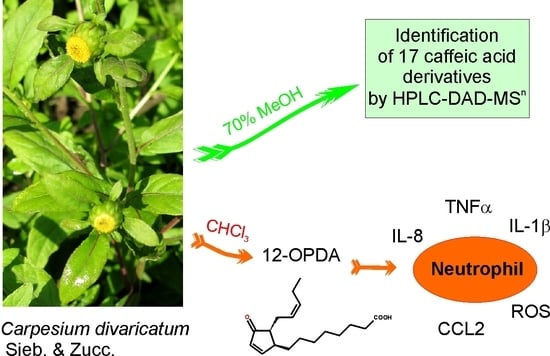
Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant
Abstract
1. Introduction
2. Results
2.1. Caffeic Acid Derivatives in Aerial Parts of C. divaricatum
2.2. Identification of Trans-12-Oxo-phytodienoic Acid (trans-12-OPDA)
2.3. Effect of trans-12-OPDA on Lipopolysaccharide (LPS)-Stimulated Release of Pro-Inflammatory Cytokines from Human Neutrophils
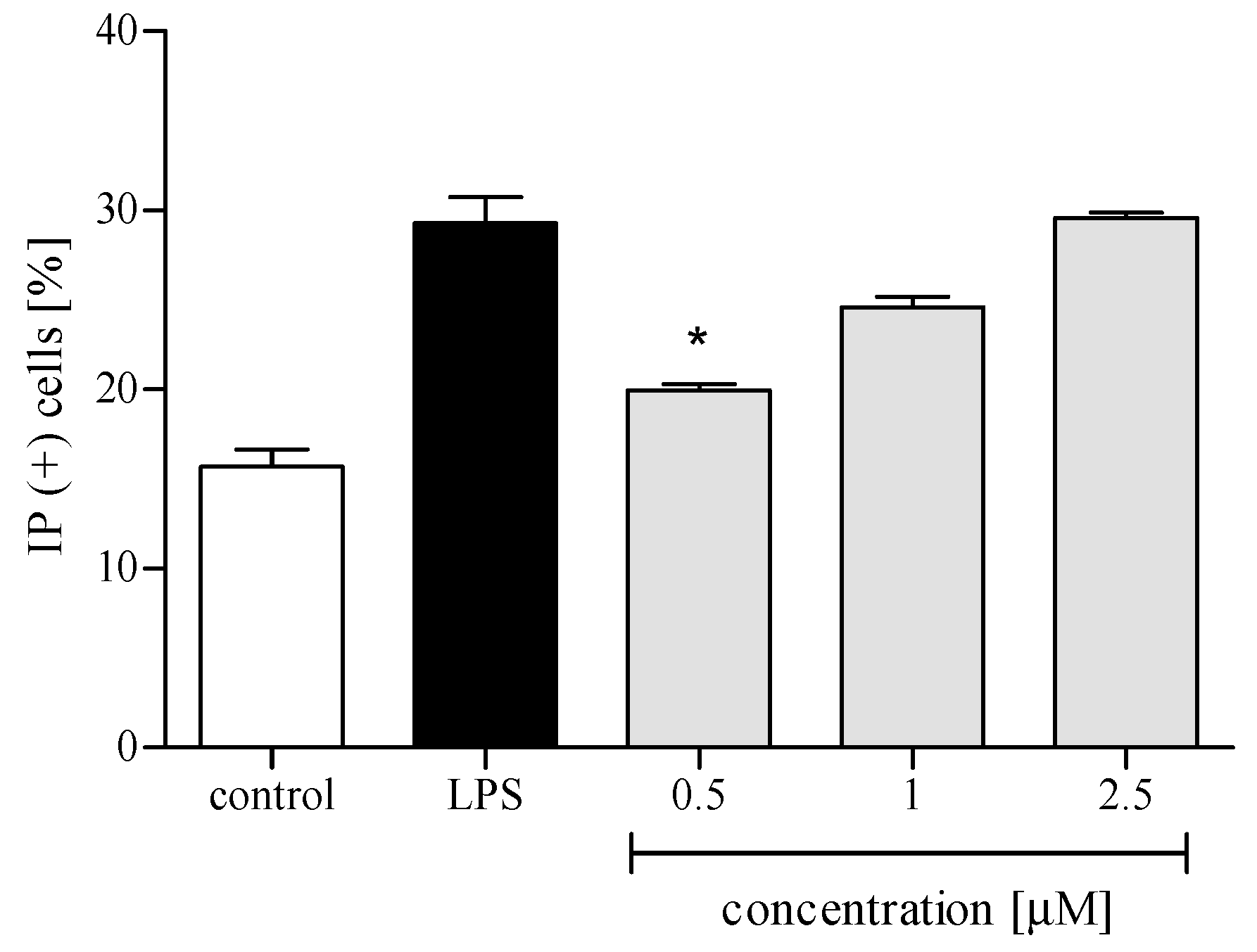
2.3.1. Cytotoxicity
2.3.2. Reactive Oxygen Species (ROS) Production
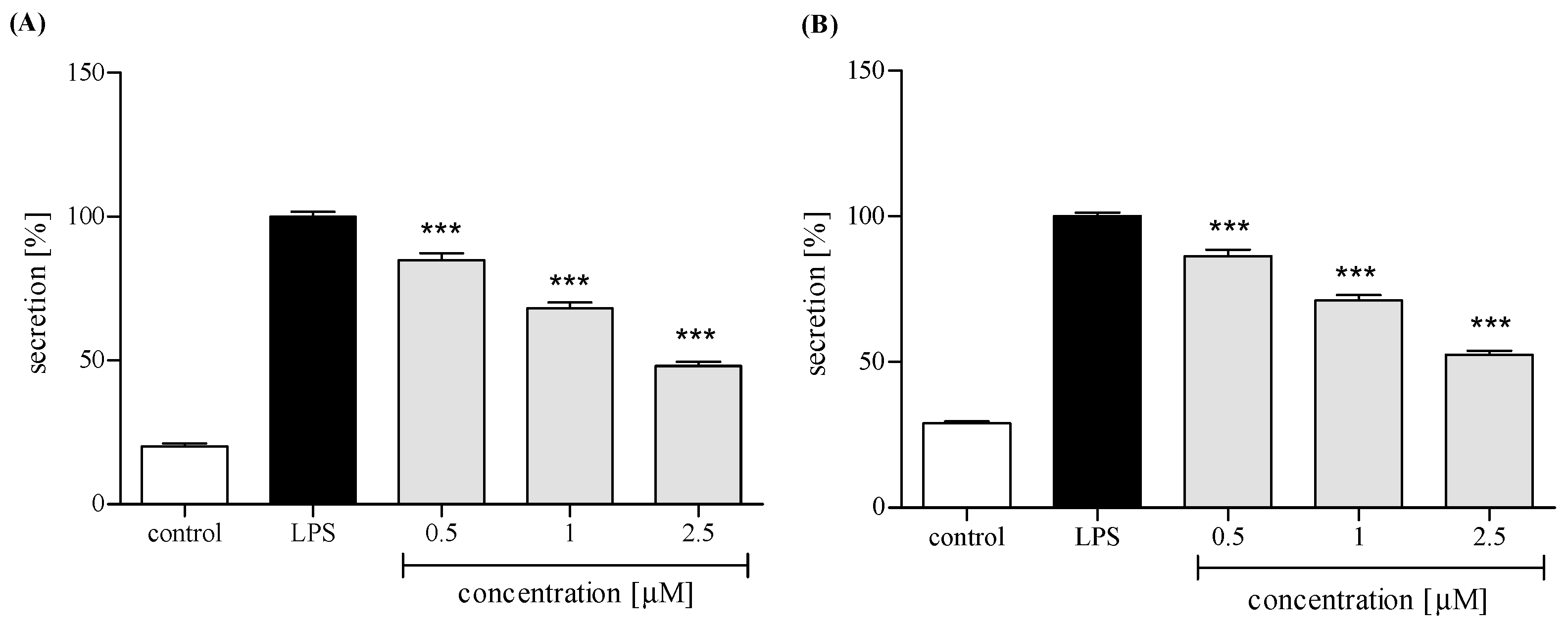
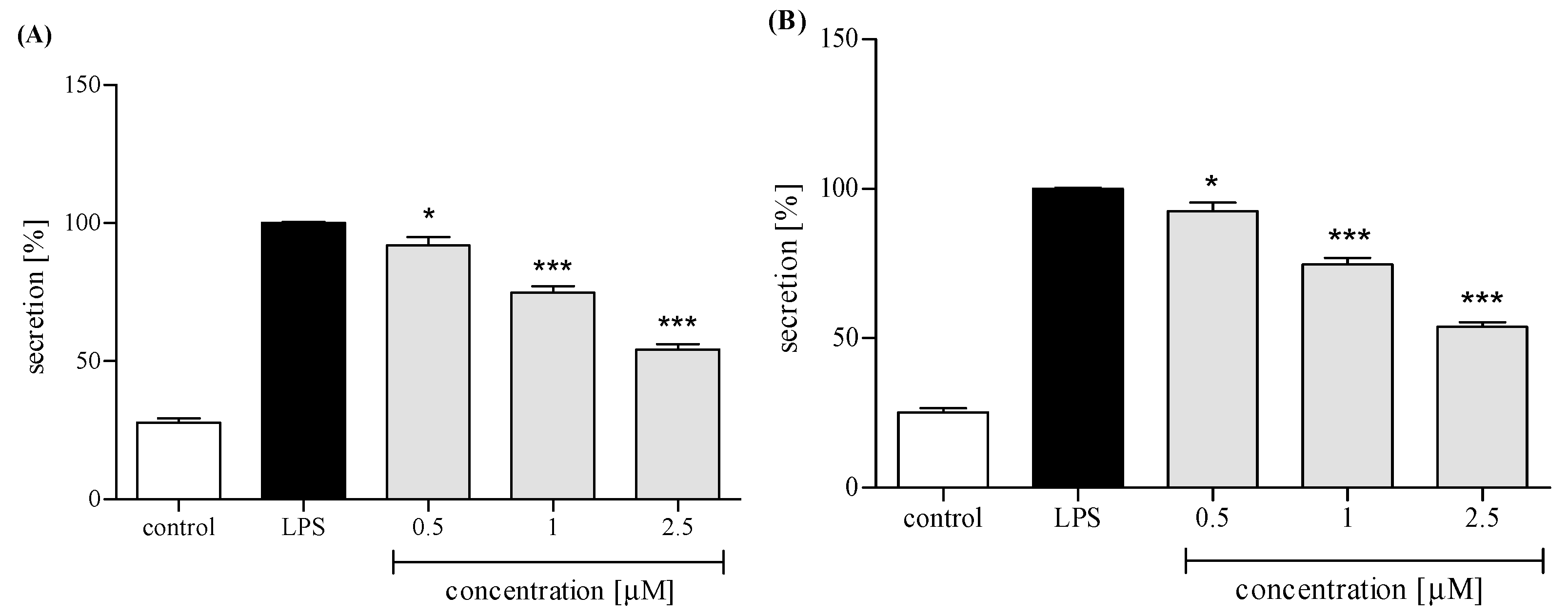
2.3.3. Release of Selected Proinflammatory Cytokines/Chemokines (IL-8, TNFα, IL-1β, CCL2)
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Materials
4.3. Plant Material
4.4. Analysis of Caffeic Acid Derivatives
4.4.1. Extract Preparation and Characterization by the HPLC-DAD-MSn Method
4.4.2. Quantification of Chlorogenic Acid (5-CQA) and 3,5-di-O-caffeoylquinic Acid (3,5-DCQA)
4.5. Isolation of Trans-12-Oxo-Phytodienoic Acid (12-OPDA)
(+) trans-12-Oxo-phytodienoic acid: (9S,13R) OPDA
4.6. Isolation of Human Neutrophils
4.7. Assessment of the Effects of Trans-12-OPDA on Lipopolysaccharide (LPS)-Stimulated Release of Pro-Inflammatory Cytokines from Human Neutrophils
4.7.1. Cytotoxicity Measurement
4.7.2. ROS Production by Neutrophils
4.7.3. IL-8, IL-1β, CCL-2, and TNFα Production by Neutrophils
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Anderberg, A.A. Inuleae. In Systematics, Evolution, and Biogeography of Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 667–680. [Google Scholar]
- Hong, L.; Zhuo, J.; Lei, Q.; Zhou, J.; Ahmed, S.; Wang, C.; Long, Y.; Li, F.; Long, C. Ethnobotany of wild plants used for starting fermented beverages in Shui communities of southwest China. J. Ethnobiol. Ethnomed. 2015, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Wang, G.-W.; Tian, X.-H.; Yang, Y.-X.; Liu, Q.-X.; Chen, L.-P.; Li, H.-L.; Zhang, W.-D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 163, 173–191. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.-J. Ethnobotanical analysis for traditional knowledge of wild edible plants in North Jeolla Province (Korea). Genet. Resour. Crop Evol. 2013, 60, 1571–1585. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, Y.; Ranjitkar, S.; Huai, H.; Wang, Y. Traditional knowledge and its transmission of wild edibles used by the Naxi in Baidi Village, northwest Yunnan province. J. Ethnobiol. Ethnomed. 2016, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Quintana, J.; Eiroa, J.L.; López, M.; Triana, J.; Bermejo, J.; Estévez, F. Potent induction of apoptosis by germacranolide sesquiterpene lactones on human myeloid leukemia cells. Eur. J. Pharmacol. 2003, 482, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, J.-H.; Si, J.-G.; Ding, G.; Zhang, Q.-B.; Zhang, H.-W.; Jia, H.-M.; Zou, Z.-M. Isolation, structure elucidation, and absolute configuration of germacrane isomers from Carpesium divaricatum. Sci. Rep. 2018, 8, 12418. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Willems, J.L.; Khamis, M.M.; Saeid, W.M.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, S.; Seger, C.; Wiesbauer, B.; Schneider, P.; Ellmerer, E.P.; Sturm, S.; Stuppner, H. Development of an HPLC-PAD-MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpinum Cass.) constituents. Phytochem. Anal. 2006, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Petereit, F.; Hensel, A. Caffeic acid derivatives from Eupatorium perfoliatum L. Molecules 2009, 14, 36–45. [Google Scholar] [CrossRef]
- Cicek, S.S.; Untersulzner, C.; Schwaiger, S.; Zidorn, C. Caffeoyl-D-glucaric acid derivatives in the genus Gnaphalium (Asteraceae: Gnaphalieae). Rec. Nat. Prod. 2012, 6, 311–315. [Google Scholar]
- Bazylko, A.; Boruc, K.; Borzym, J.; Kiss, A.K. Aqueous and ethanolic extracts of Galinsoga parviflora and Galinsoga ciliata. Investigations of caffeic acid derivatives and flavonoids by HPTLC and HPLC-DAD-MS methods. Phytochem. Lett. 2015, 11, 394–398. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Kiss, A.K. Hydroxycinnamates from elecampane (Inula helenium L.) callus culture. Acta Physiol. Plant. 2016, 38, 41. [Google Scholar] [CrossRef]
- Heilmann, J.; Müller, E.; Merfort, I. Flavonoid glucosides and dicaffeoylquinic acids from fowerheads of Buphthalmum salicifolium. Phytochemistry 1999, 51, 713–718. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Schwaiger, S.; Cervellati, R.; Seger, C.; Ellmerer, E.P.; About, N.; Renimel, I.; Godenir, C.; André, P.; Gafner, F.; Stuppner, H. Leontopodic acid—A novel highly substituted glucaric acid derivative from Edelweiss (Leontopodium alpinum Cass.) and its antioxidative and DNA protecting properties. Tetrahedron 2005, 61, 4621–4630. [Google Scholar] [CrossRef]
- McDonald, P.P.; Bald, A.; Cassatella, M. Activation of NF-κB pathway by inflammatory stimuli in human neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [PubMed]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; Del Rio, D. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55, S35–S43. [Google Scholar] [CrossRef]
- Tousch, D.; Lajoix, A.-D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.-B.; Wan, M.-Y.; Wang, P.-Y.; Zhang, C.-X.; Xu, D.-Y.; Liao, X.; Sun, H.-J. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 2018, 14, 656–668. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Chiu, C.-C.; Chen, J.Y.-F.; Chan, K.-C.; Lin, S.-D. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharm. 2012, 143, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Y.; Cao, Y.; Song, G.; Liu, Z.; Liu, X. Chicoric acid ameliorates lipopolysaccharide-induced oxidative stress via promoting the Keap1/Nrf2 transcriptional signaling pathway in BV-2 microglial cells and mouse brain. J. Agric. Food Chem. 2017, 65, 338–347. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, Z.; Li, J.; Ren, B.; Zhu, D.; Liu, Q.; Liu, Z.; Liu, X. Chicoric acid supplementation ameliorates cognitive impairment induced by oxidative stress via promotion of antioxidant defence system. RSC Adv. 2017, 7, 36149–36162. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, J.; Yuan, L.; Xiao, C.; Wang, Y.; Liu, X. Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. J. Agric. Food Chem. 2013, 61, 1509–1520. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Gevrenova, R.; Zaharieva, M.M.; Najdenski, H.; Ruseva, S.; Lozanov, V.; Balabanova, V.; Yagi, S.; Momekov, G.; Mitev, V. HPLC-UV and LC-MS analyses of acylquinic acids in Geigeria alata (DC) Oliv. & Hiern. and their contribution to antioxidant and antimicrobial capacity. Phytochem. Anal. 2016, 28, 176–184. [Google Scholar] [PubMed]
- Boukhris, M.A.; Destandau, E.; El Hakmaoui, A.; El Rhaffari, L.; Elfakir, C. A dereplication strategy for the identification of new phenolic compounds from Anvillea radiata (Coss. & Durieu). C.R. Chim. 2016, 19, 1124–1132. [Google Scholar]
- Takenaka, M.; Yan, X.; Ono, H.; Yoshida, M.; Nagata, T.; Nakanishi, T. Caffeic acid derivatives in the roots of yacon (Smallanthus sonchifolius). J. Nat. Prod. 2003, 51, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, Y.; Kim, J. Acylated flavonol glycosides from the flower of Inula britannica. J. Nat. Prod. 2000, 63, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Malarz, J.; Żylewski, M.; Kisiel, W. Acylated hydroxycinnamic acid glucosides from flowers of Telekia speciosa. Phytochem. Lett. 2015, 12, 257–261. [Google Scholar] [CrossRef]
- Stojakowska, A.; Michalska, K.; Kłeczek, N.; Malarz, J.; Beharav, A. Phenolics and terpenoids from a wild edible plant Lactuca orientalis (Boiss.) Boiss.: A preliminary study. J. Food Comp. Anal. 2018, 69, 20–24. [Google Scholar] [CrossRef]
- Maynard, D.; Gröger, H.; Dierks, T.; Dietz, K.-J. The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development. J. Exp. Bot. 2018, 69, 5341–5354. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Ingram, C.D.; Harris, T.M.; Brash, A.R. Absolute configuration of cis-12-oxophytodienoic acid of flaxseed: Implications for the mechanism of biosynthesis from the 13(S)-hydroperoxide of linolenic acid. Biochemistry 1988, 27, 18–24. [Google Scholar] [CrossRef]
- Schaller, F.; Hennig, P.; Weiler, E.W. 12-Oxophytodienoate-10,11-reductase: Occurrence of two isoenzymes of different specificity against stereoisomers of 12-oxophytodienoic acid. Plant Physiol. 1998, 118, 1345–1351. [Google Scholar] [CrossRef]
- Taki-Nakano, N.; Ohzeki, H.; Kotera, J.; Ohta, H. Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta 2014, 1840, 3413–3422. [Google Scholar] [CrossRef]
- Taki-Nakano, N.; Kotera, J.; Ohta, H. 12-oxo-phytodienoic acid, a plant-derived oxylipin, attenuates lipopolysaccharide-induced inflammation in microglia. Biochem. Biophys. Res. Commun. 2016, 473, 1288–1294. [Google Scholar] [CrossRef]
- Altiok, N.; Mezzadra, H.; Patel, P.; Koyuturk, M.; Altiok, S. A plant oxylipin, 12-oxo-phytodienoic acid, inhibits proliferation of human breast cancer cells by targeting cyclin D1. Breast Cancer Res. Treat. 2008, 109, 315–323. [Google Scholar] [CrossRef]
- Maldonado, E.M.; Salamanca, E.; Giménez, A.; Sterner, O. Antileishmanial metabolites from Lantana balansae. Rev. Bras. Farmacogn. 2016, 26, 180–183. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Compound | Retention Time (min) | UV (nm) | [M − H]− | Product Ions Main Peaks 1 | |
|---|---|---|---|---|---|
| 1 | 3-O-caffeoylquinic acid | 3.2 | 325 | 353 | 191, 179 |
| 2 | 5-O-caffeoylquinic acid | 5.1 | 325 | 353 | 191 |
| 3 | Dicaffeoylhexaric acid (I) | 7.9 | 324 | 533 | 371, 209 |
| 4 | 3,4-di-O-caffeoylquinic acid | 12.6 | 325 | 515 | 353, 335, 299, 255, 203, 191, 179, 173 |
| 5 | 1,5-di-O-caffeolyquinic acid | 12.8 | 328 | 515 | 353, 335, 191 |
| 6 | 3,5-di-O-caffeoylquinic acid | 13.0 | 327 | 515 | 353, 191 |
| 7 | tricaffeoylhexaric acid (I) | 13.5 | 327 | 695 | 533, 371, 209 |
| 8 | 4,5-di-O-caffeoylquinic acid | 13.8 | 327 | 515 | 353, 317, 299, 255, 203, 173 |
| 9 | tricaffeoylhexaric acid (II) | 14.8 | 328 | 695 | 533, 371, 209 |
| 10 | isobutyryl-dicaffeoylquinic acid | 19.0 | 326 | 585 | 497, 335, 317, 299, 255, 179 |
| 11 | isobutyryl-dicaffeoylquinic acid | 19.8 | 328 | 585 | 497, 423, 335, 179 |
| 12 | tri-O-caffeoylquinic acid | 20.1 | 327 | 677 | 515, 353 |
| 13 | 2-methylbutyryl/isovaleryl-dicaffeoylquinic acid | 22.9 | 326 | 599 | 497, 335, 299, 179 |
| 14 | 2-methylbutyryl/isovaleryl-dicaffeoylquinic acid | 23.8 | 328 | 599 | 497, 437, 335, 179 |
| 15 | isobutyryl-tricaffeoylhexaric acid | 24.7 | 328 | 765 | 603, 441 |
| 16 | 2-methylbutyryl/isovaleryl-tricaffeoylhexaric acid | 29.7 | 327 | 779 | 617, 455 |
| 17 | 2-methylbutyryl/isovaleryl-tricaffeoylhexaric acid | 30.2 | 327 | 779 | 617, 455 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłeczek, N.; Michalak, B.; Malarz, J.; Kiss, A.K.; Stojakowska, A. Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules 2019, 24, 1614. https://doi.org/10.3390/molecules24081614
Kłeczek N, Michalak B, Malarz J, Kiss AK, Stojakowska A. Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules. 2019; 24(8):1614. https://doi.org/10.3390/molecules24081614
Chicago/Turabian StyleKłeczek, Natalia, Barbara Michalak, Janusz Malarz, Anna Karolina Kiss, and Anna Stojakowska. 2019. "Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant" Molecules 24, no. 8: 1614. https://doi.org/10.3390/molecules24081614
APA StyleKłeczek, N., Michalak, B., Malarz, J., Kiss, A. K., & Stojakowska, A. (2019). Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules, 24(8), 1614. https://doi.org/10.3390/molecules24081614