Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin A Isolated from Suaeda vermiculata for Antioxidant and Cytotoxic Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structure Elucidation of Pheo-a
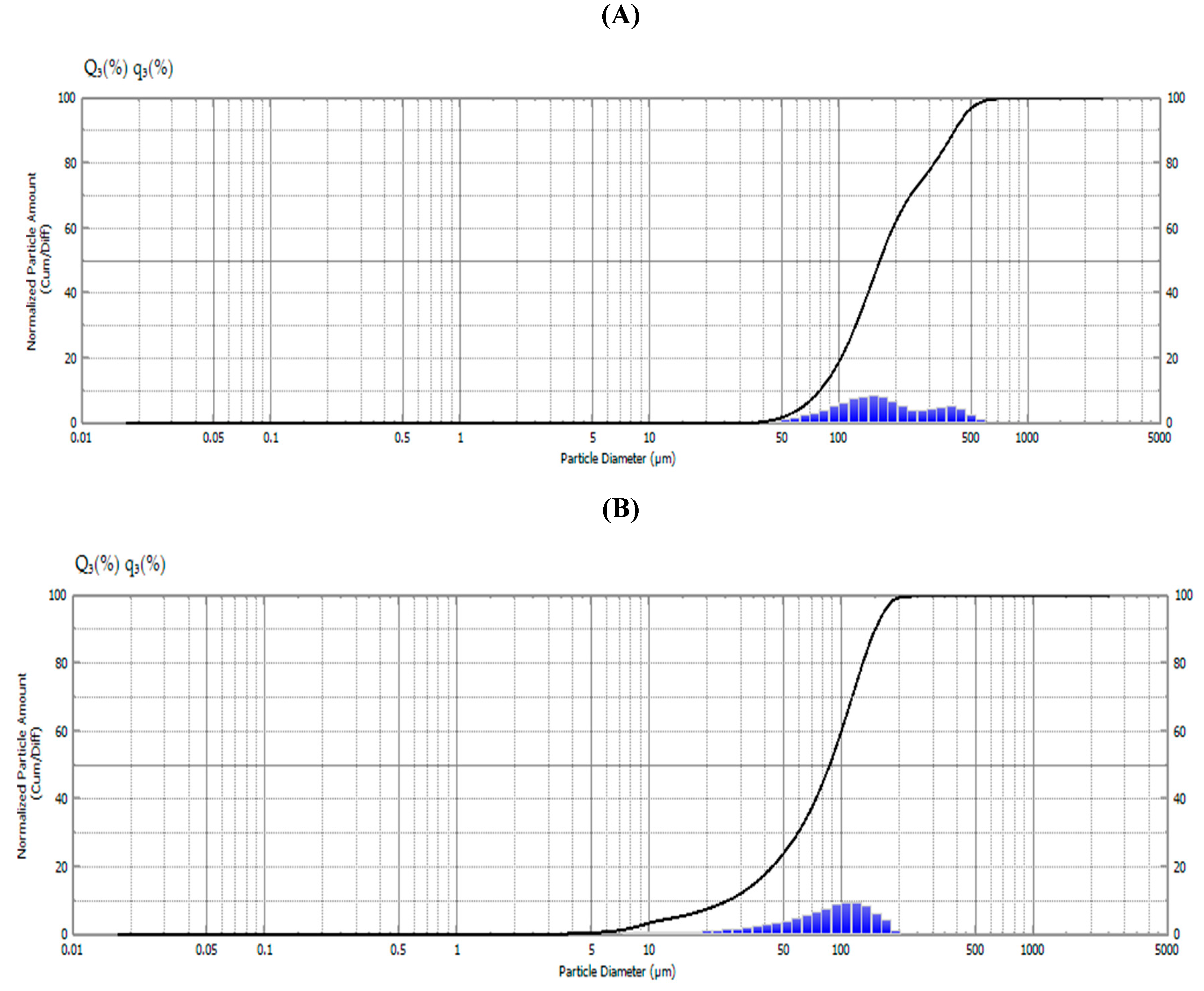
2.2. Size and Weight of Ethyl Cellulose Microparticles
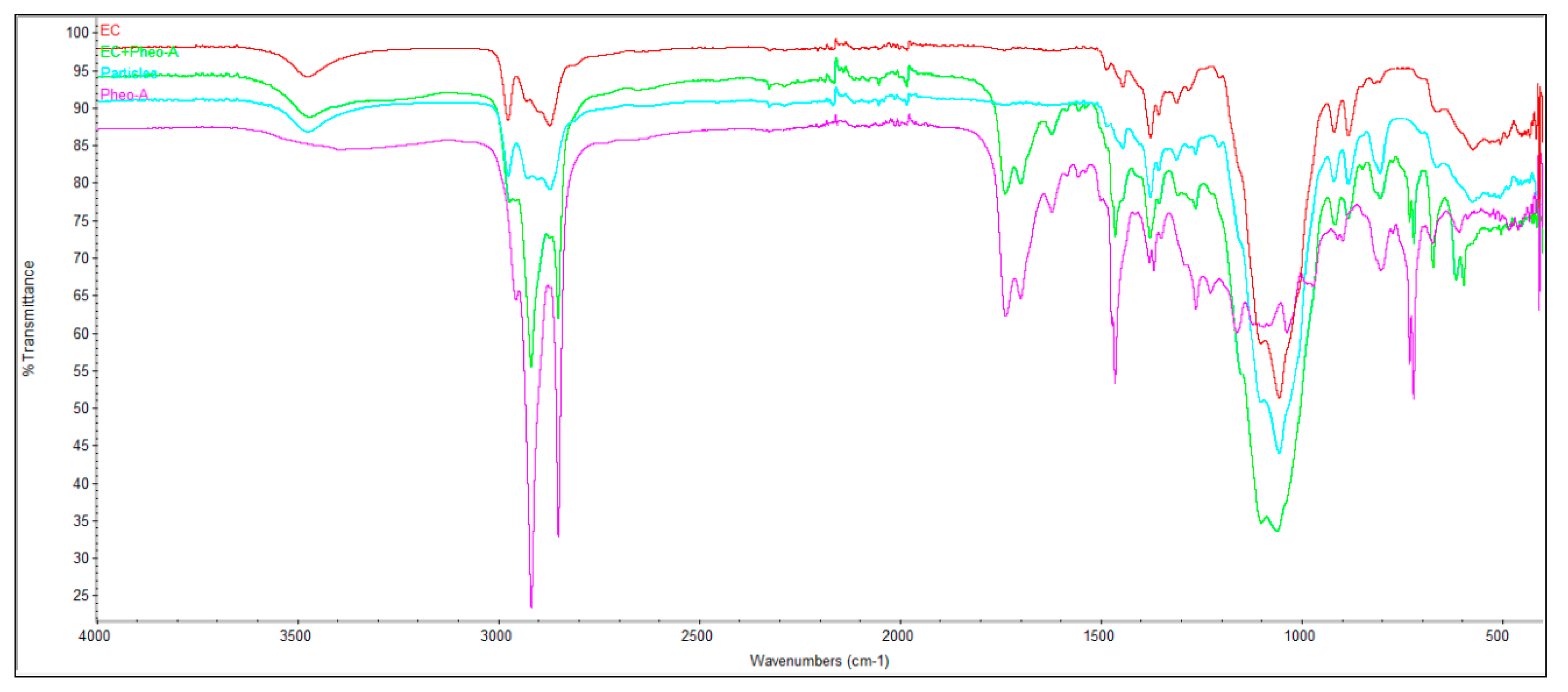
2.3. Infrared Fourier Transform Spectroscopy Analysis (FTIR)
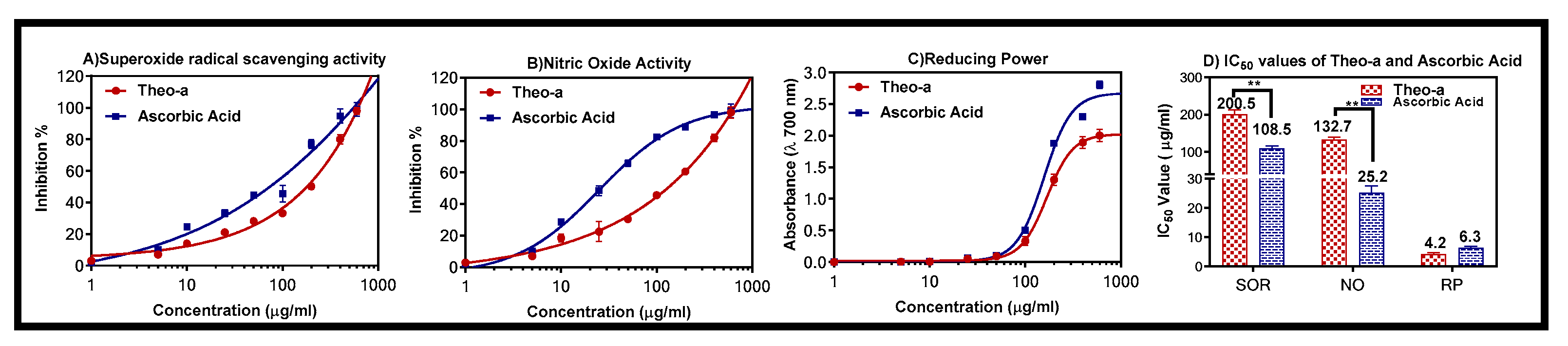
2.4. Superoxide Radical Scavenging Activity
2.5. Nitric Oxide Activity
2.6. Reducing Power Activity
2.7. Cytotoxic Assay
3. Materials and Methods
3.1. Materials
3.2. Plant Materials
3.3. Extract Preparation
3.4. Isolation and Identification of Pheo-a
3.5. Preparation of Ethyl Cellulose Microparticles Incorporated with Pheo-a
3.6. Size and Weight of Ethyl Cellulose Microparticles
3.7. Fourier-Transform Infrared Spectroscopy (FTIR)
3.8. Antioxidant Activity
3.9. Nitric Oxide Free Radical Activity (NO)
3.10. Reducing Power Method (RP)
3.11. The Cytotoxicity Assays
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Ghazalia, G.E.; Alsoqeerb, A.; Abdallaa, W.E. Effect of treated sewage effluents on heavy metals contents of three dominant wild plants at Wadi Al Rummah, Qassim Region, Saudi Arabia. Soil Water Res. 2016, 6, 51–58. [Google Scholar]
- Cybulska, I.; Brudecki, G.; Alassali, A.; Thomsen, M.; Brown, J.J. Phytochemical composition of some common coastal halophytes of the United Arab Emirates. Emir. J. Food Agric. 2014, 26, 1046–1057. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Aly, M.S.A.; Hegazy, M.M. Essential Oil Constituents and Biological Activities of the Halophytic Plants, Suaeda Vermiculata Forssk and Salsola Cyclophylla Bakera Growing in Saudi Arabia. J. Essent. Oil-Bear. Plants 2019, 1–12. [Google Scholar] [CrossRef]
- Alghamdi, A.A. Potential of some halophytic plants as animal forage in Ha’il/Saudi Arabia. Int. J. Bot. Stud. 2017, 2, 9–13. [Google Scholar]
- Orzel, L.; Was, J.; Kania, A.; Susz, A.; Rutkowska-Zbik, D.; Staron, J.; Witko, M.; Stochel, G.; Fiedor, L. Factors controlling the reactivity of divalent metal ions towards pheophytin a. JBIC J. Boil. Inorg. Chem. 2017, 22, 941–952. [Google Scholar] [CrossRef]
- Sano, Y.; Endo, K.; Tomo, T.; Noguchi, T. Modified molecular interactions of the pheophytin and plastoquinone electron acceptors in photosystem II of chlorophyll D-containing Acaryochloris marina as revealed by FTIR spectroscopy. Photosynth. Res. 2015, 125, 105–114. [Google Scholar] [CrossRef]
- Xu, H.B.; Yang, T.H.; Xie, P.; Liu, S.J.; Liang, Y.N.; Zhang, Y.; Song, Z.X.; Tang, Z.S. Pheophytin analogues from the medicinal lichen Usnea diffracta. Nat. Prod. Res. 2018, 32, 1088–1094. [Google Scholar] [CrossRef]
- Bonvino, N.P.; Liang, J.; McCord, E.D.; Zafiris, E.; Benetti, N.; Ray, N.B.; Hung, A.; Boskou, D.; Karagiannis, T.C. OliveNet: A comprehensive library of compounds from Olea europaea. Database 2018, 2018. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M. Stability of virgin olive oil. 2. Photo-oxidation studies. J. Agric. Food Chem. 2002, 50, 722–727. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef]
- Liu, C.M.; Kao, C.L.; Wu, H.M.; Li, W.J.; Huang, C.T.; Li, H.T.; Chen, C.Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Potent suppressing activity of the non-polyphenolic fraction of green tea (Camellia sinensis) against genotoxin-induced umu C gene expression in Salmonella typhimurium (TA 1535/pSK 1002)—Association with pheophytins a and b. Cancer Lett. 1997, 120, 117–123. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Tseng, C.-P.; Tsai, K.-C.; Lin, C.-F.; Wen, C.-Y.; Tsay, H.-S.; Sakamoto, N.; Tseng, C.-H.; Cheng, J.-C. Bioactivity-guided screening identifies pheophytin a as a potent anti-hepatitis C virus compound from Lonicera hypoglauca Miq. Biochem. Biophys. Commun. 2009, 385, 230–235. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Hamd, M.A.E. A Formulation, Optimization and Evaluation of Controlled Released Alginate Beads Loaded-Flurbiprofen. J. Nanomed. Nanotechnol. 2016, 07, 357. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; El-Telbany, D.F.A.; Zayed, G.; Al-Sawahli, M.M.J.J.o.P.I. Hydrogel Containing PEG-Coated Fluconazole Nanoparticles with Enhanced Solubility and Antifungal Activity. J. Pharm. Innov. 2018, 1–11. [Google Scholar] [CrossRef]
- Bittencourt, L.L.; Pedrosa, C.; Sousa, V.P.; Pierucci, A.P.; Citelli, M. Pea protein provides a promising matrix for microencapsulating iron. Plants Foods Hum. Nutr. 2013, 68, 333–339. [Google Scholar] [CrossRef]
- Ozer, A.Y.; Hincal, A.A. Studies on the masking of unpleasant taste of beclamide: Microencapsulation and tabletting. J. Microencapsul. 1990, 7, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Manjanna, K.M.; Shivakumar, B.; Kumar, T.M.P. Microencapsulation: An Acclaimed Novel Drug-Delivery System for NSAIDs in Arthritis. Crit. Rev. Ther. Drug Carr. Syst. 2010, 27, 509–545. [Google Scholar] [CrossRef]
- Idrees, A.; Rahman, N.U.; Javaid, Z.; Kashif, M.; Aslam, I.; Abbas, K.; Hussain, T. In vitro evaluation of transdermal patches of flurbiprofen with ethyl cellulose. Acta Pol. Pharm.-Drug 2014, 71, 287–295. [Google Scholar]
- Xie, Y.; Yi, Y.; Hu, X.; Shangguan, M.; Wang, L.; Lu, Y.; Qi, J.; Wu, W. Synchronous microencapsulation of multiple components in silymarin into PLGA nanoparticles by an emulsification/solvent evaporation method. Pharm. Dev. Technol. 2015, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, H.M.; Abdellatif, A.A.H.; Dennison, T.J.; Mohammed, A.R.; Sadiq, Y.; Saleem, I.Y.; Mohmmed, A.R. Colonic delivery of indometacin loaded PGA-co-PDL microparticles coated with Eudragit L100-55 from fast disintegrating tablets. Int. J. Pharm. 2017, 531, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, H.M.; Roberts, M.; El Hamd, M.A.; Abdellatif, A.A.H.; Younis, M.A. Glibenclamide Mini-tablets with an Enhanced Pharmacokinetic and Pharmacodynamic Performance. AAPS PharmSciTech 2018, 19, 2948–2960. [Google Scholar] [CrossRef]
- De Brito Filho, S.G.; Fernandes, M.G.; Chaves, O.S.; de Oliveira Chaves, M.C.; Araruna, F.B.; Eiras, C.; de Souza de Almeida Leite, J.R.; de Fátima Agra, M.; Braz-Filho, R.; de Fátima Vanderlei de Souza, M. Chemical constituents isolated from turnera subulata Sm. and electrochemical characterization of phaeophytin b. Química Nova 2014, 37, 603–609. [Google Scholar] [CrossRef]
- Schwikkard, S.L.; Mulholland, D.A.; Hutchings, A. Phaeophytins from Tapura fischeri. Phytochemistry 1998, 49, 2391–2394. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Yankuzo, H.M.; Devaraj, S.; Wong, J.K.M.; Lai, C.-S. Anti-Tumor Action, Clinical Biochemistry Profile and Phytochemical Constituents of a Pharmacologically Active Fraction of S. crispus in NMU-Induced Rat Mammary Tumour Model. PLoS ONE 2015, 10, e0126426. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Zayed, G.; El-Bakry, A.; Zaky, A.; Saleem, I.Y.; Tawfeek, H.M. Novel gold nanoparticles coated with somatostatin as a potential delivery system for targeting somatostatin receptors. Drug Dev. Ind. Pharm. 2016, 42, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.; Abou-Taleb, H.A.; El Ghany, A.A.A.; Lutz, I.; Bouazzaoui, A. Targeting of somatostatin receptors expressed in blood cells using quantum dots coated with vapreotide. Saudi Pharm. J. 2018, 26, 1162–1169. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Tawfeek, H.M. Development and evaluation of fluorescent gold nanoparticles. Drug Dev. Ind. Pharm. 2018, 44, 1679–1684. [Google Scholar] [CrossRef]
- Li, X.; Zhou, R.; Xu, K.; Xu, J.; Jin, J.; Fang, H.; He, Y. Rapid Determination of Chlorophyll and Pheophytin in Green Tea Using Fourier Transform Infrared Spectroscopy. Molecules 2018, 23, 1010. [Google Scholar] [CrossRef]
- Holt, A.S.; Jacobs, E.E. Infra-Red Absorption Spectra of Chlorophylls and Derivatives. Plant Physiol. 1955, 30, 553–559. [Google Scholar] [CrossRef]
- Wickens, A.P. Ageing and the free radical theory. Respir. Physiol. 2001, 128, 379–391. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Rosas-Romero, A.; Flerlage, N.; Burillo, J.; Codina, C. Comparison between the Radical Scavenging Activity and Antioxidant Activity of Six Distilled and Nondistilled Mediterranean Herbs and Aromatic Plants. J. Agric. Food Chem. 2002, 50, 6882–6890. [Google Scholar] [CrossRef]
- Wink, M.; Zhou, S. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Subramoniam, A.; Asha, V.V.; Nair, S.A.; Sasidharan, S.P.; Sureshkumar, P.K.; Rajendran, K.N.; Karunagaran, D.; Ramalingam, K. Chlorophyll revisited: Anti-inflammatory activities of chlorophyll a and inhibition of expression of TNF-alpha gene by the same. Inflammation 2012, 35, 959–966. [Google Scholar] [CrossRef]
- Cho, K.-J.; Han, S.H.; Kim, B.Y.; Hwang, S.-G.; Park, K.-K.; Yang, K.-H.; Chung, A.-S. Chlorophyllin Suppression of Lipopolysaccharide-Induced Nitric Oxide Production in RAW 264.7 Cells. Toxicol. Appl. Pharmacol. 2000, 166, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yadala, P.; Viswanathswamy, A. In vitro antioxidant and cytotoxic activity of rutin and piperine and their synergistic effect. Int. J. Pharm. Pharm. Sci. 2016, 8, 78–82. [Google Scholar]
- Jensen, W.B. The origin of the Soxhlet extractor. J. Chem. Educ. 2007, 84, 1913. [Google Scholar] [CrossRef]
- De Oliveira Amorim, A.P.; de Carvalho, A.R., Jr.; Lopes, N.P.; Castro, R.N.; de Oliveira, M.C.; de Carvalho, M.G. Chemical compounds isolated from Talinum triangulare (Portulacaceae). Food Chem. 2014, 160, 204–208. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of beta-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Dastmalch, S.; Nazila, D. Theophylline-Ethylcellulose Microparticles: Screening of the Process and Formulation Variables for Preparation of Sustained Release Particles. Iran. J. Basic Med. Sci. 2012, 15, 608–624. [Google Scholar]
- Abdellatif, A.A.H.; Abdelhafez, W.A.; Sarhan, H.A. Somatostatin Decorated Quantum Dots Nanoparticles for Targeting of Somatostatin Receptors. Iran. J. Pharm. Res. 2018, 17, 513–524. [Google Scholar]
- Abdellatif, A.A.; Tawfeek, H.M. Transfersomal Nanoparticles for Enhanced Transdermal Delivery of Clindamycin. AAPS PharmSciTech 2016, 17, 1067–1074. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, R. Flavonoids are scavengers of superoxide anions. J. Ethnopharmacol. 1988, 23, 345. [Google Scholar] [CrossRef]
- Hirano, K.; Hosoi, A.; Matsushita, H.; Iino, T.; Ueha, S.; Matsushima, K.; Seto, Y.; Kakimi, K. The nitric oxide radical scavenger carboxy-PTIO reduces the immunosuppressive activity of myeloid-derived suppressor cells and potentiates the antitumor activity of adoptive cytotoxic T lymphocyte immunotherapy. OncoImmunology 2015, 4, e1019195. [Google Scholar] [CrossRef][Green Version]
- Kováčik, J.; Klejdus, B.; Bačkor, M. Nitric oxide signals ROS scavenger-mediated enhancement of PAL activity in nitrogen-deficient Matricaria chamomilla roots: Side effects of scavengers. Radic. Biol. Med. 2009, 46, 1686–1693. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.A.; Al-Omar, M.S.; El-Readi, M.Z.; Alhowail, A.H.; Aldubayan, M.A.; Abdellatif, A.A.H. Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin A Isolated from Suaeda vermiculata for Antioxidant and Cytotoxic Activities. Molecules 2019, 24, 1501. https://doi.org/10.3390/molecules24081501
Mohammed HA, Al-Omar MS, El-Readi MZ, Alhowail AH, Aldubayan MA, Abdellatif AAH. Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin A Isolated from Suaeda vermiculata for Antioxidant and Cytotoxic Activities. Molecules. 2019; 24(8):1501. https://doi.org/10.3390/molecules24081501
Chicago/Turabian StyleMohammed, Hamdoon A., Mohsen S. Al-Omar, Mahmoud Zaki El-Readi, Ahmad H. Alhowail, Maha A. Aldubayan, and Ahmed A. H. Abdellatif. 2019. "Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin A Isolated from Suaeda vermiculata for Antioxidant and Cytotoxic Activities" Molecules 24, no. 8: 1501. https://doi.org/10.3390/molecules24081501
APA StyleMohammed, H. A., Al-Omar, M. S., El-Readi, M. Z., Alhowail, A. H., Aldubayan, M. A., & Abdellatif, A. A. H. (2019). Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin A Isolated from Suaeda vermiculata for Antioxidant and Cytotoxic Activities. Molecules, 24(8), 1501. https://doi.org/10.3390/molecules24081501








