Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt
Abstract
1. Introduction
2. Results
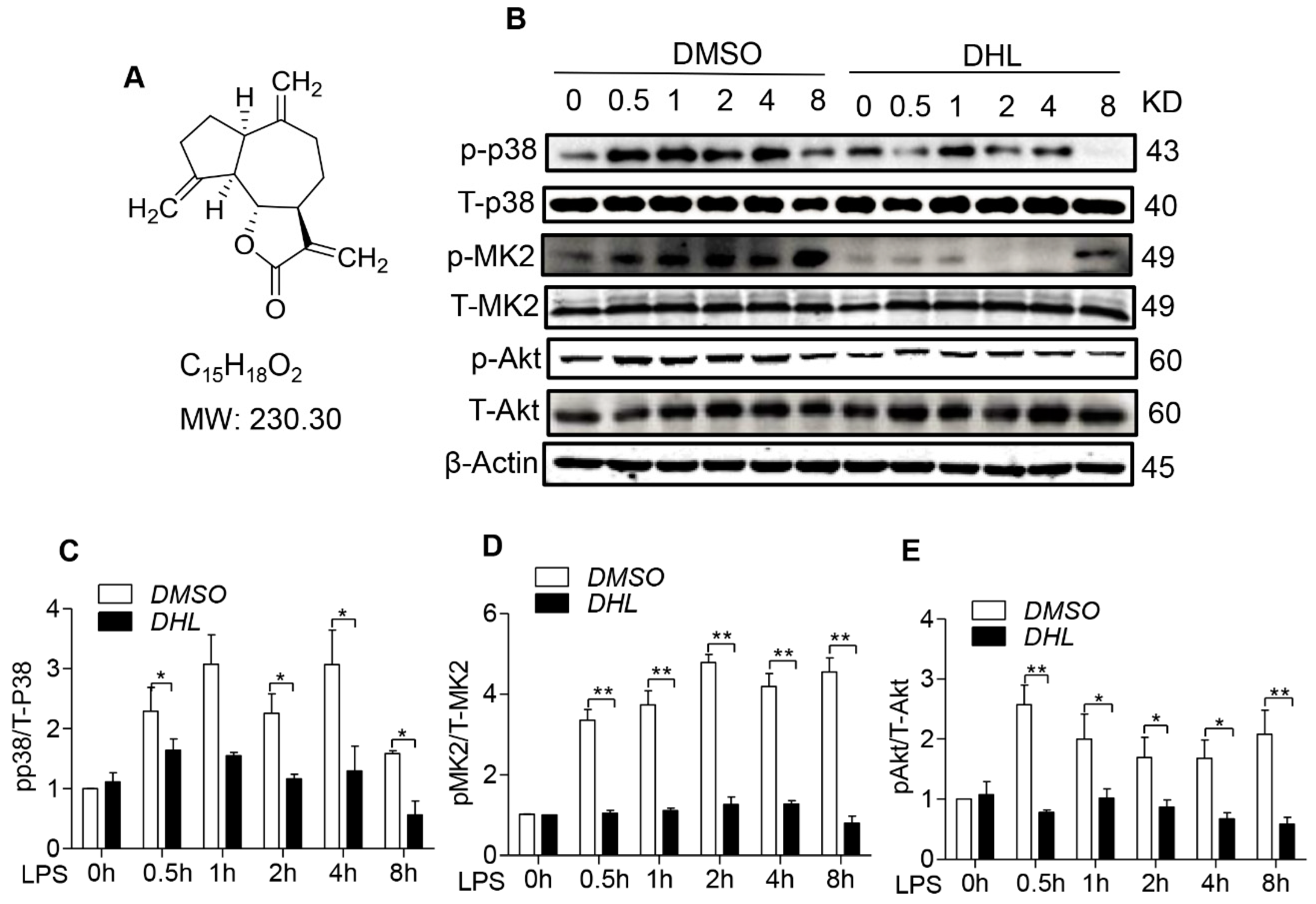
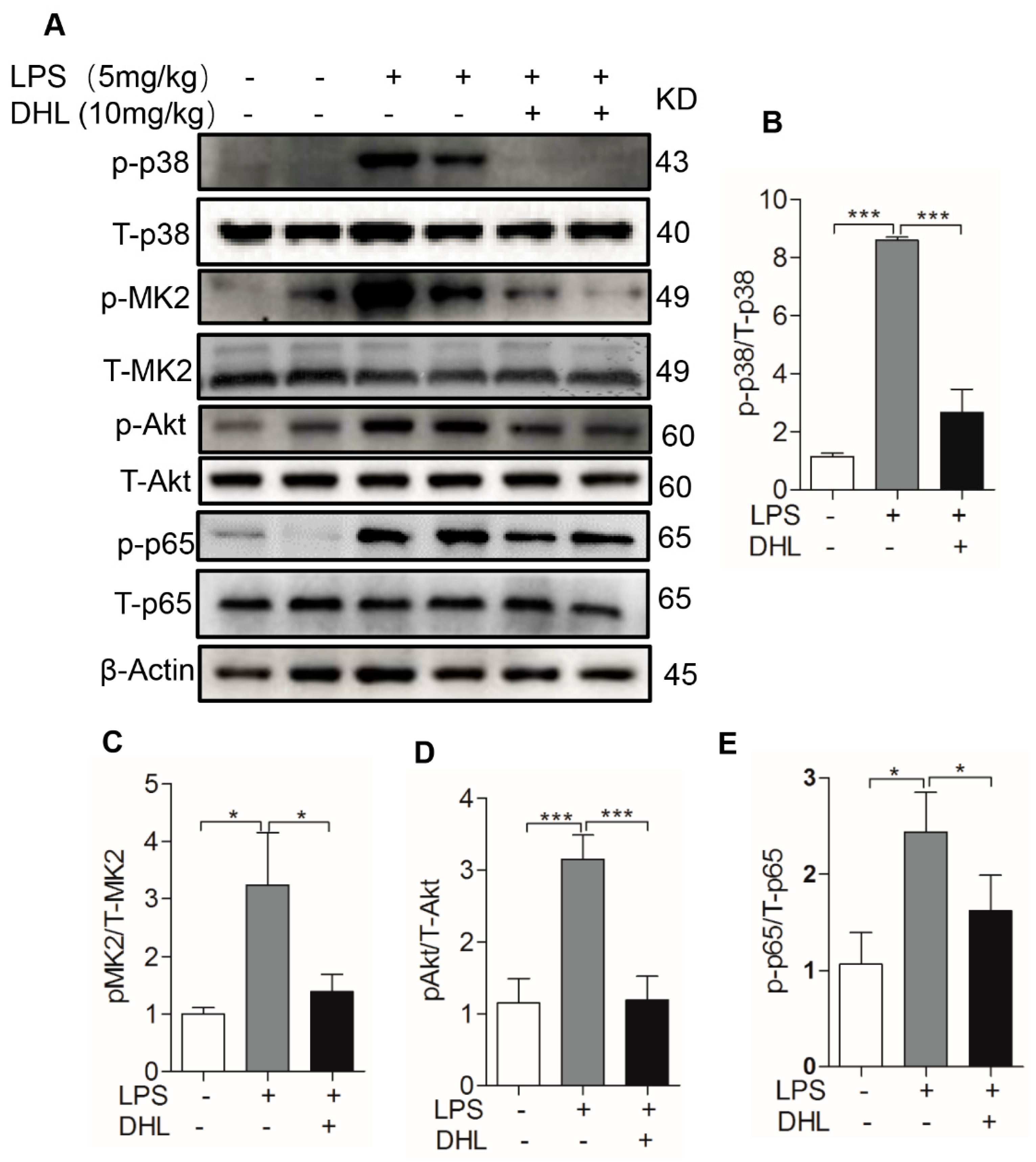
2.1. DHL Suppressed the Activation of p38/MK2 and Akt Signaling Pathway
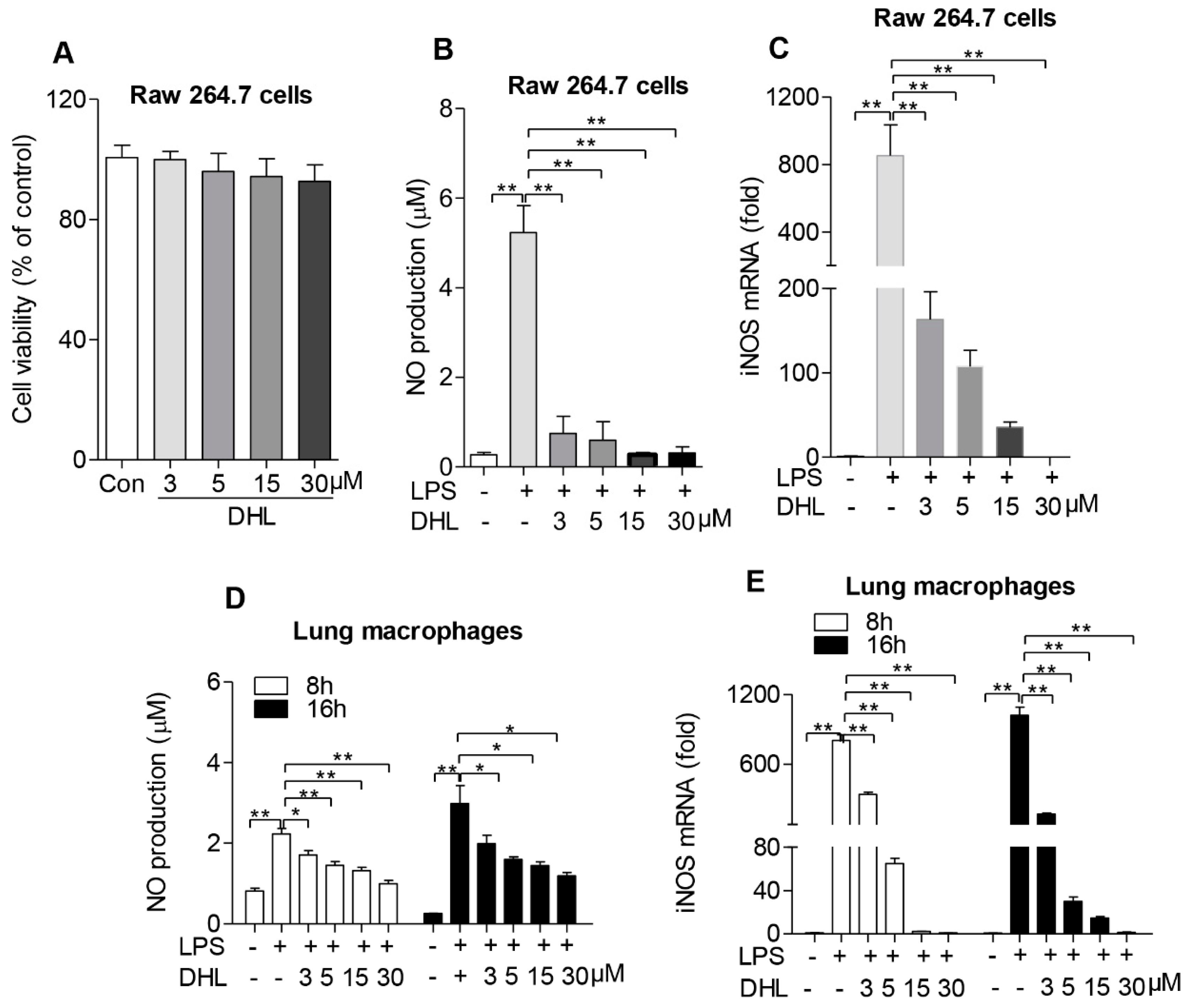
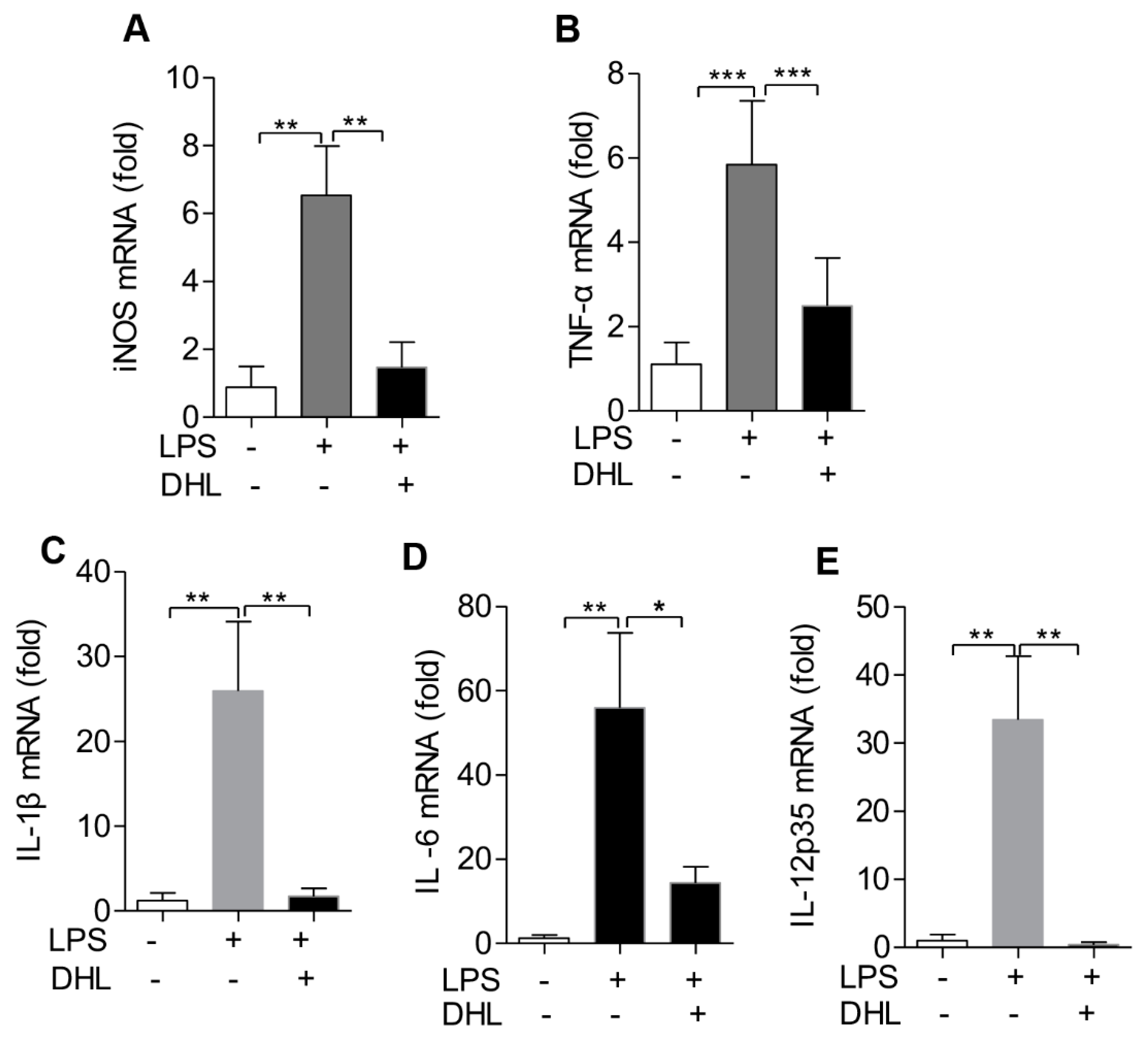
2.2. DHL Reduced the Expression of iNOS and Inhibited NO Production in both Raw264.7 Cells and Lung Macrophages
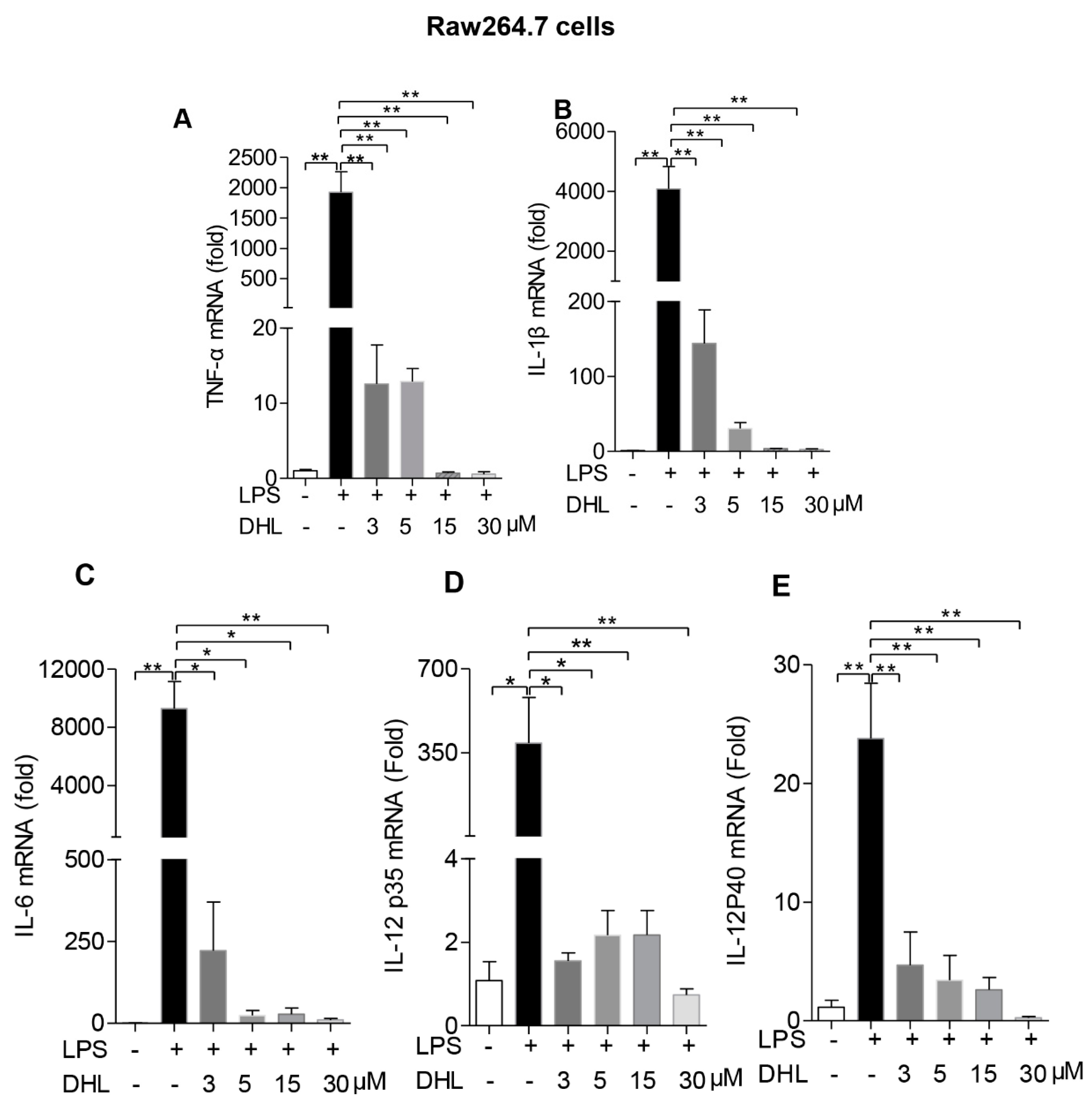
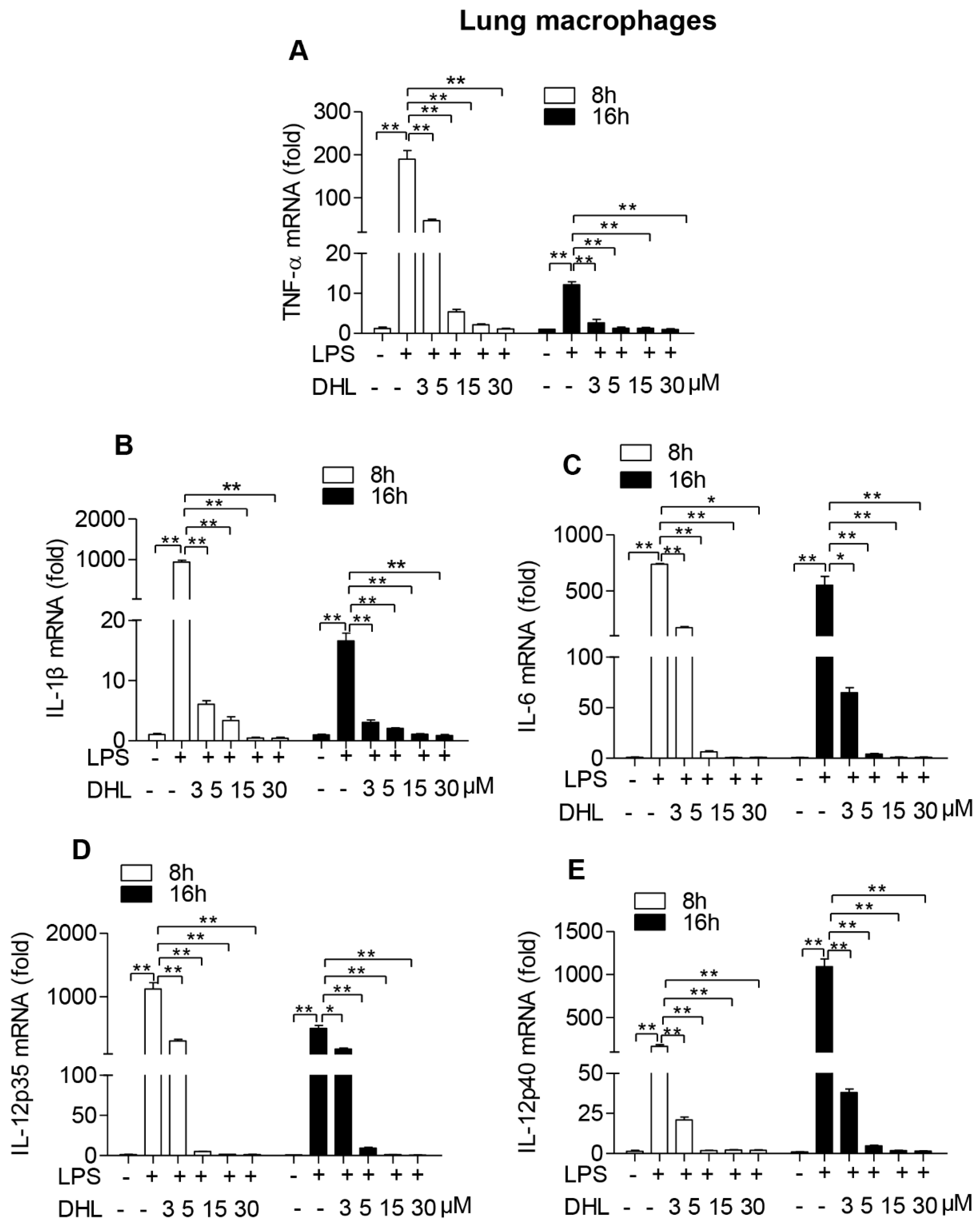
2.3. DHL Inhibited LPS-Induced Proinflammatory Cytokines in both RAW264.7 Cells and Lung Macrophages
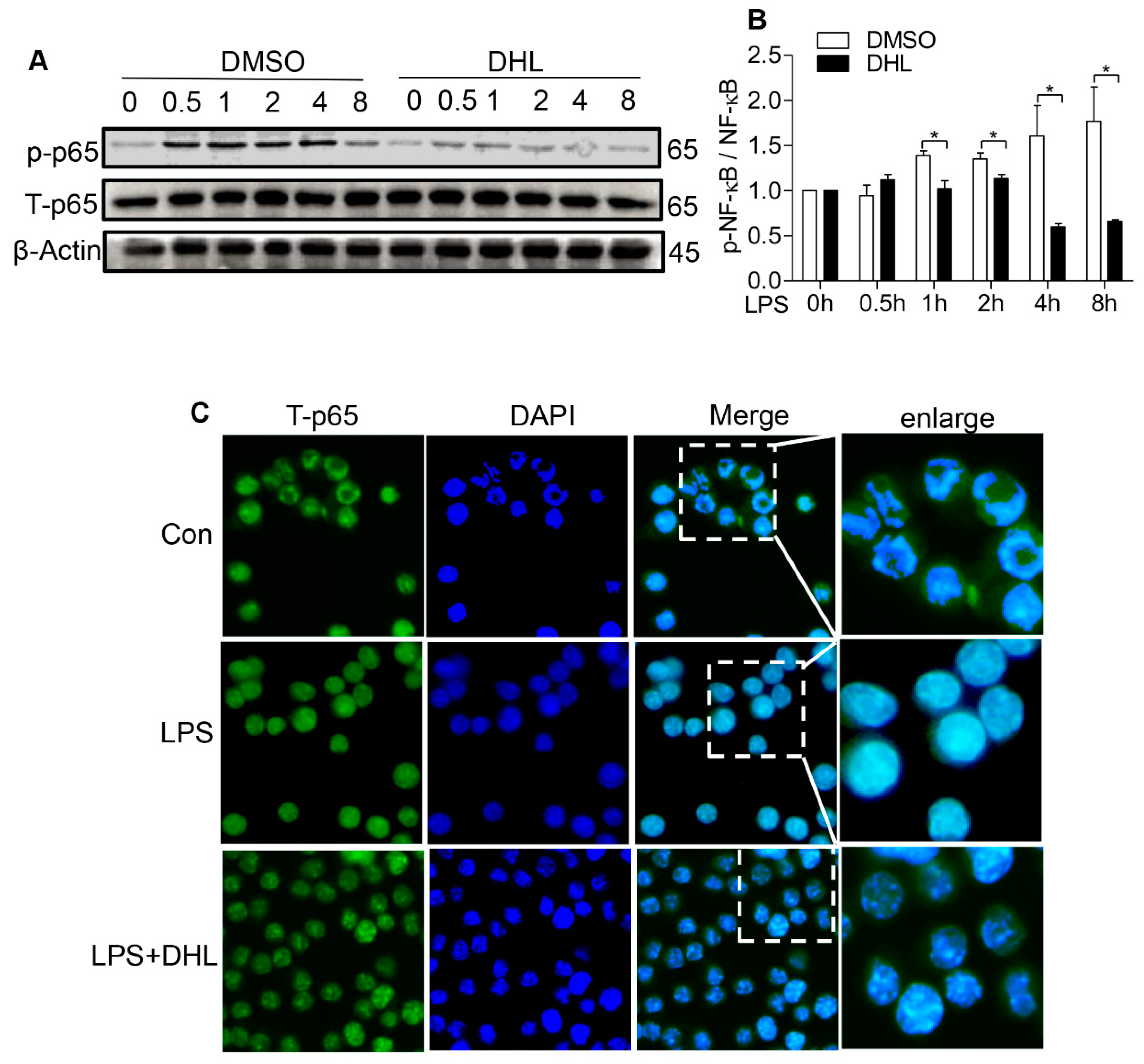
2.4. DHL Suppressed LPS-Induced Phosphorylation and Nuclear Translocation of NF-κB in RAW 264.7 Cells
2.5. DHL Attenuated LPS-Induced Acute Lung Injury
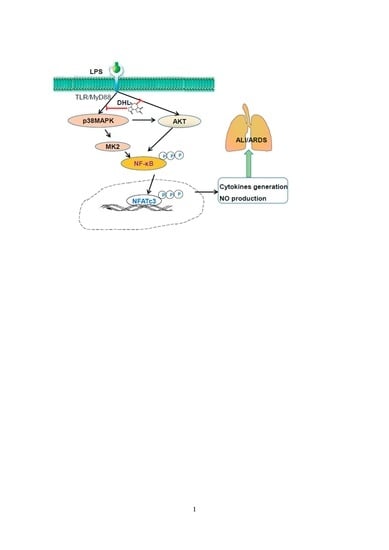
2.6. DHL Reduced the Production of Proinflammatory Cytokines in LPS-induced ALI via p38 MAPK/ Akt/ NF-κB Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Animals
4.3. Cell Culture and Treatment
4.4. Cell Viability Assay
4.5. Determination of NO Production
4.6. RNA Isolation, Reverse Transcription and Quantitative PCR
4.7. Western Blot Analysis
4.8. Immunofluorescence Assay
4.9. ALI Model
4.10. Histopathological Analysis
4.11. BALF Analysis
4.12. MPO Activity Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hayes, M.; Curley, G.; Ansari, B.; Laffey, J.G. Clinical review: Stem cell therapies for acute lung injury/acute respiratory distress syndrome—Hope or hype? Crit. Care 2012, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.R.; Ware, L.B. Biomarkers of ALI/ARDS: Pathogenesis, discovery, and relevance to clinical trials. Sem. Resp. Crit. Care Med. 2013, 34, 537–548. [Google Scholar]
- Wu, Y.X.; He, H.Q.; Nie, Y.J.; Ding, Y.H.; Sun, L.; Qian, F. Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacol. Sin. 2018, 39, 85–96. [Google Scholar] [CrossRef]
- Lee, J.P.; Li, Y.C.; Chen, H.Y.; Lin, R.H.; Huang, S.S.; Chen, H.L.; Kuan, P.; Liao, M.; Chen, C.; Kuan, Y. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol. Sin. 2010, 31, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiu, H.; Zhang, S.; Zhang, G. The Role of Macrophages in the Pathogenesis of ALI/ARDS. Mediat. Inflamm. 2018, 2018, 1264913. [Google Scholar]
- Dong, H.; Li, J.; Lv, Y.; Zhou, Y.; Wang, G.; Hu, S.; He, X.; Yang, P.; Zhou, Z.; Xiang, X.; et al. Comparative analysis of the alveolar macrophage proteome in ALI/ARDS patients between the exudative phase and recovery phase. BMC Immunol. 2013, 14, 25. [Google Scholar] [CrossRef]
- Wu, G.; Xu, G.; Chen, D.W.; Gao, W.X.; Xiong, J.Q.; Shen, H.Y.; Gao, Y.Q. Hypoxia Exacerbates Inflammatory Acute Lung Injury via the Toll-Like Receptor 4 Signaling Pathway. Front. Immunol. 2018, 9, 1667. [Google Scholar] [CrossRef]
- Alber, A.; Howie, S.E.; Wallace, W.A.; Hirani, N. The role of macrophages in healing the wounded lung. Int. J. Exp. Pathol. 2012, 93, 243–251. [Google Scholar] [CrossRef]
- Hansel, T.T.; Barnes, P.J. New drugs for exacerbations of chronic obstructive pulmonary disease. Lancet 2009, 374, 744–755. [Google Scholar] [CrossRef]
- Ding, Y.H.; Song, Y.D.; Wu, Y.X.; He, H.Q.; Yu, T.H.; Hu, Y.D.; Zhang, D.P.; Jiang, H.C.; Yu, K.K.; Li, X.Z.; et al. Isoalantolactone suppresses LPS-induced inflammation by inhibiting TRAF6 ubiquitination and alleviates acute lung injury. Acta Pharmacol. Sin. 2019, 40, 64–74. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.L.; Quan, H.F.; Yan, L.; Pei, X.Y.; Wang, R.; Peng, X.D. Effects of Betaine on LPS-Stimulated Activation of Microglial M1/M2 Phenotypes by Suppressing TLR4/NF-kappaB Pathways in N9 Cells. Molecules 2019, 24, 367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Li, F.; Yin, C.; Shi, J.; Gong, Q. Icariside II attenuates lipopolysaccharide-induced neuroinflammation through inhibiting TLR4/MyD88/NF-kappaB pathway in rats. Biomed. Pharmacother. 2018, 111, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, X.; Sun, W.; Kang, N.; Liu, Y.; Yang, S.; Xu, Q.M.; Wang, C.M.; Chen, X.P. Total tanshinones exhibits anti-inflammatory effects through blocking TLR4 dimerization via the MyD88 pathway. Cell Death Dis. 2017, 8, e3004. [Google Scholar] [CrossRef]
- Menon, M.B.; Gropengiesser, J.; Fischer, J.; Novikova, L.; Deuretzbacher, A.; Lafera, J.; Schimmeck, H.; Czymmeck, N.; Ronkina, N.; Kotlyarov, A. p38(MAPK)/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nat. Cell Biol. 2017, 19, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, G.Y.; Lee, H.H. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-kappaB signaling pathways. Drug Des. Dev. Ther. 2014, 8, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Guo, S.; Yang, C.; Yang, J.; Chen, Y.; Shaukat, A.; Zhao, G.; Wu, H.C.; Deng, G.Z. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-kappaB pathway. Int. Immunopharmacol. 2018, 64, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, W.; Yang, L.; Dong, W.; Chen, W.; Mao, Y.; Xu, P.; Li, D.; Yuan, H.; Li, Y. MiR-135a Protects Vascular Endothelial Cells Against Ventilator-Induced Lung Injury by Inhibiting PHLPP2 to Activate PI3K/Akt Pathway. Cell. Physiol. Biochem. 2018, 48, 1245–1258. [Google Scholar] [CrossRef]
- Luo, X.; Yu, Z.; Deng, C.; Zhang, J.; Ren, G.; Sun, A.; Mani, S.; Wang, Z.; Dou, W. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci. Rep. 2017, 7, 16374. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zeng, M.; Lin, D.; Wang, Y.; Wen, X.; Xu, C.; Yang, L.; Fan, X.; Gong, Y.; et al. Apelin-13 Administration Protects Against LPS-Induced Acute Lung Injury by Inhibiting NF-kappaB Pathway and NLRP3 Inflammasome Activation. Cell. Phys. Biochem. 2018, 49, 1918–1932. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int. J. Mol. Sci. 2015, 16, 10888–10906. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Wang, C.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Ma, J.; Zhang, B.; et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-kappaB/COX-2 signaling pathway by targeting IKKbeta. Am. J. Cancer Res. 2017, 7, 1270–1284. [Google Scholar] [PubMed]
- Li, W.; Ma, Y.B.; Mao, Y.Q.; Lin, T. Dehydrocostus lactone suppresses cell growth and induces apoptosis in recombinant human papilloma virus18 HaCaT cells via the PI3K/Akt signaling pathway. Mol. Med. Rep. 2018, 17, 7925–7930. [Google Scholar] [PubMed]
- Singireesu, S.; Mondal, S.K.; Misra, S.; Yerramsetty, S.; Suresh, B.K. Dehydrocostus lactone induces prominent apoptosis in kidney distal tubular epithelial cells and interstitial fibroblasts along with cell cycle arrest in ovarian epithelial cells. Biomed. Pharmacother. 2018, 99, 956–969. [Google Scholar] [CrossRef]
- Gong, Q.; Li, Y.; Ma, H.; Guo, W.; Kan, X.; Xu, D.; Liu, J.; Fu, S. Peiminine Protects against Lipopolysaccharide-Induced Mastitis by Inhibiting the AKT/NF-kappaB, ERK1/2 and p38 Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 2637. [Google Scholar] [CrossRef]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukocyte Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, H.; Zhou, D.; Xi, H.; Shan, L. Arctigenin attenuates lipopolysaccharide-induced acute lung injury in rats. Inflammation 2015, 38, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wu, H.; Wang, C.; Xiao, Z.; Xu, F. Regulatory T Cells and Acute Lung Injury: Cytokines, Uncontrolled Inflammation, and Therapeutic Implications. Front. Immunol. 2018, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- He, H.Q.; Wu, Y.X.; Nie, Y.J.; Wang, J.; Ge, M.; Qian, F. LYRM03, an ubenimex derivative, attenuates LPS-induced acute lung injury in mice by suppressing the TLR4 signaling pathway. Acta Pharm. Sin. 2017, 38, 342–350. [Google Scholar] [CrossRef]
- Belchamber, K.B.R.; Donnelly, L.E. Macrophage Dysfunction in Respiratory Disease. Cell Differ. 2017, 62, 299–313. [Google Scholar]
- Zhang, L.; Yao, Z.; Ji, G. Herbal Extracts and Natural Products in Alleviating Non-alcoholic Fatty Liver Disease via Activating Autophagy. Front. Pharmacol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Bae, S.; Lee, K.W.; Jeong, H.C.; Park, B.H.; Bae, W.J.; Han, C.H.; Kim, S.W. Effects of a combination of herbal extracts (modified Ojayeonjonghwan (Wuzi Yanzong wan)) on partial urethral obstruction-induced detrusor overactivity in rats: Impact on the nitric oxide pathway and oxidative stress. BMC Complem. Altern. Med. 2019, 19, 64. [Google Scholar] [CrossRef]
- Coricello, A.; Adams, J.D.; Lien, E.J.; Nguyen, C.; Perri, F.; Williams, T.J.; Aiello, F. A Walk in Nature. Sesquiterpene Lactones as Multi-Target Agents Involved in Inflammatory Pathways. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Mao, H.; Wang, C.; Yang, N.; Zhang, Z.; Han, J. Dehydrocostus Lactone Enhances Chemotherapeutic Potential of Doxorubicin in Lung Cancer by Inducing Cell Death and Limiting Metastasis. Med. Sci. Monitor 2018, 24, 7850–7861. [Google Scholar] [CrossRef]
- Zarpelon, A.C.; Fattori, V.; Souto, F.O.; Pinto, L.G.; Pinho-Ribeiro, F.A.; Ruiz-Miyazawa, K.W.; Turato, W.M.; Cunha, T.M.; da Costa, F.B.; Cunha, F.Q.; et al. The Sesquiterpene Lactone, Budlein A, Inhibits Antigen-Induced Arthritis in Mice: Role of NF-kappaB and Cytokines. Inflammation 2017, 40, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Hong, J.E.; Lim, S.S.; Kwon, G.T.; Kim, J.; Kim, J.S.; Lee, K.W.; Park, J.H.Y. The hexane extract of Saussurea lappa and its active principle, dehydrocostus lactone, inhibit prostate cancer cell migration. J. Med. Food 2012, 15, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; El Mohtadi, M.; Slevin, M.; Gilmore, W.; Ashworth, J.J. The Effect of C-Reactive Protein Isoforms on Nitric Oxide Production by U937 Monocytes/Macrophages. Front. Immunol. 2018, 9, 1500. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, B.; Xu, G.; Yang, Z.; Tang, M.; Ma, A.; Jing, T.; Xu, X.L.; Zhang, X.; et al. POH1 deubiquitinates pro-interleukin-1beta and restricts inflammasome activity. Nature Commun. 2018, 9, 4225. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F. Ruthenium pyridyl thiocyanate complex increased the production of pro-inflammatory TNFalpha and IL1beta cytokines by the LPS stimulated mammalian macrophages in vitro. Mol. Biol. Rep. 2018. [Google Scholar] [CrossRef]
- Cronin, J.G.; Kanamarlapudi, V.; Thornton, C.A.; Sheldon, I.M. Signal transducer and activator of transcription-3 licenses Toll-like receptor 4-dependent interleukin (IL)-6 and IL-8 production via IL-6 receptor-positive feedback in endometrial cells. Mucosal Immunol. 2016, 9, 1125–1136. [Google Scholar] [CrossRef]
- Poroyko, V.; Meng, F.; Meliton, A.; Afonyushkin, T.; Ulanov, A.; Semenyuk, E.; Latif, O.; Tesic, V.; Birukova, A.A.; Birukov, K.G. Alterations of lung microbiota in a mouse model of LPS-induced lung injury. Am. J. Physiol. 2015, 309, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Lu, Q.; Wang, K.; Lu, J.; Gu, X.; Zhu, D. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J. Cell. Physiol. 2018, 233, 6615–6631. [Google Scholar] [CrossRef]
- Wyns, H.; Plessers, E.; De Backer, P.; Meyer, E.; Croubels, S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet. Immunol. Immunop. 2015, 166, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.; Gonzalez-Rivera, B.L.; Redus, L.; Parrott, J.M.; O’Connor, J.C. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm. Behav. 2012, 62, 202–209. [Google Scholar] [CrossRef]
- Garcia-Pineres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001, 276, 39713–39720. [Google Scholar] [CrossRef]
- Dang, Y.; Mu, Y.; Wang, K.; Xu, K.; Yang, J.; Zhu, Y. Papaverine inhibits lipopolysaccharide-induced microglial activation by suppressing NF-kappaB signaling pathway. Drug Des. Dev. Ther. 2016, 10, 851–859. [Google Scholar] [CrossRef]
- Ehlting, C.; Ronkina, N.; Bohmer, O.; Albrecht, U.; Bode, K.A.; Lang, K.S. Distinct functions of the mitogen-activated protein kinase-activated protein (MAPKAP) kinases MK2 and MK3: MK2 mediates lipopolysaccharide-induced signal transducers and activators of transcription 3 (STAT3) activation by preventing negative regulatory effects of MK3. J. Biol. Chem. 2011, 286, 24113–24124. [Google Scholar] [PubMed]
- Gorska, M.M.; Liang, Q.; Stafford, S.J.; Goplen, N.; Dharajiya, N.; Guo, L. MK2 controls the level of negative feedback in the NF-kappaB pathway and is essential for vascular permeability and airway inflammation. J. Exp. Med. 2007, 204, 1637–1652. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, G.J.; Choi, J.K.; Choi, Y.A.; Jeong, N.H.; Park, P.H. 4-(Hydroxymethyl)catechol Extracted From Fungi in Marine Sponges Attenuates Rheumatoid Arthritis by Inhibiting PI3K/Akt/NF-kappaB Signaling. Front. Pharmacol. 2018, 9, 726. [Google Scholar] [CrossRef]
- Qian, F.; Deng, J.; Wang, G.; Ye, R.D.; Christman, J.W. Pivotal Role of Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 in Inflammatory Pulmonary Diseases. Curr. Protein Pept. 2016, 17, 332–342. [Google Scholar] [CrossRef]
- Nie, Y.; Sun, L.; Wu, Y.; Yang, Y.; Wang, J.; He, H. AKT2 Regulates Pulmonary Inflammation and Fibrosis via Modulating Macrophage Activation. J. Immunol. 2017, 198, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Deng, J.; Gantner, B.N.; Flavell, R.A.; Dong, C.; Christman, J.W. Map kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am. J. Physiol. 2012, 302, 866–874. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Dehydrocostus Lactone are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Y.; Wang, Z.; Chai, G.; Xiong, Y.; Li, B.; Zhang, H.; Xin, R.; Qian, X.; Tang, Z.; Wu, J.; et al. Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt. Molecules 2019, 24, 1510. https://doi.org/10.3390/molecules24081510
Nie Y, Wang Z, Chai G, Xiong Y, Li B, Zhang H, Xin R, Qian X, Tang Z, Wu J, et al. Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt. Molecules. 2019; 24(8):1510. https://doi.org/10.3390/molecules24081510
Chicago/Turabian StyleNie, Yunjuan, Zhongxuan Wang, Gaoshang Chai, Yue Xiong, Boyu Li, Hui Zhang, Ruiting Xin, Xiaohang Qian, Zihan Tang, Jiajun Wu, and et al. 2019. "Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt" Molecules 24, no. 8: 1510. https://doi.org/10.3390/molecules24081510
APA StyleNie, Y., Wang, Z., Chai, G., Xiong, Y., Li, B., Zhang, H., Xin, R., Qian, X., Tang, Z., Wu, J., & Zhao, P. (2019). Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt. Molecules, 24(8), 1510. https://doi.org/10.3390/molecules24081510