Labdane and Abietane Diterpenoids from Juniperus oblonga and Their Cytotoxic Activity
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. X-ray Diffraction Analyses
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Single Crystal X-ray Diffraction Analysis
3.5.1. Crystallographic Data for 3
3.5.2. Crystallographic Data for 6
3.6. Cytotoxicity Assay
3.7. Calculations of the CD Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, R.P. The leaf essential oils and chemotaxonomy of Juniperus sect. Juniperus. Biochem. Syst. Ecol. 1998, 26, 637–645. [Google Scholar] [CrossRef]
- Emami, S.A.; Asili, J.; Mohagheghi, Z.; Hassanzadeh, M.K. Antioxidant activity of leaves and fruits of Iranian conifers. Evid.-Based Complement. Altern. 2007, 4, 313–319. [Google Scholar] [CrossRef] [PubMed]
- San, F.A.; Gordaliza, M.; Salinero, M.A.; Jm, M.D.C. Abietane acids: Sources, biological activities, and therapeutic uses. Planta Med. 1993, 59, 485–490. [Google Scholar]
- Agrawal, O.P.; Bharadwaj, S.; Mathur, R. Antifertility effects of fruits of Juniperus communis. Planta Med. 1980, 40, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Li, E.W.; Jia, Z.J. Terpenes from Juniperus przewalskii and their antitumor activities. Pharmazie 2002, 57, 343–345. [Google Scholar] [PubMed]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazević, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L. Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar]
- Akdogan, M.; Koyu, A.; Ciris, M.; Yildiz, K. Anti-hypercholesterolemic activity of J. communis Oil in rats: A biochemical and histopathological investigation. Biomed. Res. 2012, 23, 321–328. [Google Scholar]
- Kagawa, K.; Tokura, K.; Uchida, K.; Kakushi, H.; Shike, T.; Kikuchi, J.; Nakai, H.; Dorji, P.; Subedi, L. Platelet aggregation inhibitors in a Bhutanese medicinal plant, shug chher. Chem. Pharm. Bull. 1993, 41, 1604–1607. [Google Scholar] [CrossRef][Green Version]
- Asili, J.; Emami, S.A.; Rahimizadeh, M.; Fazly-Bazzaz, B.S.; Hassanzadeh, M.K. Chemical and Antimicrobial Studies of Juniperus communis subsp. hemisphaerica and Juniperus oblonga Essential Oils. J. Essent. Oil Bear. Plants 2008, 11, 96–105. [Google Scholar] [CrossRef]
- Emami, S.A.; Asgary, S.; Naderi, G.A.; Ardekani, M.R.S.; Aslani, S.; Airin, A.; Kasher, T.; Sahebkar, A. Investigation of antioxidant and anti-glycation properties of essential oils from fruits and branchlets of Juniperus oblonga. Rev. Bras. Farmacogn. 2012, 22, 985–993. [Google Scholar] [CrossRef]
- Zhang, F.G.; Ren-Jie, H.U.; Zhang, S.Y.; Zhang, Y.W.; Gao, W.Y.; Duan, H.Q. Anticancer activity of diterpenes from Veronica sibirica in vitro. Chin. Tradit. Herbal Drugs 2005, 39, 967–970. [Google Scholar]
- Lin, F.M.; Tsai, C.H.; Yang, Y.C.; Tu, W.C.; Chen, L.R.; Liang, Y.S.; Wang, S.Y.; Shyur, L.F.; Chien, S.C.; Cha, T.L. A novel diterpene suppresses CWR22Rv1 tumor growth in vivo through antiproliferation and proapoptosis. Cancer Res. 2008, 68, 6634–6642. [Google Scholar] [CrossRef]
- Konoshima, T.; Konishi, T.; Takasaki, M.; Yamazoe, K.; Tokuda, H. Anti-tumor-promoting activity of the diterpene from Excoecaria agallocha. II. Biol. Pharm. Bull. 2001, 24, 1440–1442. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Toma, W.; Gracioso, J.S.; de Almeida, A.B.A.; Batista, L.M.; Magri, L.; de Paula, A.C.B.; Soares, F.R.; Nunes, D.S.; Bruto, A.R.M.S. Natural trans-crotonin: The antiulcerogenic effect of another diterpene isolated from the bark of Croton cajucara Benth. Biol. Pharm. Bull. 2002, 25, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Correia, C.A.B.; Vasconcelos dos Santos, M.; David, J.M.; David, J.P.; Duarte, G.P.; Magalhaes, P.J.C. Mechanisms underlying the cardiovascular effects of a labdenic diterpene isolated from Moldenhawera nutans in normotensive rats. Vascul. Pharmacol. 2007, 46, 60–66. [Google Scholar] [CrossRef]
- Singh, M.; Pal, M.; Sharma, R.P. Biological activity of the labdane diterpenes. Planta Med. 1999, 65, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C.; Dimas, K.; Hatziantoniou, S.; Anastasaki, T.; Angelopoulou, D. Cytotoxic and anti-inflammatory activity of labdane and cis-clerodane type diterpenes. Planta Med. 2001, 67, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Rajouani, N.; Ait Itto, M.Y.; Benharref, A.; Auhmani, A.; Daran, J.C. 6-Hydroxy-7-isopropyl-1,1,4a-trimethyl-2,3,4,4a,10,10a-hexahydrophenanthren-9(1H)-one. Acta Crystallogr. E 2008, 64, 762. [Google Scholar] [CrossRef]
- Wenchiung, S.U.; Fang, J.; Cheng, Y. Abietanes and kauranes from leaves of Cryptomeria japonica. Phytochemistry 1994, 35, 1279–1284. [Google Scholar]
- Kobayashi, M.; Ishida, K.; Terabayashi, S.; Mitsuhashi, H. 10-Hydroxypheophytins and norlabdane diterpene from the leaves of Cupressus funebris Endl. Chem. Pharm. Bull. 1991, 39, 3348–3349. [Google Scholar] [CrossRef]
- Mirzaei, H.H.; Firuzi, O.; Schneider, B.; Baldwin, I.T.; Jassbi, A.R. Cytotoxic diterpenoids from the roots of Salvia lachnocalyx. Rev. Bras. Farmacogn. 2017, 27, 475–479. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Wu, T.R.; Cheng, M.C.; Wang, Y. Five new compounds from the heartwood of Juniperus formosana Hayata. Chem. Pharm. Bull. 1990, 38, 3195–3201. [Google Scholar] [CrossRef][Green Version]
- Kuo, Y.H.; Lin, N.H.; Lin, Y.T. Two New Diterpens Phenols-7α-Methoxydeoxocryptojaponol and 7β-Hydroxydeoxocryptojaponol. J. Chin. Chem. Soc. 2013, 27, 19–22. [Google Scholar] [CrossRef]
- Mangoni, L.; Caputo, R. Sempervirol, a novel type of diterpene phenol. Tetrahedron Lett. 1967, 8, 673–675. [Google Scholar] [CrossRef]
- Ulubelen, A. New Diterpenoids from the Roots of Salvia triloba. Planta Med. 1990, 56, 82–83. [Google Scholar] [CrossRef]
- Tanaka, R.; Ohtsu, H.; Matsunaga, S. Abietane diterpene acids and other constituents from the leaves of Larix kaempferi. Phytochemistry 1997, 46, 1051–1057. [Google Scholar] [CrossRef]
- Harmatha, J.; Budesinsky, M.; Trka, A. The structure of yatein, determination of the positions, and configurations of benzyl groups in lignans of the 2,3-dibenzylbutyrolactone type. Coll. Czech. Chem. Commun. 1982, 47, 644–663. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, H.H.; Piao, X.L.; Kim, J.S.; Kang, S.S.; Shin, K.H. Anti-estrogenic activity of lignans from Acanthopanax chiisanensis root. Arch. Pharm. Res. 2005, 28, 186–189. [Google Scholar] [CrossRef]
- Sankar, S.S.; Gilbert, R.D.; Fornes, R.E. 13C-NMR studies of some hydroxycoumarins and related compounds. Magn. Reson. Chem. 1982, 19, 222–224. [Google Scholar] [CrossRef]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 9, 457–463. [Google Scholar] [CrossRef]
- Echeverría, J.; Gonzálezteuber, M.; Urzúa, A. Antifungal activity against Botrytis cinerea of labdane-type diterpenoids isolated from the resinous exudate of Haplopappus velutinus Remy (Asteraceae). Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chinou, I.; Demetzos, C.; Harvala, C.; Verbist, J. Cytotoxic and antibacterial labdane-type diterpenes from the aerial parts of Cistus incanus subsp. creticus. Planta Med. 1994, 60, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Dellar, J.E.; Cole, M.D.; Waterman, P.G. Antimicrobial abietane diterpenoids from Plectranthus elegans. Phytochemistry 1996, 41, 735–738. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
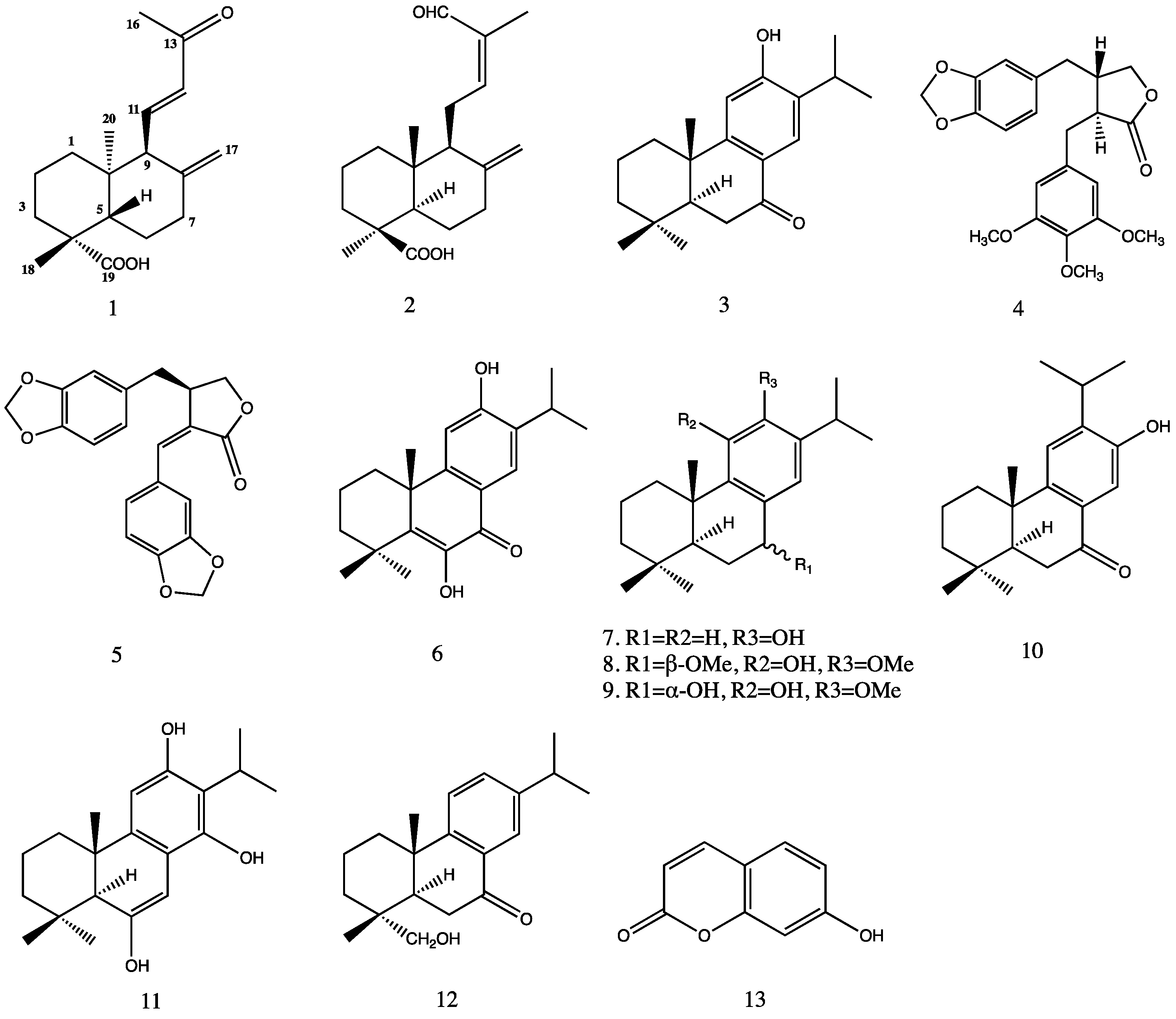
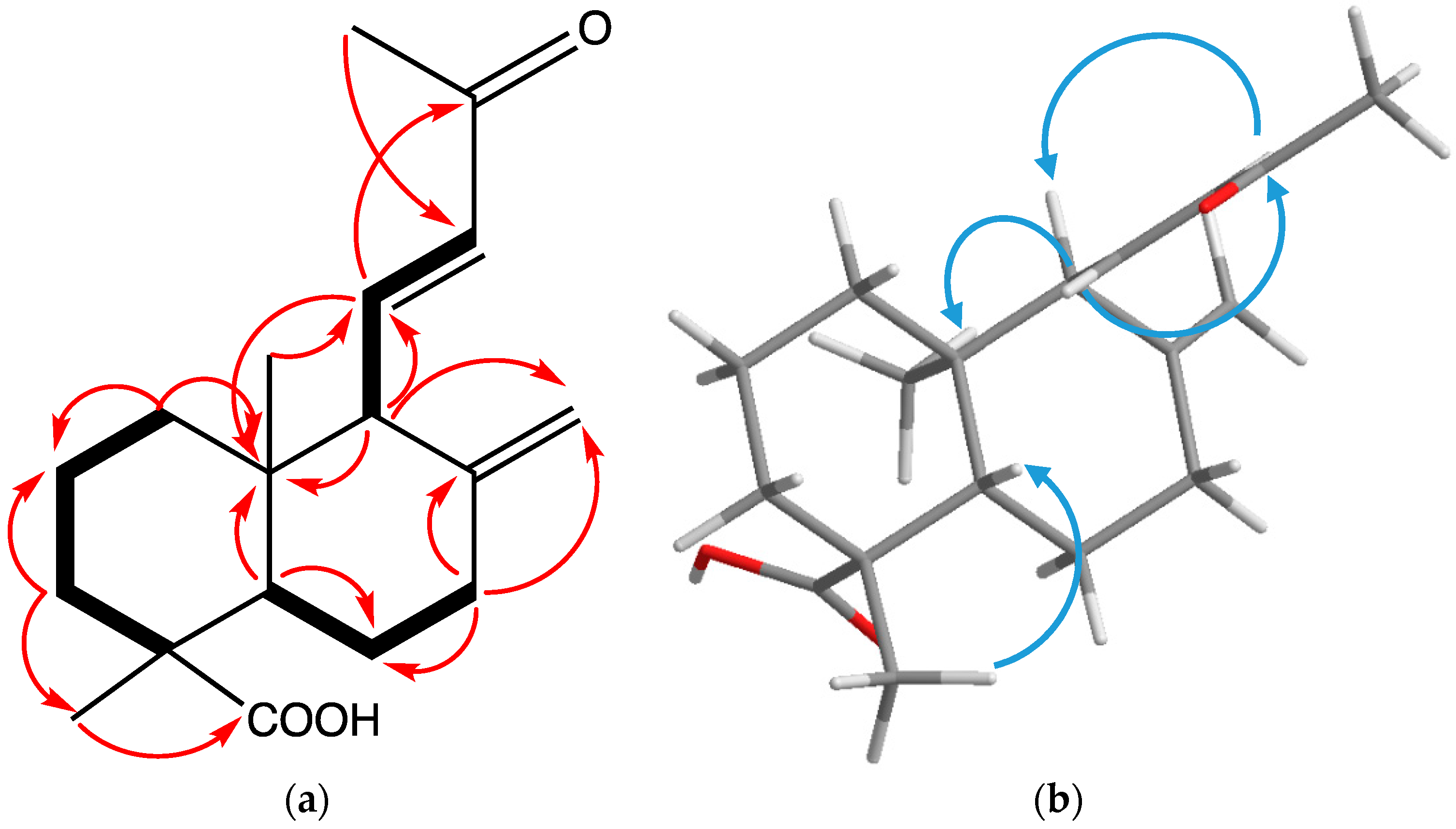
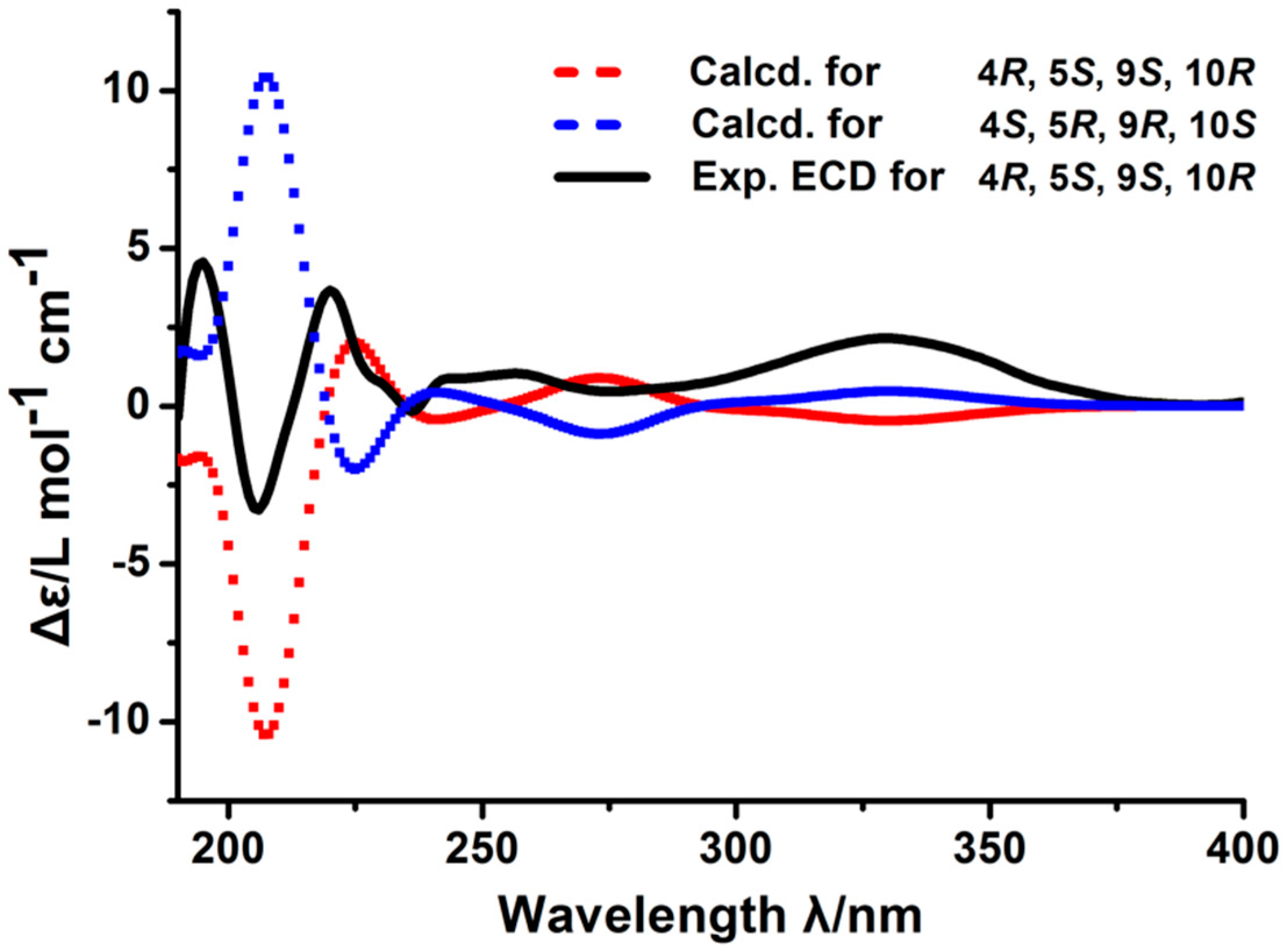

| Position | δC | δH, J (Hz) | 1H-1H-COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 42.2 | 1.42 m, 1.14 ddd 4.0, 13.0 | H-2 | C-2, C-10, C-20 | |
| 2 | 20.9 | 1.88 td 3.0,13.0, 1.43 m | H-1, H-3 | C-3, C-5 | |
| 3 | 39.3 | 2.14m, 1.08 ddd 3.4, 13.0 | H-2 | C-2, C-4, C-5, C-18, C-19 | |
| 4 | 45.1 | ||||
| 5 | 56.4 | 1.44 m | H-6 | C-6, C-10, C-19, C-20 | H-18 |
| 6 | 26.4 | 2.00m, 1.93 ddd 3.8, 13.0 | H-7 | C-5, C-7 | |
| 7 | 38.3 | 2.45 dt 3, 12, 2.06 ddd 5.0,13.0 | H-6 | C-5, C-8, C-6, C-17 | |
| 8 | 149.9 | ||||
| 9 | 61.4 | 2.57 d 10 | H-11 | C-5, C-10, C-11, C-12, C-17, C-20 | H-12 |
| 10 | 41.0 | ||||
| 11 | 148.7 | 6.95 dd 5.0 10.0 | H-9, H-12 | C-8, C-9, C-10, C-13 | H-12, H-14, H-20 |
| 12 | 134.6 | 6.08 d 5.0 | H-11 | C-8, C-9, C-13, C-16 | H-11 |
| 13 | 200.9 | ||||
| 16 | 27.1 | 2.27 s | C-11, C-12, C-13 | H-11, | |
| 17 | 108.9 | 4.80 d 1.5, 4.42 d 1.5 | C-7, C-8, C-9 | ||
| 18 | 29.4 | 1.21 s | C-2, C-3, C-4, C-5, C-19 | H-5 | |
| 19 | 181.2 | ||||
| 20 | 14.2 | 0.83 s | C-5, C-9, C-10 | H-11 |
| Compound | HepG2 | HeLa | MCF-7 | LO2 |
|---|---|---|---|---|
| 1 | 18.39 | 26.36 | 28.45 | 20.57 |
| 2 | 16.06 | 23.95 | 21.08 | 10.61 |
| 3 | 35.05 | 12.29 | 9.2 | 26.62 |
| 5 | 0.11 | 0.22 | 10.09 | 9.05 |
| 6 | 86.57 | 83.83 | 83.99 | 27.76 |
| 8 | 7.84 | 0 | 1.35 | 17.07 |
| 9 | 24.33 | 0 | 10.96 | 22.21 |
| 10 | 48.67 | 63.4 | 43.4 | 19.08 |
| 11 | 67.12 | 22.01 | 29.88 | 23.29 |
| 13 | 32.71 | 3.6 | 17.96 | 26.59 |
| Doxorubicin a | 42.21 | 49.51 | 46.46 | 46.7 |
| Compounds | Cytotoxicity (IC50: μM) a | ||
|---|---|---|---|
| HepG2 | MCF-7 | Hela | |
| 6 | 48.73 ± 1.31 | 58.39 ± 2.45 | 24.41 ± 2.05 |
| 10 | 64.94 ± 2.64 | 79.98 ± 1.20 | 56.93 ± 2.39 |
| Doxorubicin b | 3.18 ± 1.19 | 3.44 ± 1.59 | 3.64 ± 1.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Khutsishvili, M.; Alizade, V.; Atha, D.; Borris, R.P. Labdane and Abietane Diterpenoids from Juniperus oblonga and Their Cytotoxic Activity. Molecules 2019, 24, 1561. https://doi.org/10.3390/molecules24081561
Qiao Y, Khutsishvili M, Alizade V, Atha D, Borris RP. Labdane and Abietane Diterpenoids from Juniperus oblonga and Their Cytotoxic Activity. Molecules. 2019; 24(8):1561. https://doi.org/10.3390/molecules24081561
Chicago/Turabian StyleQiao, Yilin, Manana Khutsishvili, Valida Alizade, Daniel Atha, and Robert P. Borris. 2019. "Labdane and Abietane Diterpenoids from Juniperus oblonga and Their Cytotoxic Activity" Molecules 24, no. 8: 1561. https://doi.org/10.3390/molecules24081561
APA StyleQiao, Y., Khutsishvili, M., Alizade, V., Atha, D., & Borris, R. P. (2019). Labdane and Abietane Diterpenoids from Juniperus oblonga and Their Cytotoxic Activity. Molecules, 24(8), 1561. https://doi.org/10.3390/molecules24081561





