Cytotoxic Withanolides from the Whole Herb of Physalis angulata L.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Compounds 1–2
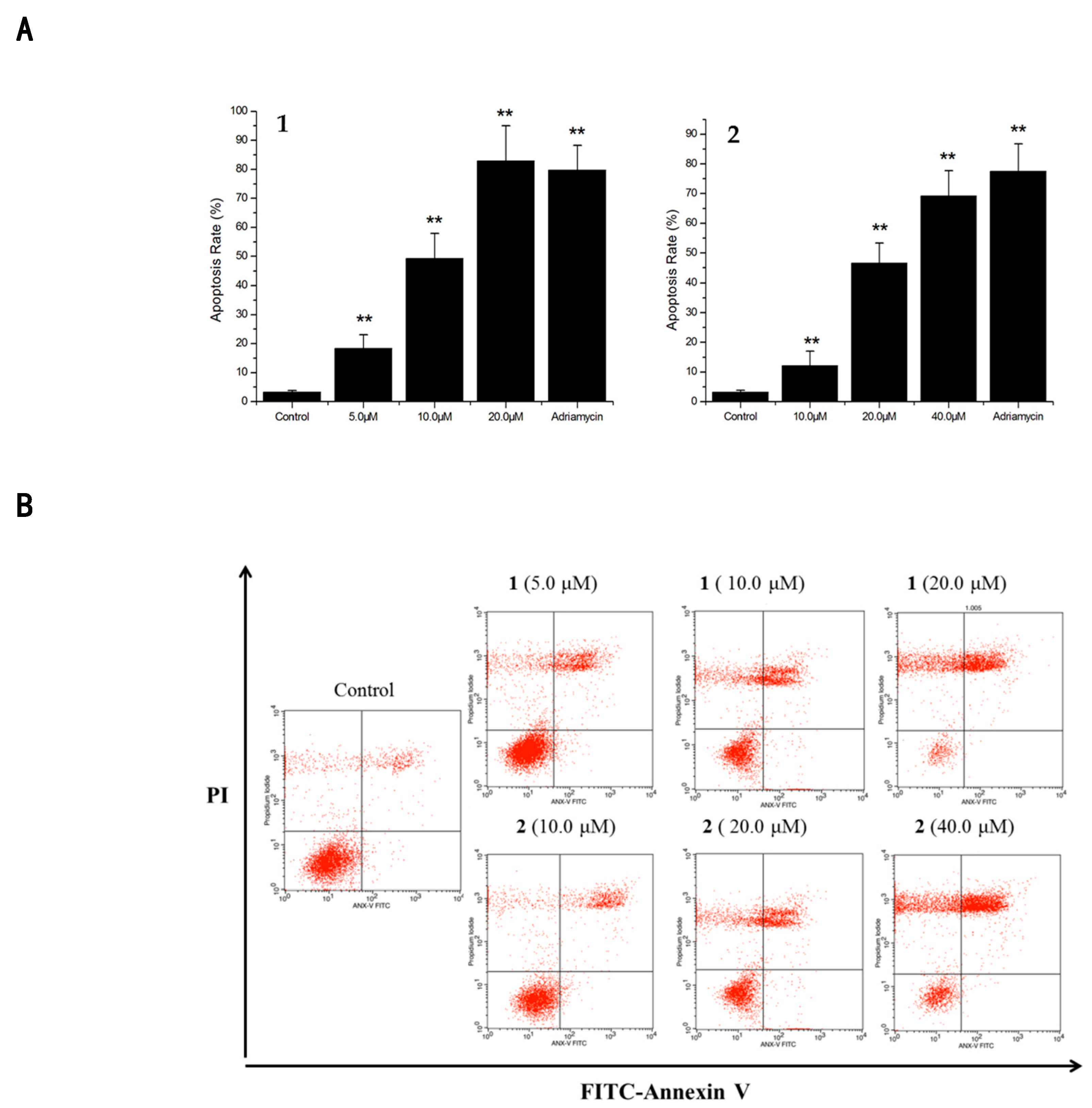
2.2. Cytotoxicities and Cell Apoptosis
2.3. Identification of Physalis Angulata L. by DNA Barcoding Techniques
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Extraction and Isolation
3.3. Characterization of New Compounds 1 and 2
3.4. ECD Calculations
3.5. Cell Culture
3.6. MTT Assay for Cytotoxicities
3.7. Cell Apoptosis Assay
3.8. Plant Material and Identification by DNA Barcoding Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.N.; Tong, W.Y. Chemical Constituents and Biological Activities of Plants from the Genus Physalis. Chem. Biodiver. 2016, 13, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Chen, M.G.; Hu, L.; Zhu, D.N. Chemical Constituents of Whole Plant of Physalis angulata L. Chin. Pharm. J. 2013, 48, 1715–1718. [Google Scholar]
- Glotter, E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991, 8, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhao, F.; Jiang, Z.H.; Chen, L.X.; Zhao, Q.; Liu, H.X.; Yao, X.S.; Qiu, F. Steroids and flavonoids from Physalis alkekengi var. franchetii and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2008, 71, 642–646. [Google Scholar] [CrossRef]
- Sun, C.P.; Qiu, C.Y.; Yuan, T.; Nie, X.F.; Sun, H.X.; Zhang, Q.; Li, H.X.; Ding, L.Q.; Zhao, F.; Chen, L.X.; et al. Antiproliferative and anti-inflammatory withanolides from Physalis angulata. J. Nat. Prod. 2016, 79, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Xia, G.Y.; He, H.; Huang, J.; Qiu, F.; Zi, X.L. New withanolides with TRAIL-sensitizing effect from Physalis pubescens L. RSC Adv. 2016, 6, 52925–52936. [Google Scholar] [CrossRef]
- Damu, A.G.; Kuo, P.C.; Su, C.R.; Kuo, T.H.; Chen, T.H.; Bastow, K.F.; Lee, K.H.; Wu, T.S. Isolation, structures and structure-cytotoxic activity relationships of withanolides and physalins from Physalis angulata. J. Nat. Prod. 2007, 70, 1146–1152. [Google Scholar] [CrossRef]
- Guan, Y.Z.; Shan, S.M.; Zhang, W.; Luo, J.G.; Kong, L.Y. Withanolides from Physalis minima and their inhibitory effects on nitric oxide production. Steroids 2014, 82, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Glotter, E.; Sahai, M.; Kirson, I.; Gottlieb, H.E. Physapubenolide and pubescenin, two new ergostane-type steroids from Physalis pubescens L. J. Chem. Soc. Perkin Trans. 1 1985, 1, 2241–2245. [Google Scholar] [CrossRef]
- Shingu, K.; Yahara, S.; Okabe, H.; Nohara, T. Three new withanolides, Physagulins E, F and G from Physalis angulata L. Chem. Pharm.Bull. 1992, 40, 2448–2452. [Google Scholar] [CrossRef]
- Nagafuji, S.; Okabe, H.; Akahane, H.; Abe, F. Trypanocidal constituents in plants 4. Withanolides from the aerial parts of Physalis angulata. Biol. Pharm. Bull. 2004, 27, 193–197. [Google Scholar] [CrossRef]
- He, H.; Zang, L.H.; Feng, Y.S.; Chen, L.X.; Kang, N.; Tashiro, S.; Onodera, S.; Ikejima, T. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation and autophagy plays a protective role against apoptosis through p38-NF-κB survival pathway in A375-S2 cells. J. Ethnopharmacol. 2013, 148, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Janua´rio, A.H.; Filho, E.R.; Pietro, R.C.; Kashima, S.; Sato, D.N.; Franca, S.C. Antimycobacterial physalins from Physalis angulata L. (Solanaceae). Phytother. Res. 2002, 16, 445–448. [Google Scholar] [CrossRef]
- Arai, M.A.; Uchida, K.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Physalin H from Solanum nigrum as an Hh signaling inhibitor blocks GLI1–DNA-complex formation. Beilstein J. Org.Chem. 2014, 10, 134–140. [Google Scholar] [CrossRef]
- Kawai, M.; Makino, B.; Yamamura, H.; Butsugan, Y. Upon “Physalin L” isolated from Physalis minima. Phytochemistry 1996, 43, 661–663. [Google Scholar] [CrossRef]
- Lin, R.; Guan, Y.Z.; Li, R.J.; Xu, X.M.; Luo, J.G.; Kong, L.Y. 13,14-seco-Withanolides from Physalis minima with Potential Anti-inflammatory Activity. Chem. Biodiver. 2016, 13, 884–890. [Google Scholar] [CrossRef]
- Cordero, C.P.; Morantes, S.J.; Paez, A.; Rincon, J.; Aristizabal, F.A. Cytotoxicity of withanolides isolated from Acnistus arborescens. Fitoterapia 2009, 80, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, R.; Zhou, M.; Yang, Y.; Kong, L.; Luo, J. Cytotoxic withanolides from Physalis angulata. Nat. Prod. Res. 2018, 32, 676–681. [Google Scholar] [CrossRef]
- Perez-Castorena, A.L.; Oropeza, R.F.; Vazquez, A.R.; Martinez, M.; Maldonado, E. Labdanes and withanolides from Physalis coztomatl. J. Nat. Prod. 2006, 69, 1029–1033. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. C 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cao, C.; Zhu, L.; Chen, Y.; Wang, C.H.; ShenTu, J.Z.; Zheng, Y.L. Physalin B induces G2/M cell cycle arrest and apoptosis in A549 human non-small-cell lung cancer cells by altering mitochondrial function. Anticancer Drugs 2019, 30, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Yao, H.; Han, J.P.; Liu, C.; Song, J.Y.; Shi, L.C.; Zhu, Y.J.; Gao, T.; Pang, X.H.; Luo, K.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

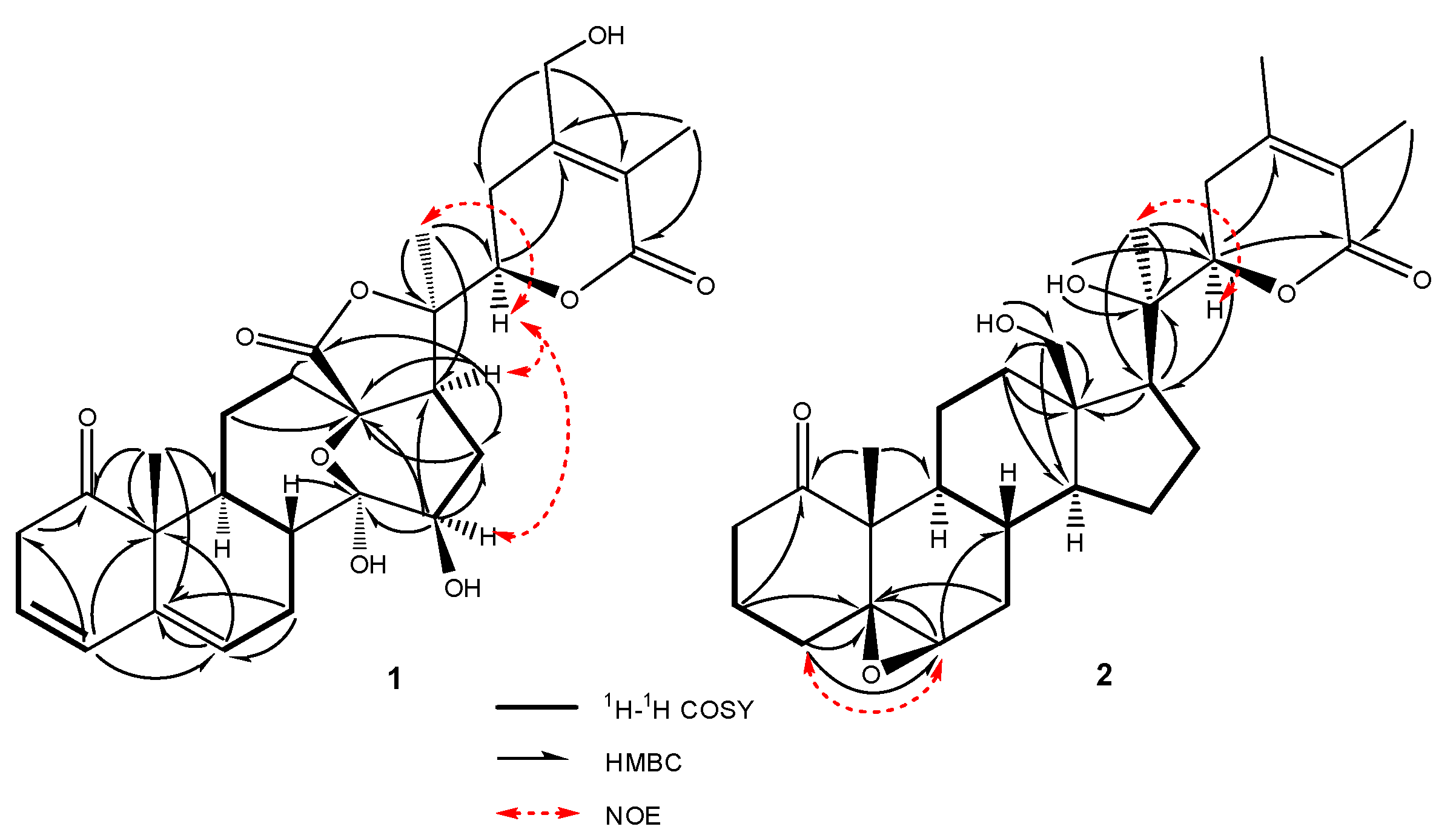

| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J, Hz) | δC (ppm) | δH (J, Hz) | δC (ppm) | |
| 1 | 210.2 | 212.5 | ||
| 2 | 3.40 (d, 20.5) 2.62 (dd, 20.5, 4.0) | 40.4 | 2.22 (m) 2.71 (dt, 14.2, 8.3) | 34.5 |
| 3 | 5.65 (dt, 9.4,4.0) | 122.0 | 1.80 (m) | 17.6 |
| 4 | 6.08 (d, 9.4) | 128.3 | 1.15 (m), 1.90 (m) | 29.5 |
| 5 | 139.3 | 63.76 | ||
| 6 | 5.69(dd, 5.2, 2.2) | 127.3 | 3.15 (br s) | 59.40 |
| 7 | 2.27(m), 2.02(m) | 26.4 | 1.33 (m), 2.00 (dd, 11.0, 2.0) | 31.32 |
| 8 | 1.81(m) | 44.5 | 1.31(m) | 28.57 |
| 9 | 2.04(m) | 32.9 | 1.16 (m) | 41.94 |
| 10 | 52.1 | 51.63 | ||
| 11 | 2.48 (m), 1.77 (m) | 20.1 | 1.58 (m), 1.78 (m) | 20.96 |
| 12 | 2.38 (m), 1.83 (m) | 38.2 | 0.91 (td, 13.0, 3.5), 2.31(m) | 33.43 |
| 13 | 81.0 | 46.66 | ||
| 14 | 98.6 | 1.09 (m) | 54.50 | |
| 15 | 4.19 (dd, 7.7, 4.6) | 74.3 | 1.08 (m), 1.58 (m) | 23.27 |
| 16 | 1.85 (m), 1.41 (m) | 24.0 | 1.58 (m), 1.80 (m) | 21.00 |
| 17 | 2.46 (m) | 57.7 | 1.43(t, 9.2) | 55.22 |
| 18 | 175.3 | 3.32 (m), 3.43 (dd, 11.8, 6.6) | 56.96 | |
| 19 | 1. 31 (s) 0 | 20.5 | 1.02 (s) | 12.84 |
| 20 | 84.7 | 73.82 | ||
| 21 | 1.46 (s) | 25.5 | 1.24 (s) | 20.83 |
| 22 | 4.66 (dd, 12.6, 3.4) | 76.5 | 4.10 (dd, 13.2, 3.5) | 80.21 |
| 23 | 2.64 (m), 2.40 (m) | 26.0 | 2.22 (m), 2.33 (m) | 30.98 |
| 24 | 152.6 | 149.99 | ||
| 25 | 119.4 | 120.18 | ||
| 26 | 164.6 | 165.76 | ||
| 27 | 1.73 (s) | 11.5 | 1.75 (s) | 12.15 |
| 28 | 4.21 (d, 14.8) 4.16 (d, 14.8) | 60.0 | 1.91 (s) | 20.06 |
| OH-18 | 5.12 (dd, 6.6, 4.7) | |||
| OH-20 | 5.36 (s) | |||
| Cell Lines | IC50 (μM) Values of Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Adriamycin | |
| P388 | 8.03 ± 0.02 | 17.60 ± 0.11 | >30 | 19.21 | 7.85 ± 0.13 | 12.50 ± 0.22 | 5.50 ± 0.12 | 13.30 ± 0.77 | 3.20 ± 0.01 |
| Hela | 21.75 ± 0.23 | >30 | >30 | >30 | 3.55 ± 0.15 | 8.50 ± 0.16 | 2.82 ± 0.03 | 10.51 ± 0.51 | 1.50 ± 0.02 |
| A549 | 11.36 ± 0.26 | 23.51 ± 0.17 | 17.73 ± 0.39 | 22.20 ± 0.75 | 2.10 ± 0.09 | 15.98 ± 0.13 | 1.91 ± 0.07 | 7.62 ± 0.02 | 2.30 ± 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Fan, J.; Liu, Z.; Li, X.; Zhang, F.; Zhang, Y.; Sun, Y.; Li, L.; Liu, X.; Hua, E. Cytotoxic Withanolides from the Whole Herb of Physalis angulata L. Molecules 2019, 24, 1608. https://doi.org/10.3390/molecules24081608
Meng Q, Fan J, Liu Z, Li X, Zhang F, Zhang Y, Sun Y, Li L, Liu X, Hua E. Cytotoxic Withanolides from the Whole Herb of Physalis angulata L. Molecules. 2019; 24(8):1608. https://doi.org/10.3390/molecules24081608
Chicago/Turabian StyleMeng, Qinghong, Jiajia Fan, Zhiguo Liu, Xiwen Li, Fangbo Zhang, Yanlin Zhang, Yi Sun, Li Li, Xia Liu, and Erbing Hua. 2019. "Cytotoxic Withanolides from the Whole Herb of Physalis angulata L." Molecules 24, no. 8: 1608. https://doi.org/10.3390/molecules24081608
APA StyleMeng, Q., Fan, J., Liu, Z., Li, X., Zhang, F., Zhang, Y., Sun, Y., Li, L., Liu, X., & Hua, E. (2019). Cytotoxic Withanolides from the Whole Herb of Physalis angulata L. Molecules, 24(8), 1608. https://doi.org/10.3390/molecules24081608








