Rhinoclactones A-E, Resorcylic Acid Analogs from Desert Plant Endophytic Fungus Rhinocladiella similis
Abstract
1. Introduction
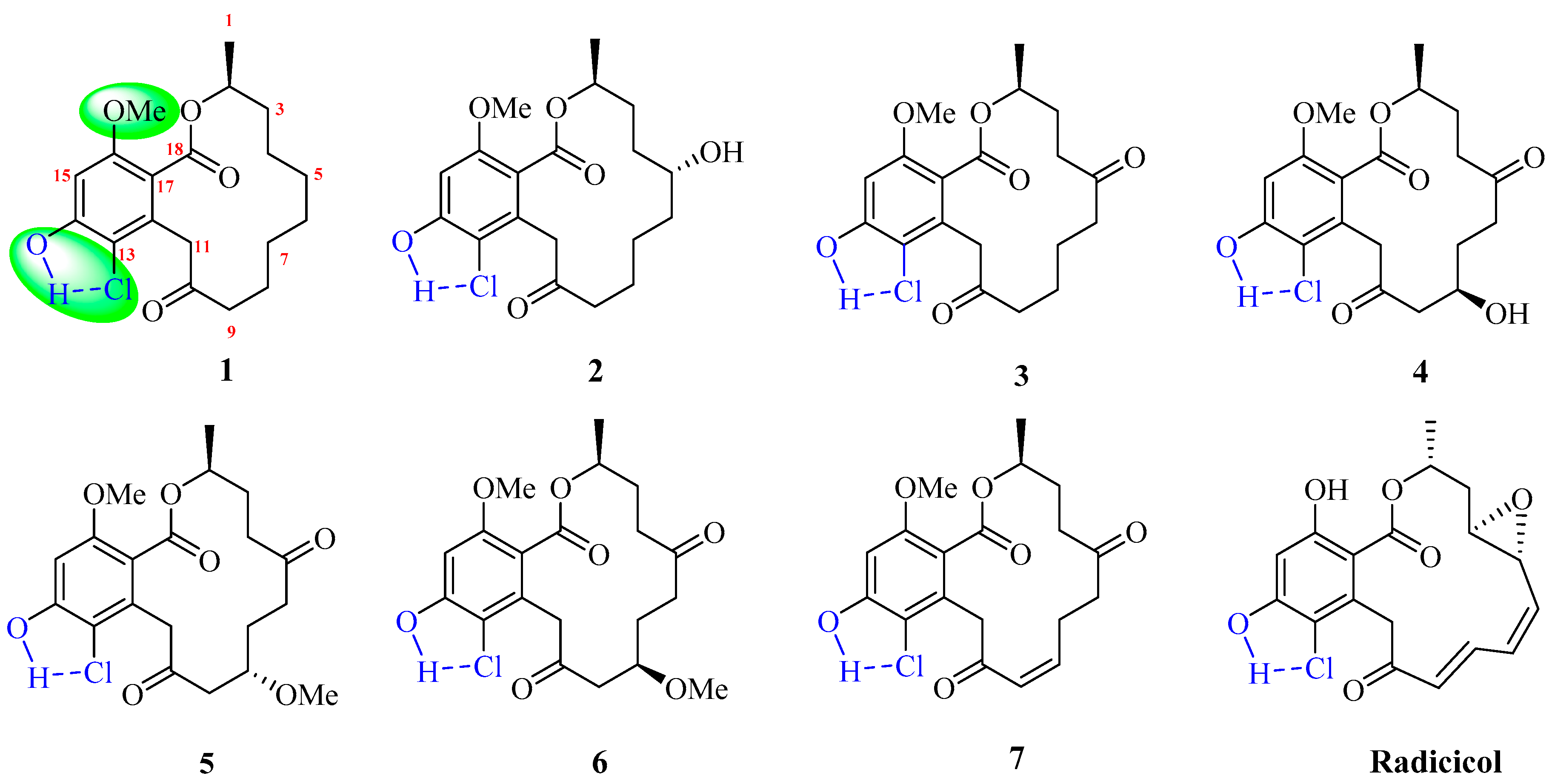
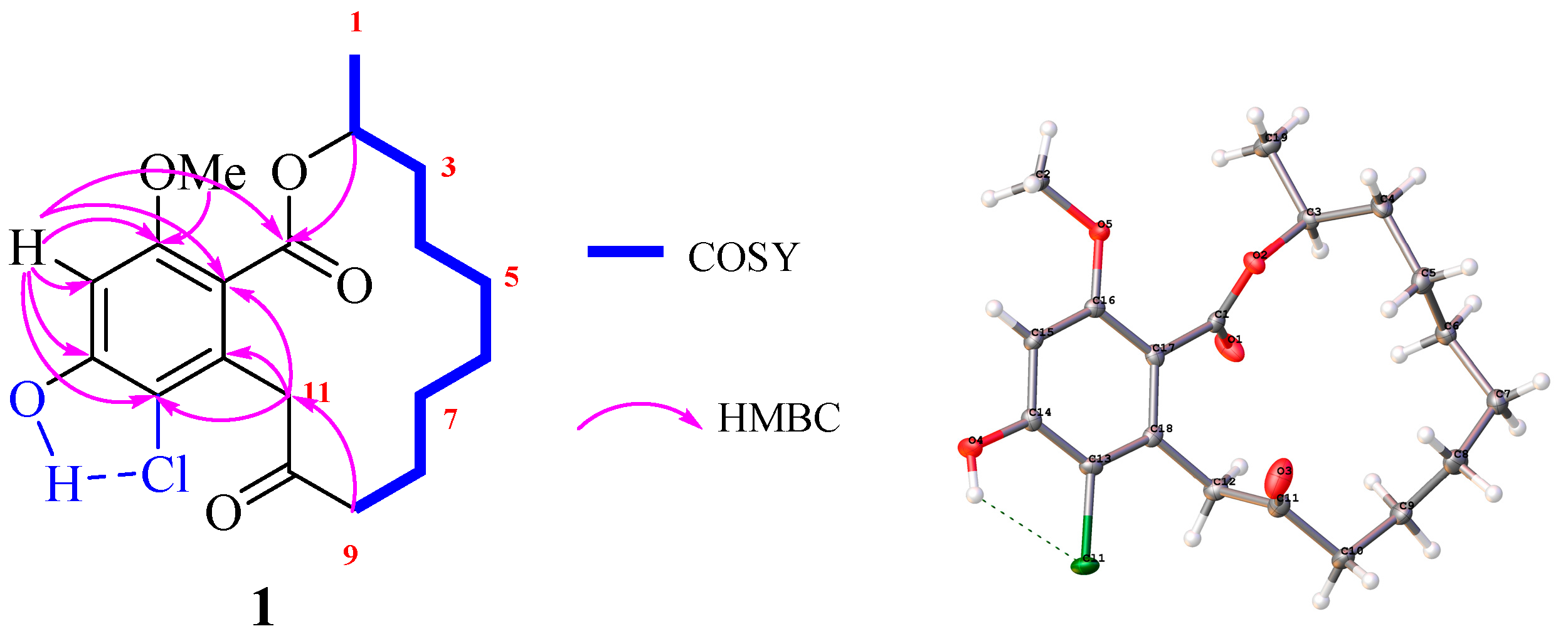
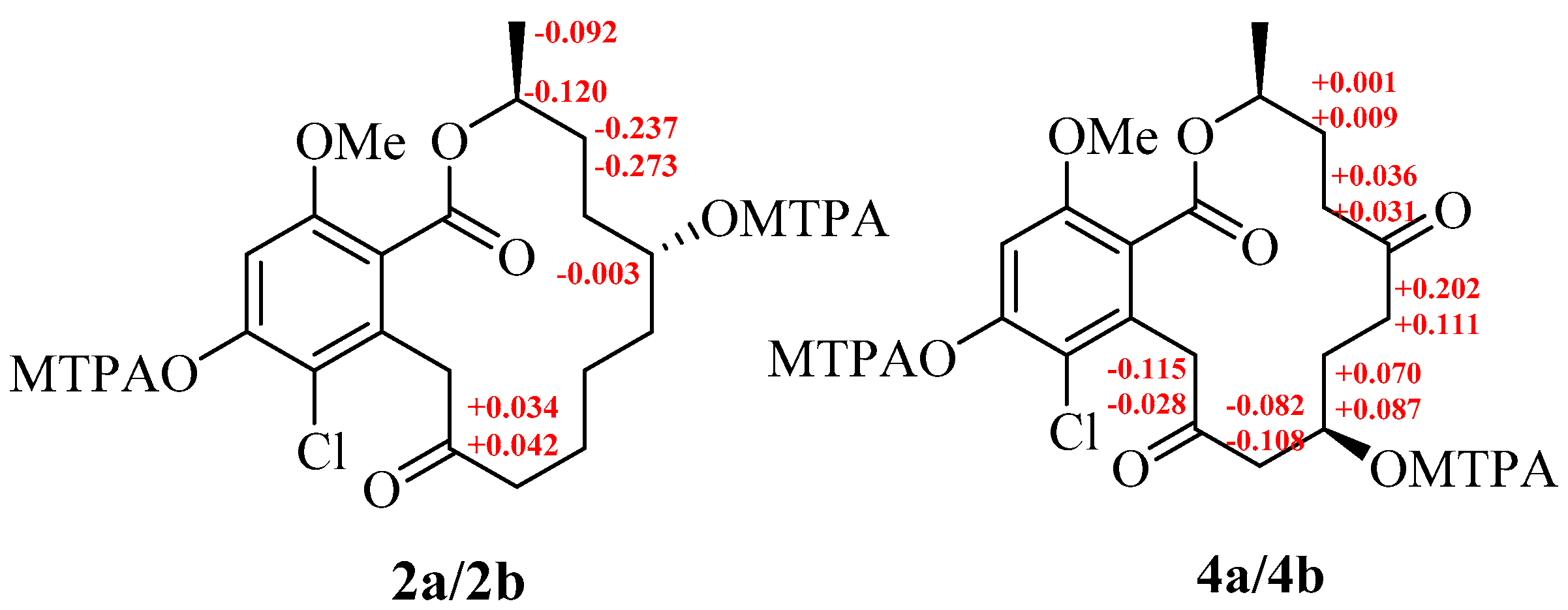
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. X-ray Crystallographic Analysis of 1
3.5. Cytotoxic Evaluation (MTT Assay)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Winssinger, N.; Barluenga, S. Chemistry and biology of resorcylic acid lactones. Chem. Commun. 2007, 1, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R. ChemInform Abstract: A Biosynthetic Classification of Fungal and Streptomycete Fused-Ring Aromatic Polyketides. Chem. Biol. Chem. 2001, 2, 612–627. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Zhou, Z.; Su, S.; Roberts, S.A.; Montfort, W.R.; Zeng, J.; Chen, M.; Zhang, W.; Zhan, J.; et al. Rational reprogramming of fungal polyketide first-ring cyclization. Proc. Natl. Acad. Sci. USA 2013, 110, 5398–5403. [Google Scholar] [CrossRef]
- Shen, W.; Mao, H.; Huang, Q.; Dong, J. Benzenediol lactones: A class of fungal metabolites with diverse structural features and biological activitie. Eur. J. Med. Chem. 2015, 97, 747–777. [Google Scholar] [CrossRef] [PubMed]
- Winssinger, N.; Fontaine, J.G.; Barluenga, S. Hsp90 Inhibition with Resorcylic Acid Lactones (RALs). Curr. Top. Med. Chem. 2009, 9, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Ayers, S.; Graf, T.N.; Adcock, A.F.; Kroll, D.J.; Matthew, S.; Carcache de Blanco, E.J.; Shen, Q.; Swanson, S.M.; Wani, M.C.; Pearce, C.J.; et al. Resorcylic Acid Lactones with Cytotoxic and NF-κB Inhibitory Activities and Their Structure–Activity Relationships. J. Nat. Prod. 2011, 74, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Akinaga, S.; Agatsuma, T. International Patent 2004. No. WO 2004/024141, 25 March 2004. [Google Scholar]
- Ninomiya-Tsuji, J.; Kajino, T.; Ono, K.; Ohtomo, T.; Matsumoto, M.; Shiina, M.; Mihara, M.; Tsuchiya, M.; Kunihiro, M. A Resorcylic Acid Lactone, 5Z-7-Oxozeaenol, Prevents Inflammation by Inhibiting the Catalytic Activity of TAK1 MAPK Kinase Kinase. J. Biol. Chem. 2003, 278, 18485–18490. [Google Scholar] [CrossRef]
- Tan, X.M.; Chen, A.J.; Wu, B.; Zhang, G.S.; Ding, G. Advance of swainsonine biosynthesis. Chin. Chem. Lett. 2018, 29, 417–422. [Google Scholar] [CrossRef]
- Li, L.Y.; Sun, B.D.; Zhang, G.S.; Deng, H.; Wang, M.H.; Tan, X.M.; Zhang, X.Y.; Jia, H.M.; Zhang, T.; Zou, Z.M.; et al. Polyketides with different post-modifications from desert endophytic fungus Paraphoma sp. Nat. Prod. Res. 2017, 32, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Zhang, X.Y.; Sun, B.D.; Deng, H.; Zou, Z.M.; Ding, G. Phenolic acid analogs from the endophytic fungus Embellisia chlamydospora isolated from desert medicinal plant Artemisia desertorum. Mycosystema 2018, 37, 88–94. [Google Scholar]
- Li, L.Y.; Song, B.; Chen, A.J.; Sun, B.D.; Zhang, G.S.; Deng, H.; Ding, G. Advance on secondary metabolites of grassland and desert plants endophytic fungi. Microbiol. China 2018, 45, 1146–1160. [Google Scholar]
- Zhang, X.Y.; Liu, Z.L.; Sun, B.D.; Niu, S.N.; Wang, M.H.; Tan, X.M.; Zou, Z.M.; Ding, G. Bioactive resorcylic acid lactones with different ring systems from desert plant endophytic fungus Chaetosphaeronema hispidulum. J. Agric. Food Chem. 2018, 66, 8976–8982. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.M.; Li, L.Y.; Sun, L.Y.; Sun, B.D.; Niu, S.B.; Wang, M.H.; Zhang, X.Y.; Sun, W.S.; Zhang, G.S.; Deng, H.; et al. Spiciferone analogs from an endophytic fungus Phoma betae collected from desert plants in West China. J. Antibiot. 2018, 71, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, L.Y.; Shang, H.; Liu, Y.; Yu, M.; Ding, G.; Zou, Z.M. Trematosphones A and B, Two Unique Dimeric Structures from the Desert Plant Endophytic Fungus Trematosphaeria terricola. Org. Lett. 2019, 21, 2139–2142. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Raja, H.A.; Day, C.S.; Chen, W.; Swanson, S.M.; Oberlies, N.H. Greensporones: Resorcylic Acid Lactones from an Aquatic Halenospora sp. J. Nat. Prod. 2014, 77, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Ikuko, O.; Takenori, K.; Yoel, K.; Hiroshi, K. High-Field FT NMR Application of Mosher’s Method. The Absolute Configurations of Marine Terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar]
- Zhou, H.; Qiao, K.J.; Gao, Z.Z.; Vederas, J.C.; Tang, Y. Insights into Radicicol Biosynthesis via Heterologous Synthesis of Intermediates and Analogs. J. Biol. Chem. 2010, 285, 41412–41421. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Niu, S.B.; Li, L.; Ding, G.; Yu, M.; Zhang, G.S.; Wang, M.H.; Li, L.Y.; Zhang, T.; Jia, H.M.; et al. Trichoderpyrone, a Unique Polyketide Hybrid with a Cyclopentenone–Pyrone Skeleton from the Plant Endophytic Fungus Trichoderma gamsii. J. Nat. Prod. 2017, 80, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (1–7) are available from the authors. |
| No. | 1 | 2 | 4 | 5 | 6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| δHa | δCb | δHa | δCb | δHa | δCb | δHa | δCb | δHa | δCb | |
| 1 | 1.27, d (7.2) | 19.6 | 1.28, d (7.8) | 19.6 | 1.28, d (7.8) | 19.5 | 1.27, d (7.2) | 20.6 | 1.29, d (7.2) | 19.5 |
| 2 | 5.17, m | 70.4 | 5.20, m | 72.0 | 5.12, m | 70.9 | 4.90, m | 71.4 | 5.16, m | 70.9 |
| 3 | 1.60, m | 34.7 | 1.48, m | 30.8 | 2.16, m | 27.9 | 1.50, m | 29.5 | 2.21, m | 27.9 |
| 1.60, m | 1.28, m | 1.60, m | 1.50, m | 1.58, m | ||||||
| 4 | 1.33, m | 22.1 | 1.51, m | 31.6 | 2.62, m | 37.3 | 2.64, ddd (18.5, 10.0, 4.0) | 35.2 | 2.66, m | 35.4 |
| 1.33, m | 1.28, m | 2.37, m | 2.16, ddd (18.5, 5.0, 5.0) | 2.38, m | ||||||
| 5 | 1.36, m | 25.7 | 3.45, m | 70.3 | 209.7 | 209.6 | 209.6 | |||
| 1.36, m | ||||||||||
| 6 | 1.34, m | 24.9 | 1.64, m | 34.6 | 2.64, m | 35.2 | 2.43, ddd (15.0, 7.5, 3.5) | 40.6 | 2.55, m | 36.7 |
| 1.34, m | 1.29, m | 2.64, m | 2.24, ddd (15.0, 11.5, 3.5) | 2.34, m | ||||||
| 7 | 1.70, m | 22.7 | 1.40, m | 22.4 | 1.87, m | 30.2 | 2.09, m | 21.3 | 1.89, m | 26.6 |
| 1.50, m | 1.40, m | 1.87, m | 1.76, m | 1.89, m | ||||||
| 8 | 1.70, m | 25.9 | 1.41, m | 22.8 | 3.82, m | 65.6 | 3.56, m | 79.1 | 3.37, m | 75.3 |
| 1.52, m | 1.17, m | |||||||||
| 8-OMe | 3.27 s | 57.1 | 3.27 s | 55.5 | ||||||
| 9 | 2.71, ddd (14.5, 9.5, 3.0) | 40.5 | 2.79, ddd (17.0, 7.5, 3.0) | 39.5 | 2.63, dd (14.0, 3.5) | 48.8 | 3.00, dd (14.5, 8.0) | 45.5 | 2.68, dd (14.5, 3.5) | 45.2 |
| 2.30, ddd (14.5, 9.0, 3.0) | 2.46, ddd (17.0, 10.5, 3.5) | 2.33, dd (14.0, 9.5) | 2.52, dd (14.5, 1.5) | 2.21, dd (14.5, 9.0) | ||||||
| 10 | 204.4 | 204.1 | 203.9 | 205.0 | 203.4 | |||||
| 11 | 4.13, d (18.5) | 44.1 | 4.03, d (18.5) | 44.5 | 4.10, d (18.0) | 45.3 | 4.07, d (18.5) | 46.8 | 4.12, d (17.5) | 44.9 |
| 3.97, d (18.5) | 3.97, d (18.5) | 3.89, d (18.0) | 4.01, d (18.5) | 3.89, d (17.5) | ||||||
| 12 | 132.6 | 132.3 | 132.4 | 133.2 | 132.4 | |||||
| 13 | 113.7 | 113.5 | 113.5 | 114.8 | 113.4 | |||||
| 14 | 154.9 | 154.6 | 155.3 | 156.8 | 155.2 | |||||
| 15 | 6.68, s | 99.3 | 6.68, s | 99.2 | 6.71, s | 99.5 | 6.67, s | 100.5 | 6.69, s | 99.5 |
| 16 | 156.4 | 156.1 | 156.6 | 157.3 | 156.7 | |||||
| 17 | 117.9 | 118.6 | 117.8 | 118.6 | 117.9 | |||||
| 18 | 167.0 | 166.7 | 167.0 | 168.2 | 167.0 | |||||
| 16-OMe | 3.77, s | 55.5 | 3.79, s | 55.6 | 3.78, s | 55.6 | 3.74, s | 56.5 | 3.78, s | 55.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, X.; Tan, X.; Sun, B.; Wu, B.; Yu, M.; Zhang, T.; Zhang, Y.; Ding, G. Rhinoclactones A-E, Resorcylic Acid Analogs from Desert Plant Endophytic Fungus Rhinocladiella similis. Molecules 2019, 24, 1405. https://doi.org/10.3390/molecules24071405
Li L, Zhang X, Tan X, Sun B, Wu B, Yu M, Zhang T, Zhang Y, Ding G. Rhinoclactones A-E, Resorcylic Acid Analogs from Desert Plant Endophytic Fungus Rhinocladiella similis. Molecules. 2019; 24(7):1405. https://doi.org/10.3390/molecules24071405
Chicago/Turabian StyleLi, Luying, Xiaoyan Zhang, Xiangmei Tan, Bingda Sun, Bin Wu, Meng Yu, Tao Zhang, Yonggang Zhang, and Gang Ding. 2019. "Rhinoclactones A-E, Resorcylic Acid Analogs from Desert Plant Endophytic Fungus Rhinocladiella similis" Molecules 24, no. 7: 1405. https://doi.org/10.3390/molecules24071405
APA StyleLi, L., Zhang, X., Tan, X., Sun, B., Wu, B., Yu, M., Zhang, T., Zhang, Y., & Ding, G. (2019). Rhinoclactones A-E, Resorcylic Acid Analogs from Desert Plant Endophytic Fungus Rhinocladiella similis. Molecules, 24(7), 1405. https://doi.org/10.3390/molecules24071405







