4.49 |
| Stomach | 77.05 ± 5.96 | 86.84 ± 5.80 | 90.33 ± 4.15 |
| Small Intestine | 81.78 ± 11.74 | 85.77 ± 7.12 | 89.88 ± 5.97 |
| Large Intestine | 90.67 ± 11.24 | 93.63 ± 10.12 | 101.04 ± 6.64 |
| Feces | 101.15 ± 11.12 | 97.03 ± 9.54 | 96.78 ± 3.98 |
| Urine | 100.87 ± 1.80 | 101.58 ± 3.71 | 99.43 ± 3.31 |
| P.O. | I.V. | |
|---|---|---|
| (50 mg/kg) | (5 mg/kg) | |
| Tmax (min) | 270.00 ± 73.48 | - |
| Cmax (μg/mL) | 0.73 ± 0.31 | 1.64 ± 0.17 |
| T1/2 (min) | 439.84 ± 60.98 | 11.45 ± 5.76 |
| AUC (min μg/mL) | 272.06 ±77.82 | 179.77 ± 66.46 |
| Absolute Bioavailability (%) | 15.13 | |
| Time (h) | Feces | Urine |
|---|---|---|
| Concentration (μg/kg) | Concentration (μg/kg) | |
| 0–4 | 0.273 ± 0.236 | 0.094 ± 0.055 |
| 4–8 | 3.482 ± 4.401 | 0.094 ± 0.062 |
| 8–12 | 9.724 ± 3.036 | 0.068 ± 0.038 |
| 12–24 | 9.546 ± 3.182 | 0.086 ± 0.049 |
| Total Amount (μg) (% of administered dose) | 21.669 ± 11.479 (0.094 ± 0.053%) | 0.932 ± 0.602 (0.004 ± 0.002%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.-H.; Li, I.-C.; Lin, T.-W.; Chen, W.-P.; Lee, L.-Y.; Chen, C.-C.; Kuo, C.-F. Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia. Molecules 2019, 24, 1624. https://doi.org/10.3390/molecules24081624
Hu J-H, Li I-C, Lin T-W, Chen W-P, Lee L-Y, Chen C-C, Kuo C-F. Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia. Molecules. 2019; 24(8):1624. https://doi.org/10.3390/molecules24081624
Chicago/Turabian StyleHu, Jun-Hao, I-Chen Li, Ting-Wei Lin, Wan-Ping Chen, Li-Ya Lee, Chin-Chu Chen, and Chia-Feng Kuo. 2019. "Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia" Molecules 24, no. 8: 1624. https://doi.org/10.3390/molecules24081624
APA StyleHu, J.-H., Li, I.-C., Lin, T.-W., Chen, W.-P., Lee, L.-Y., Chen, C.-C., & Kuo, C.-F. (2019). Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia. Molecules, 24(8), 1624. https://doi.org/10.3390/molecules24081624





