A BODIPY-Bridged Bisphenoxyl Diradicaloid: Solvent-Dependent Diradical Character and Physical Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
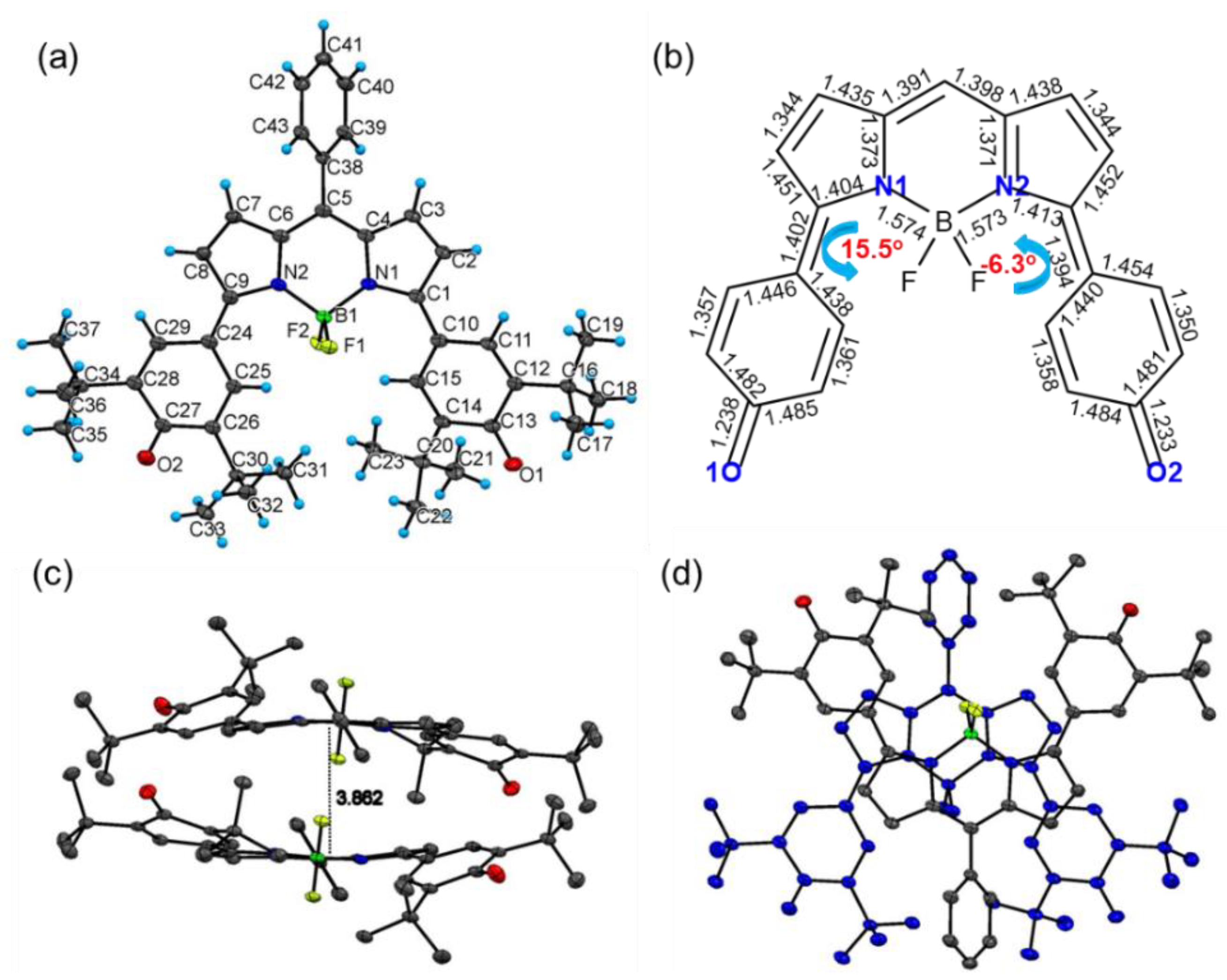
2.2. X-Ray Crystallographic Structure
2.3. Solvent-Dependent Optical and Magnetic Properties
2.4. Solvent-Dependent Electrochemical Properties
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–8526. [Google Scholar] [CrossRef]
- Nakano, M. Excitation Energies and Properties of Open-Shell Singlet Molecules; Springer: New York, NY, USA, 2014. [Google Scholar]
- Sun, Z.; Zeng, Z.; Wu, J. Zethrenes, extended p-quinodimethanes, and periacenes with a singlet biradical ground state. Acc. Chem. Res. 2014, 47, 2582–2591. [Google Scholar] [CrossRef]
- Zeng, Z.; Shi, X.; Chi, C.; López Navarrete, J.T.; Casado, J.; Wu, J. Pro-aromatic and anti-aromatic π-conjugated molecules: An irresistible wish to be diradicals. Chem. Soc. Rev. 2015, 44, 6578–6596. [Google Scholar] [CrossRef]
- Kubo, T. Recent progress in quinoidal singlet biradical molecules. Chem. Lett. 2015, 44, 111–122. [Google Scholar] [CrossRef]
- Gopalakrishna, T.Y.; Zeng, W.; Lu, X.; Wu, J. From open-shell singlet diradicaloids to polyradicaloids. Chem. Commun. 2018, 54, 2186–2199. [Google Scholar] [CrossRef]
- Dimroth, K.; Umbach, W.; Blocher, K.H. Bis-phenoxy-radicals of the polyphenyl series. Angew. Chem. Int. Ed. 1963, 2, 620–621. [Google Scholar] [CrossRef]
- West, R.; Jorgenson, J.A.; Stearly, K.L.; Calabrese, J.C. Synthesis, structure and semiconductivity of a p-terphenoquinone. J. Chem. Soc. Chem. Commun. 1991, 2, 1234–1235. [Google Scholar] [CrossRef]
- Boldt, P.; Bruhnke, D.; Gerson, F.; Scholz, M.; Jones, P.G.; Bar, F. Synthesis and structure of a p-terphenoquinone and paramagnetic species derived therefrom. Helv. Chim. Acta 1993, 76, 1739–1751. [Google Scholar] [CrossRef]
- Takahashi, T.; Matsuoka, K.; Takimiya, K.; Otsubo, T.; Aso, Y. Extensive quinoidal oligothiophenes with dicyanomethylene groups at terminal positions as highly amphoteric redox molecules. J. Am. Chem. Soc. 2005, 127, 8928–8929. [Google Scholar] [CrossRef]
- Ueda, A.; Nishida, S.; Fukui, K.; Ise, T.; Shiomi, D.; Sato, K.; Takui, T.; Nakasuji, K.; Morita, Y. Three-dimensional intramolecular exchange interaction in a curved and nonalternant π-conjugated system: Corannulene with two phenoxyl radicals. Angew. Chem. Int. Ed. 2010, 49, 1678–1682. [Google Scholar] [CrossRef]
- Schmidt, D.; Son, M.; Lim, M.J.; Lin, M.-J.; Krummenacher, I.; Braunschweig, H.; Kim, D.; Wurthner, F. Perylene bisimide radicals and biradicals: Synthesis and molecular properties. Angew. Chem. Int. Ed. 2015, 54, 13980–13984. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, L.; Phan, H.; Huang, X.; Herng, T.S.; Zhou, J.; Zeng, W.; Ding, J.; Luo, S.; Wu, J.; et al. A Stable N-annulated perylene-bridged bisphenoxyl diradicaloid and the corresponding boron trifluoride complex. Chem. Eur. J. 2017, 23, 9419–9424. [Google Scholar] [CrossRef] [PubMed]
- Naoda, K.; Shimizu, D.; Kim, O.J.; Furukawa, K.; Kim, D.; Osuka, A. Thienylquinonoidal porphyrins and hexaphyrins with singlet diradical ground states. Chem. Eur. J. 2017, 23, 8969–8979. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Phan, H.; Herng, T.S.; Gopalakrishna, T.Y.; Liu, C.; Zeng, W.; Ding, J.; Wu, J. Toward stable superbenzoquinone diradicaloids. Angew. Chem. Int. Ed. 2016, 55, 5012–5016. [Google Scholar]
- Lee, S.; Miao, F.; Phan, H.; Herng, T.S.; Ding, J.; Wu, J.; Kim, D. Radical and diradical formation in naphthalene diimides through simple chemical oxidation. ChemPhysChem 2017, 18, 591–595. [Google Scholar] [CrossRef]
- Rausch, R.; Schmidt, D.; Bialas, D.; Krummenacher, I.; Braunschweig, H.; Würthner, F. Stable organic (bi)radicals by delocalization of spin density into the electron-poor chromophore core of isoindigo. Chem. Eur. J. 2018, 24, 3420–3424. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Lee, S.; Son, M.; Aratani, N.; Ishida, M.; Samanta, A.; Yanada, H.; Chang, Y.-T.; Furuta, H.; Kim, D.; et al. A diradical approach towards BODIPY-based dyes with intense near-infrared absorption around λ = 1100 nm. Angew. Chem. Int. Ed. 2017, 55, 2815–2819. [Google Scholar] [CrossRef]
- Miao, F.; Lim, Z.L.; Hu, P.; Dong, S.; Qi, Q.; Zhang, X.; Wu, J. BODIPY blocked anthroxyl radicals: The substituent effect on reactivity and fluorescence turn-on detection of a hydroxyl radical. Org. Biomol. Chem. 2017, 15, 3188–3191. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, C.; Feng, Z.; Yu, Y.; Wang, J.; Hao, E.; Wei, Y.; Mu, X.; Jiao, L. Highly regioselective α-chlorination of the BODIPY chromophore with copper(II) chloride. Org. Lett. 2015, 17, 4632–4635. [Google Scholar] [CrossRef]
- Feng, J.; Gopalakrishna, T.Y.; Phan, H.; Wu, J. Hexakis(3,6-di-tert-butyl-4-oxo-2,5-cyclohexadien-1-ylidene)cyclohexane: Closed-shell [6]radialene or open-shell hexa-radicaloid? Chem. Eur. J. 2018, 24, 9499–9503. [Google Scholar] [CrossRef]
- Bleaney, B.; Bowers, K.D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond. Ser. A 1952, 214, 451–453. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| Solution | λmax (nm) | ε (105 M−1cm−1) |
|---|---|---|
| Toluene | 775 832 a | 1.11 0.811 |
| DCM | 774 837 a | 1.14 0.876 |
| THF | 770 826 a | 1.14 0.862 |
| Acetone | 767 830 a | 1.23 0.922 |
| DMF | 772 a 819 | 1.01 1.50 |
| DMSO | 782 830 | 0.954 0.893 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, F.; Phan, H.; Wu, J. A BODIPY-Bridged Bisphenoxyl Diradicaloid: Solvent-Dependent Diradical Character and Physical Properties. Molecules 2019, 24, 1446. https://doi.org/10.3390/molecules24081446
Miao F, Phan H, Wu J. A BODIPY-Bridged Bisphenoxyl Diradicaloid: Solvent-Dependent Diradical Character and Physical Properties. Molecules. 2019; 24(8):1446. https://doi.org/10.3390/molecules24081446
Chicago/Turabian StyleMiao, Fang, Hoa Phan, and Jishan Wu. 2019. "A BODIPY-Bridged Bisphenoxyl Diradicaloid: Solvent-Dependent Diradical Character and Physical Properties" Molecules 24, no. 8: 1446. https://doi.org/10.3390/molecules24081446
APA StyleMiao, F., Phan, H., & Wu, J. (2019). A BODIPY-Bridged Bisphenoxyl Diradicaloid: Solvent-Dependent Diradical Character and Physical Properties. Molecules, 24(8), 1446. https://doi.org/10.3390/molecules24081446






