Characterization and Discrimination of Chinese Marinated Pork Hocks by Volatile Compound Profiling Using Solid Phase Microextraction Gas Chromatography-Mass Spectrometry/Olfactometry, Electronic Nose and Chemometrics
Abstract
1. Introduction
2. Results and Discussion
2.1. Volatile Profile of Marinated Pork Hocks Characterized by GC-MS/O
2.1.1. Volatile Composition of Marinated Pork Hocks
2.1.2. OAVs of the Odour-Active Compounds
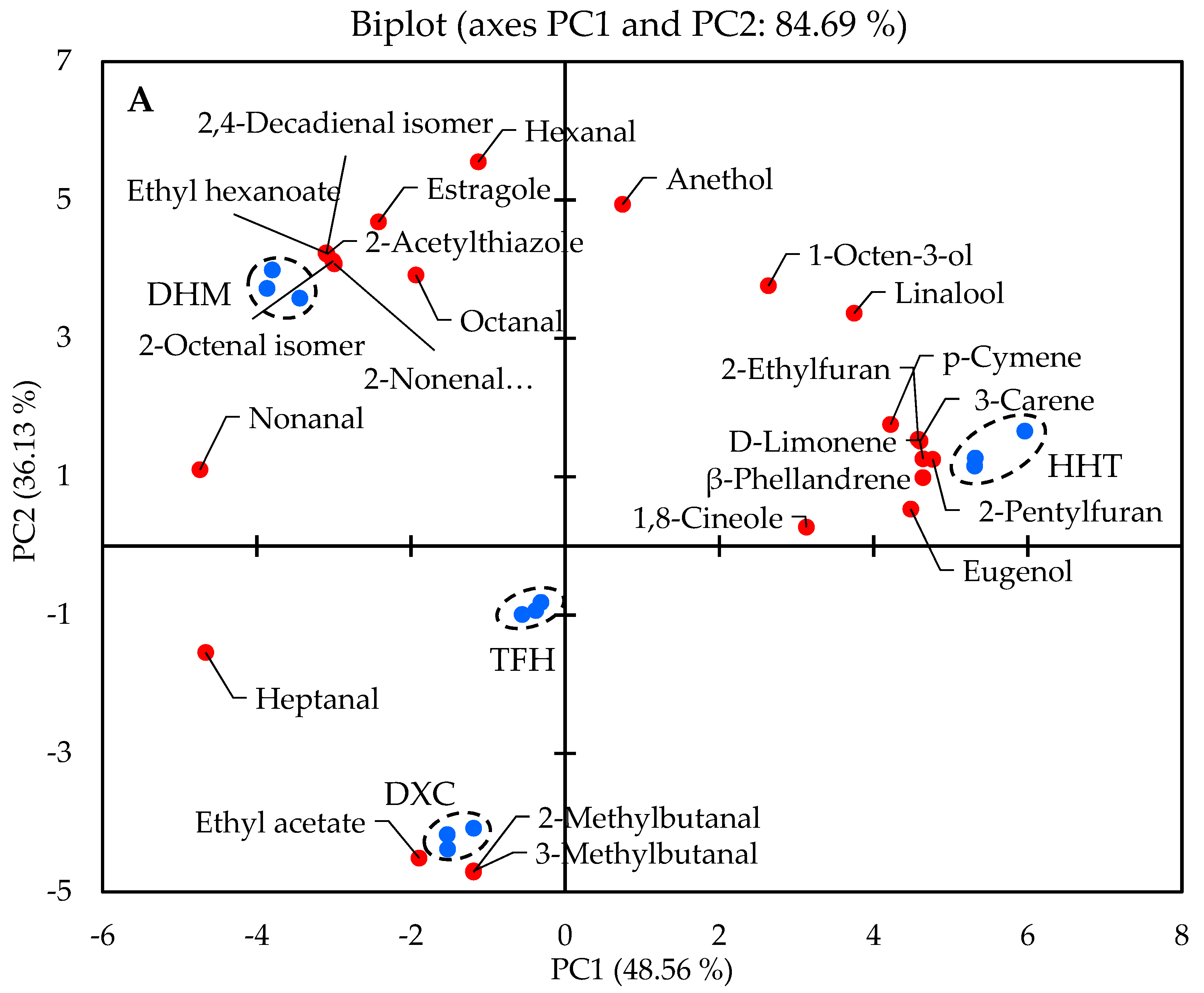
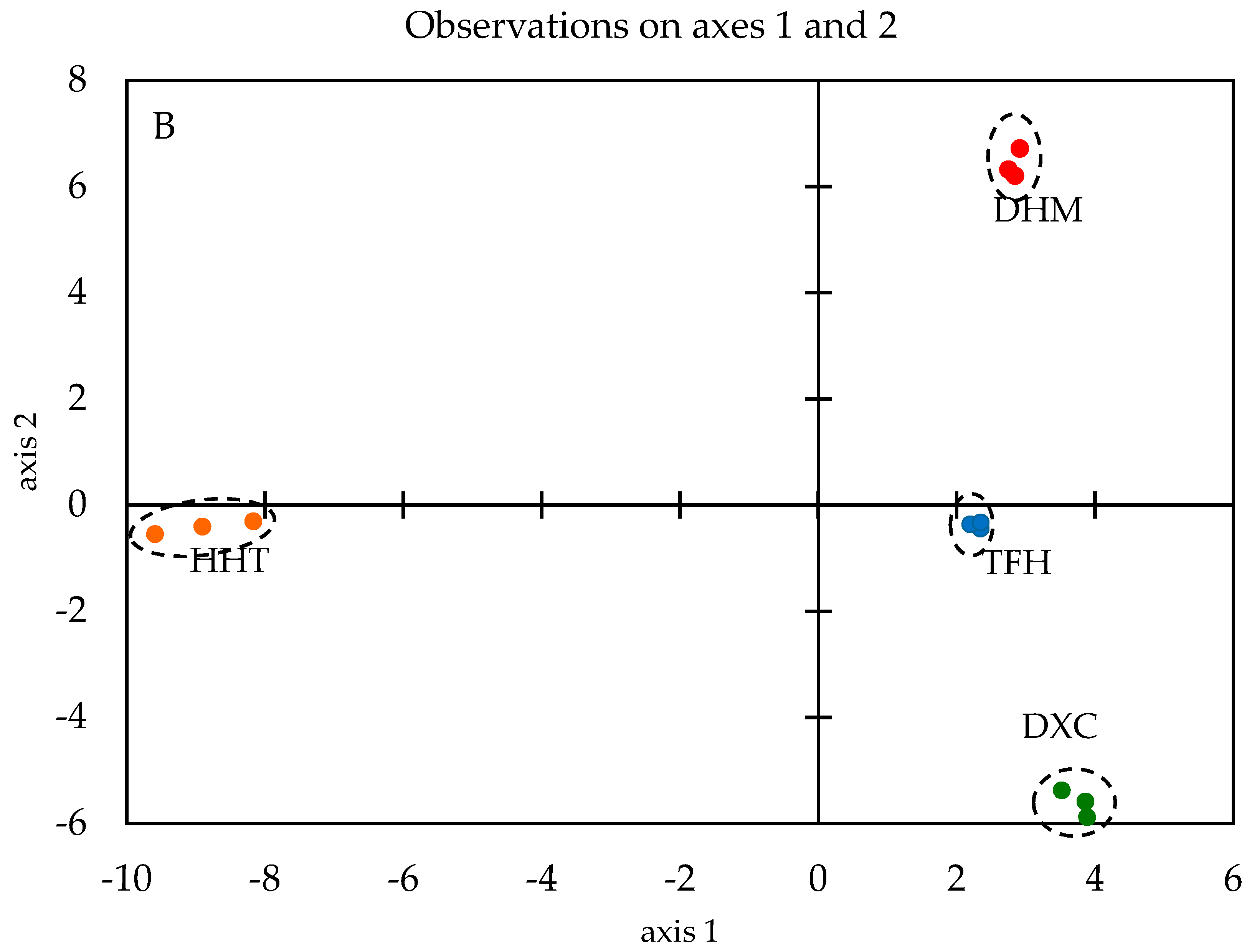
2.2. Discrimination of Marinated Pork Hocks by GC-MS/O
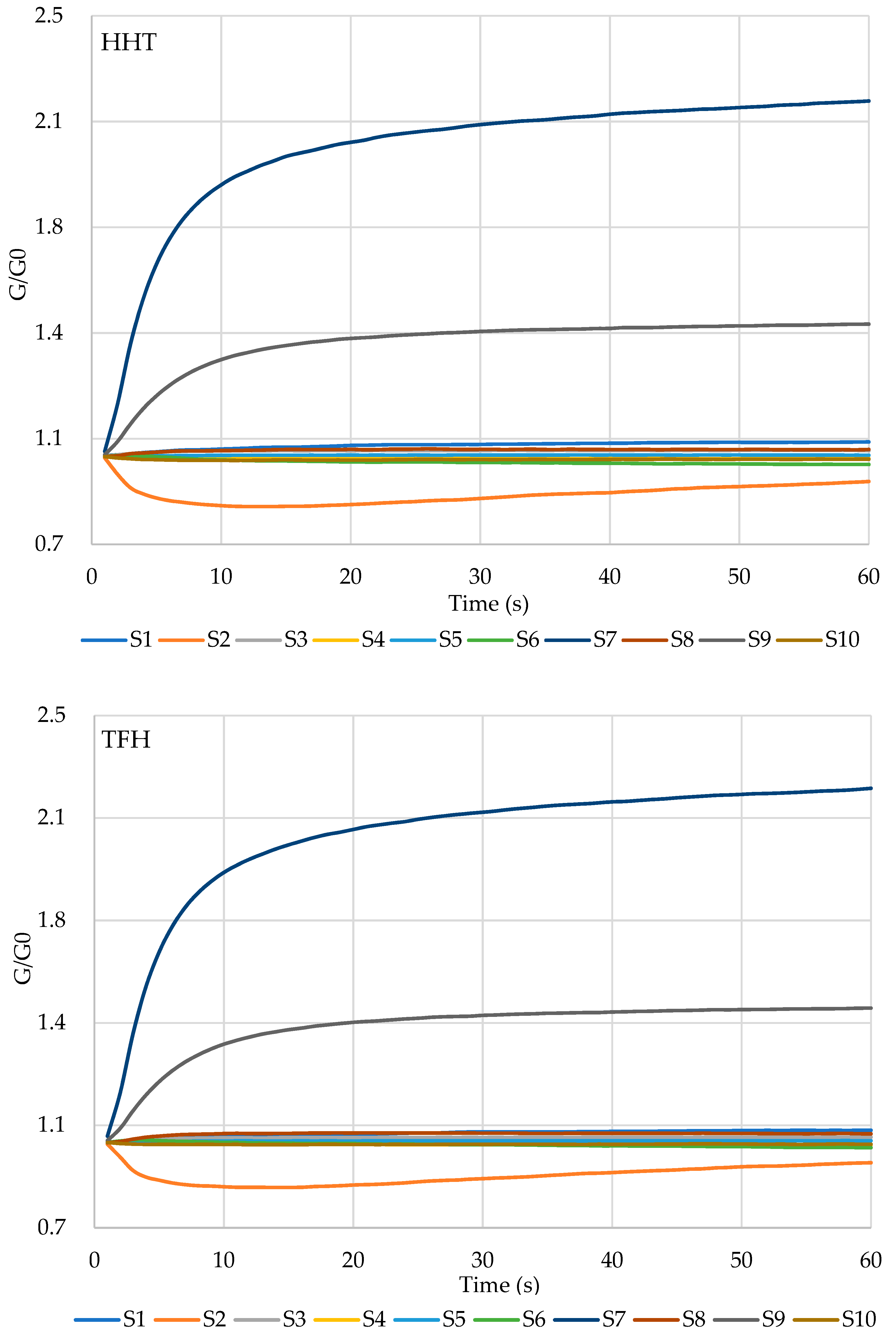
2.3. Volatile Profile of Marinated Pork Hocks Characterized by E-Nose
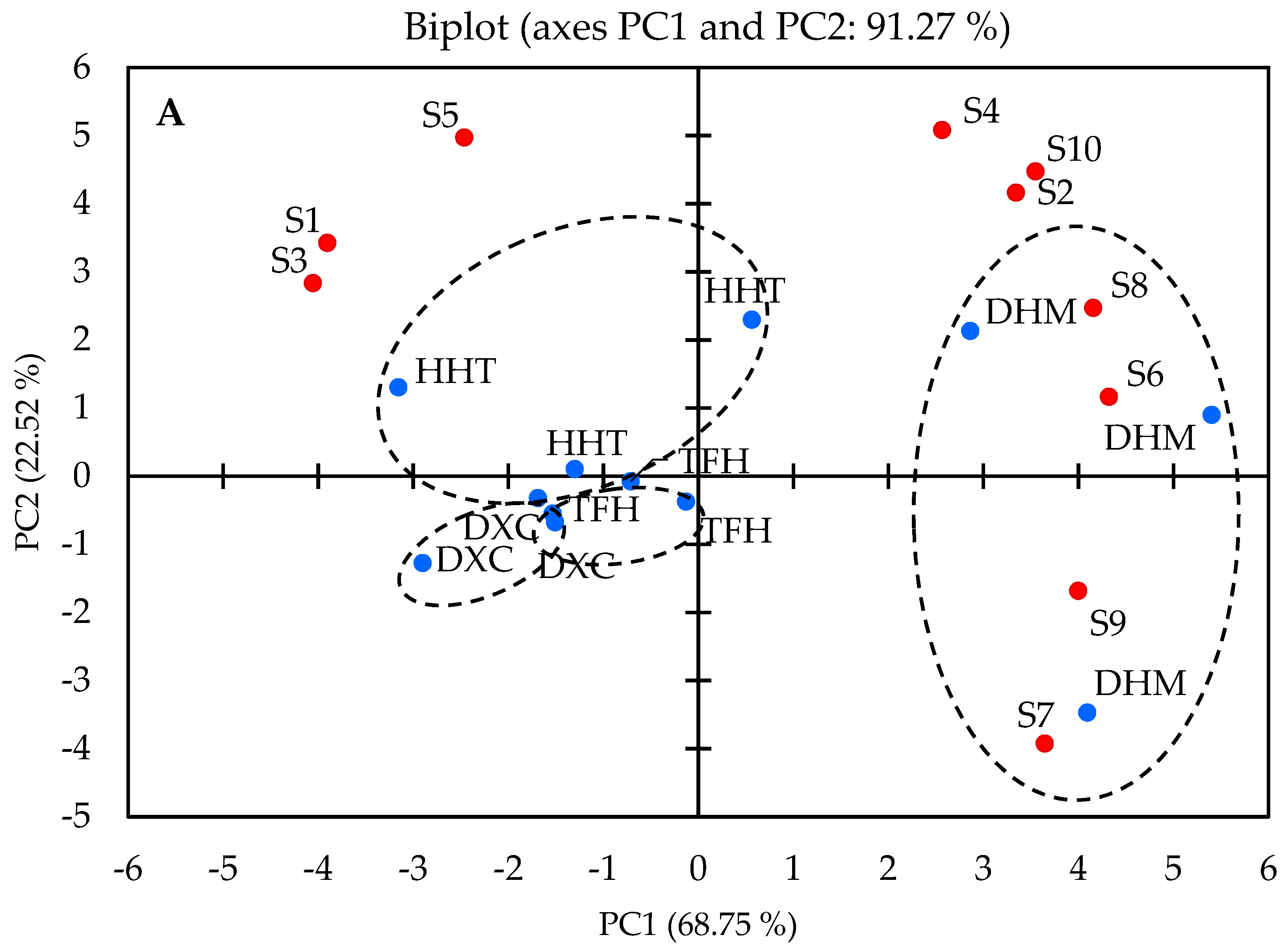
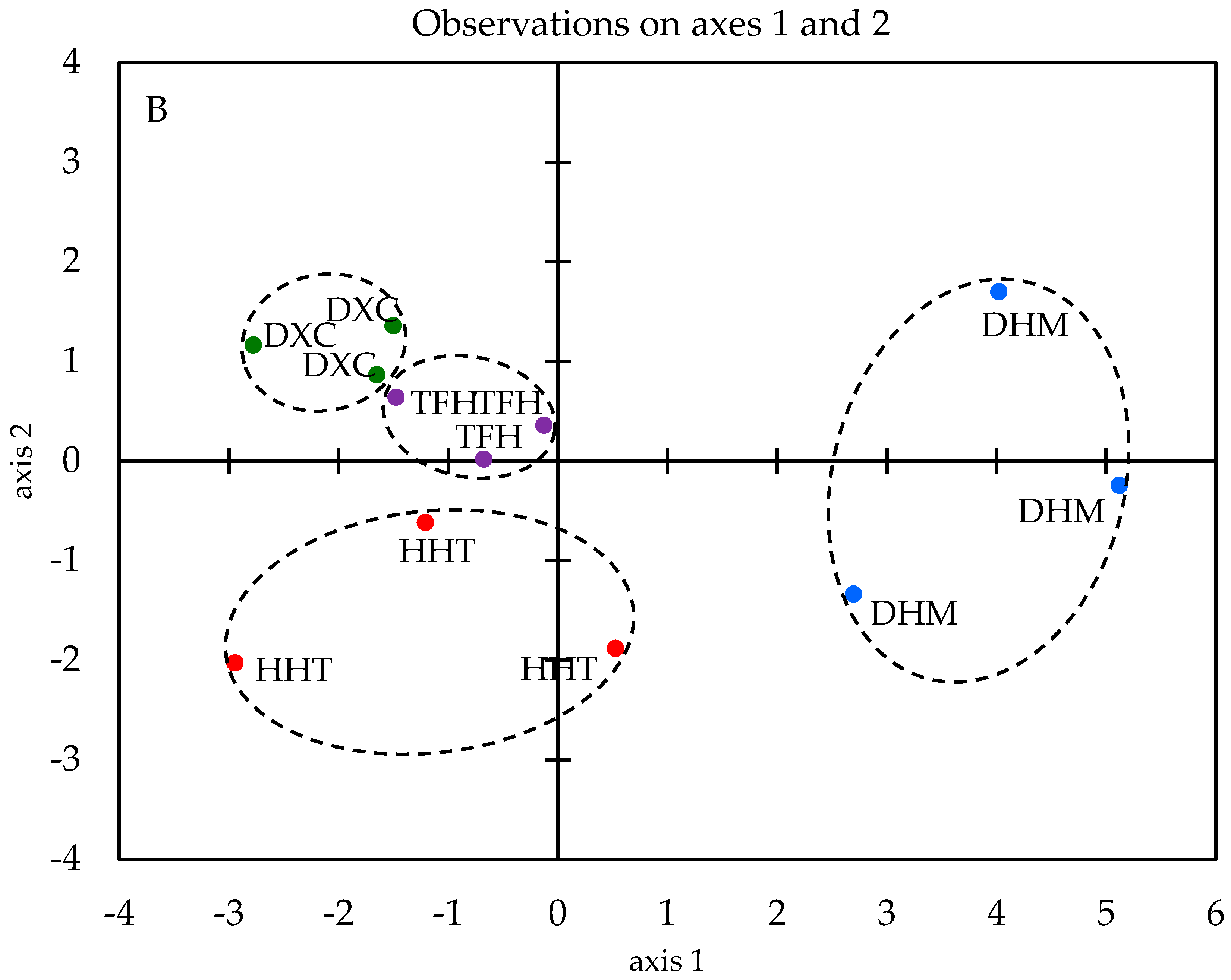
2.4. Discrimination of Marinated Pork Hocks by E-Nose
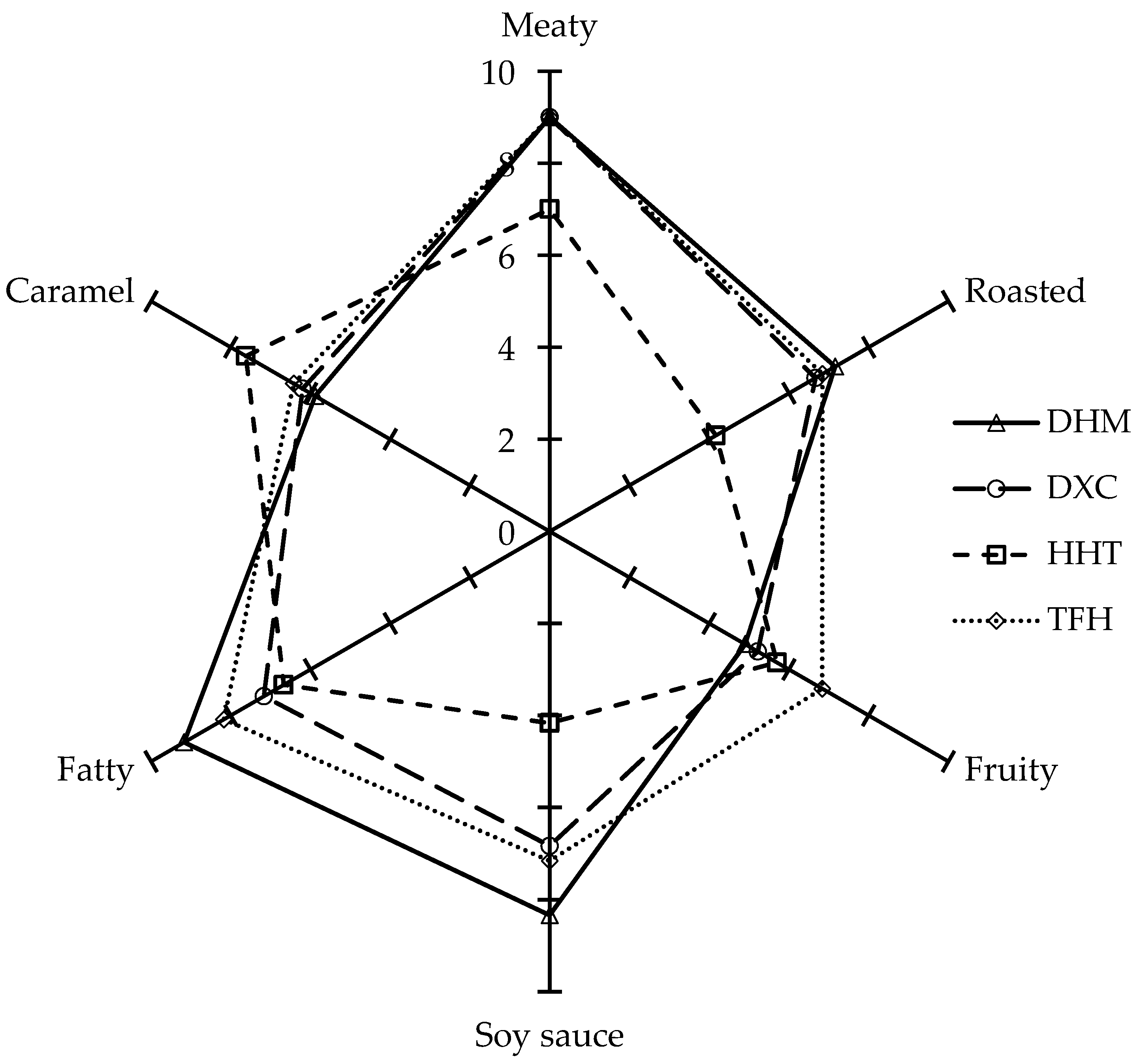
2.5. Sensory Analysis of Marinated Pork Hocks
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Solid Phase Microextraction of Volatile Compounds
3.3. GC-MS/O Analysis of Volatile Compounds
3.4. Identification and Quantification of Volatile Compounds
3.5. E-Nose Analysis of Marinated Pork Hocks
3.6. Sensory Evaluation of Marinated Pork Hocks
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, X.; Wang, C.; Zhang, C.; Li, X.; Wang, J.; Li, H.; Tang, C. A combination of quantitative marinating and Maillard reaction to enhance volatile flavor in Chinese marinated chicken. J. Sci. Food Agric. 2017, 97, 823–831. [Google Scholar] [CrossRef]
- Shahidi, F. Flavor of Meat, Meat Products and Seafood; Springer: London, UK, 1998. [Google Scholar]
- Li, H.; Li, X.; Zhang, C.H.; Wang, J.Z.; Tang, C.H.; Chen, L.L. Flavor compounds and sensory profiles of a novel Chinese marinated chicken. J. Sci. Food Agric. 2016, 96, 1618–1626. [Google Scholar] [CrossRef]
- Petricevic, S.; Marusic Radovcic, N.; Lukic, K.; Listes, E.; Medic, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sun, B.; Zheng, F.; Wang, S. Volatile flavor constituents in roasted pork of Mini-pig. Food Chem. 2008, 109, 506–514. [Google Scholar] [CrossRef]
- Wang, W.; Feng, X.; Zhang, D.; Li, B.; Sun, B.; Tian, H.; Liu, Y. Analysis of volatile compounds in Chinese dry-cured hams by comprehensive two-dimensional gas chromatography with high-resolution time-of-flight mass spectrometry. Meat Sci. 2018, 140, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zheng, F.; Chen, H.; Huang, M.; Xie, J.; Feng, C.; Sun, B. Analysis of volatiles in Dezhou Braised Chicken by comprehensive two-dimensional gas chromatography/high resolution-time of flight mass spectrometry. LWT Food Sci. Technol. 2015, 60, 1235–1242. [Google Scholar]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef]
- Feng, T.; Zhuang, H.; Ye, R.; Jin, Z.; Xu, X.; Xie, Z. Analysis of volatile compounds of Mesona Blumes gum/rice extrudates via GC–MS and electronic nose. Sens. Actuators B Chem. 2011, 160, 964–973. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Fonseca, S. Volatile compounds of Celta dry-cured ‘lacon’ as affected by cross-breeding with Duroc and Landrace genotypes. J. Sci. Food Agric. 2014, 94, 2978–2985. [Google Scholar] [CrossRef]
- Gu, S.-Q.; Wang, X.-C.; Tao, N.-P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Liu, L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE–GC–MS, e-nose and sensory evaluation. Food Chem. 2016, 207, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, H.; Ma, C. Aroma-active compounds of Beijing roast duck. Flavour Fragr. J. 2009, 24, 186–191. [Google Scholar] [CrossRef]
- Yu, H.-Z.; Chen, S.-S. Identification of characteristic aroma-active compounds in steamed mangrove crab (Scylla serrata). Food Res. Int. 2010, 43, 2081–2086. [Google Scholar] [CrossRef]
- Tao, N.P.; Wu, R.; Zhou, P.G.; Gu, S.Q.; Wu, W. Characterization of odor-active compounds in cooked meat of farmed obscure puffer (takifugu obscurus) using gas chromatography-mass spectrometry-olfactometry. J. Food Drug Anal. 2014, 22, 431–438. [Google Scholar] [CrossRef]
- Thomas, C.; Mercier, F.; Tournayre, P.; Martin, J.L.; Berdagué, J.-L. Identification and origin of odorous sulfur compounds in cooked ham. Food Chem. 2014, 155, 207–213. [Google Scholar] [CrossRef]
- Mi, S.; Shang, K.; Li, X.; Zhang, C.-H.; Liu, J.-Q.; Huang, D.-Q. Characterization and discrimination of selected China’s domestic pork using an LC-MS-based lipidomics approach. Food Control 2019, 100, 305–314. [Google Scholar] [CrossRef]
- Mi, S.; Shang, K.; Li, X.; Zhang, C.-H.; Liu, J.-Q.; Huang, D.-Q. Characterization and authentication of Taihe black-boned silky fowl (Gallus gallus domesticus Brisson) muscles based on mineral profiling using ICP-MS. Microchem. J. 2019, 144, 26–33. [Google Scholar] [CrossRef]
- Yang, J.; Pan, J.; Zhu, S.; Zou, Y. Application of pca and slda methods for the classification and differentiation of cooked pork from chinese indigenous pig breeds and a hybrid pig breed. Int. J. Food Prop. 2014, 17, 1518–1528. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Iu, B.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J.; Roura, E. Low intramuscular fat (but high in PUFA) content in cooked cured pork ham decreased Maillard reaction volatiles and pleasing aroma attributes. Food Chem. 2016, 196, 76–82. [Google Scholar]
- Liu, J.; Liu, M.; He, C.; Song, H.; Guo, J.; Wang, Y.; Yang, H.; Su, X. A comparative study of aroma-active compounds between dark and milk chocolate: Relationship to sensory perception. J. Sci. Food Agric. 2015, 95, 1362–1372. [Google Scholar] [CrossRef]
- Tanchotikul, U.; Hsieh, C.Y. Analysis of Volatile Flavor Components in Steamed Rangia Clam by Dynamic Headspace Sampling and Simultaneous Distillation and Extraction. J. Food Sci. 2010, 56, 327–331. [Google Scholar] [CrossRef]
- Sanchezpena, C.M.; Luna, G.; Garciagonzalez, D.L.; Aparicio, R. Characterization of French and Spanish dry-cured hams: Influence of the volatiles from the muscles and the subcutaneous fat quantified by SPME-GC. Meat Sci. 2005, 69, 635–645. [Google Scholar] [CrossRef]
- Muriel, E.; Antequera, T.; Petron, M.J.; Andres, A.I.; Ruiz, J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004, 68, 391. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, S.; Fan, G.; Dong, L.; Ren, J.; Zhu, Y. Evaluation of volatile profile of Sichuan dongcai, a traditional salted vegetable, by SPME–GC–MS and E-nose. LWT Food Sci. Technol. 2015, 64, 528–535. [Google Scholar] [CrossRef]
- Buttery, R.G.; Black, D.R.; Guadagni, D.G.; Ling, L.C.; Connolly, G.; Teranishi, R. California bay oil. I. Constituents, odor properties. J. Agric. Food Chem. 1974, 22, 773–777. [Google Scholar] [CrossRef]
- Chao, Y.; Luo, L.; Zhang, H.; Xu, Y.; Yu, L.; Song, H. Common aroma-active components of propolis from 23 regions of China. J. Sci. Food Agric. 2010, 90, 1268. [Google Scholar]
- Fu, X.J.; Xu, S.Y.; Wang, Z. Kinetics of lipid oxidation and off-odor formation in silver carp mince: The effect of lipoxygenase and hemoglobin. Food Res. Int. 2009, 42, 85–90. [Google Scholar] [CrossRef]
- Taylor, A.J.; Mottram, D.S. Composition and odour of volatiles from autoxidised methyl arachidonate. J. Sci. Food Agric. 2010, 50, 407–417. [Google Scholar] [CrossRef]
- Machiels, D.; van Ruth, S.M.; Posthumus, M.A.; Istasse, L. Gas chromatography-olfactometry analysis of the volatile compounds of two commercial Irish beef meats. Talanta 2003, 60, 755–764. [Google Scholar] [CrossRef]
- Jian, Z.; Meng, W.; Xie, J.; Zhao, M.; Li, H.; Liang, J.; Shi, W.; Jie, C. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51. [Google Scholar]
- Zhang, M.; Chen, X.; Hayat, K.; Duhoranimana, E.; Zhang, X.; Xia, S.; Yu, J.; Xing, F. Characterization of odor-active compounds of chicken broth and improved flavor by thermal modulation in electrical stewpots. Food Res. Int. 2018, 109, 72–81. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of fresh watermelon juice aroma characteristics of five varieties based on gas chromatography-olfactometry-mass spectrometry. Food Res. Int. 2018, 107, 119–129. [Google Scholar] [CrossRef]
- Lou, X.W.; Zhang, Y.B.; Sun, Y.Y.; Wang, Y.; Pan, D.D.; Cao, J.X. The change of volatile compounds of two kinds of vinasse-cured ducks during processing. Poult. Sci. 2018, 97, 2607–2617. [Google Scholar] [CrossRef]
- Roldan, M.; Ruiz, J.; Pulgar, J.S.D.; Perezpalacios, T.; Antequera, T. Volatile compound profile of sous-vide cooked lamb loins at different temperature-time combinations. Meat Sci. 2015, 100, 52–57. [Google Scholar] [CrossRef]
- Yang, W.; Yu, J.; Pei, F.; Mariga, A.M.; Ma, N.; Fang, Y.; Hu, Q. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME-GC-MS and electronic nose. Food Chem. 2016, 196, 860–866. [Google Scholar] [CrossRef]
- Gao, L.; Liu, T.; An, X.; Zhang, J.; Ma, X.; Cui, J. Analysis of volatile flavor compounds influencing Chinese-type soy sauces using GC–MS combined with HS-SPME and discrimination with electronic nose. J. Food Sci. Technol. Mysore 2017, 54, 130–143. [Google Scholar] [CrossRef]
- Wu, W.; Tao, N.; Gu, S. Characterization of the key odor-active compounds in steamed meat of Coilia ectenes from Yangtze River by GC–MS–O. Eur. Food Res. Technol. 2014, 238, 237–245. [Google Scholar] [CrossRef]
- Liu, M.; Han, X.; Tu, K.; Pan, L.; Tu, J.; Tang, L.; Liu, P.; Zhan, G.; Zhong, Q.; Xiong, Z. Application of electronic nose in Chinese spirits quality control and flavour assessment. Food Control 2012, 26, 564–570. [Google Scholar] [CrossRef]
- Lee, S.M.; Kwon, G.Y.; Kim, K.O.; Kim, Y.S. Metabolomic approach for determination of key volatile compounds related to beef flavor in glutathione-Maillard reaction products. Anal. Chim. Acta 2011, 703, 204–211. [Google Scholar] [CrossRef]
- Braghieri, A.; Piazzolla, N.; Carlucci, A.; Bragaglio, A.; Napolitano, F. Sensory properties, consumer liking and choice determinants of Lucanian dry cured sausages. Meat Sci. 2016, 111, 122–129. [Google Scholar] [CrossRef]
- Peñaranda, I.; Ma, D.G.; Egea, M.; Díaz, P.; Álvarez, D.; Ma, A.O.; Ma, B.L. Sensory perception of meat from entire male pigs processed by different heating methods. Meat Sci. 2017, 134, 98. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds hexanal, octanal, nonanal, 1,8-cineole, anethole, 2-pentylfuran, heptanal, 2-ethylfuran, eugenol and 1-octen-3-ol are available from the authors. |
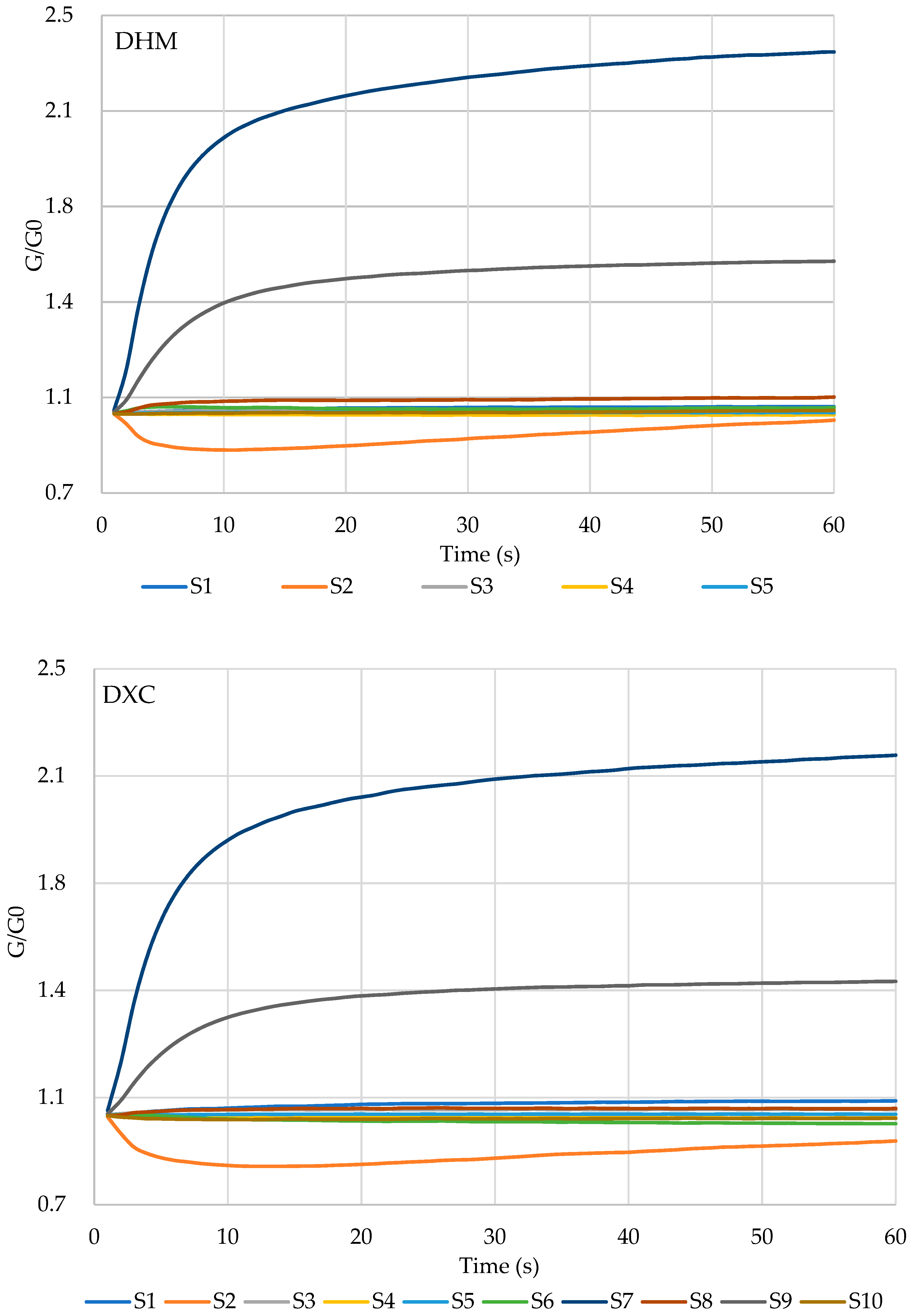
| Code | Compound | Formula | DB-wax e | DB-5 f | Identification | g Odour Descriptions | h Odour Threshold (μg·kg−1) | Relative Concentration (μg·kg−1) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DHM | DXC | HHT | TFH | ||||||||
| Aldehydes (13) | 372.0 ± 9.2a | 322.1 ± 18.7a | 142.5 ± 10.1b | 377.0 ± 3.8a | |||||||
| 1 | 2-Methylbutanal | C5H10O | 902 | 680 | MS, LRI, O | Nutty | 1 | j N.D. | 10.0 ± 1.2 | N.D. | N.D. |
| 2 | 3-Methylbutanal | C5H10O | 906 | i N.A. | MS, LRI, O | Almond, nutty | 1.1 | N.D. | 11.7 ± 1.8 | N.D. | N.D. |
| 3 | Hexanal | C6H12O | 1073 | 800 | MS, LRI, O | Green, grass, | 4 | 81.0 ± 3.5a | 14.4 ± 1.6d | 46.9 ± 4.6b | 35.6 ± 1.2c |
| 4 | Heptanal | C7H14O | 1179 | 901 | MS, LRI, O | Fatty, oily | 3 | 23.9 ± 1.5a | 25.5 ± 3.1a | N.D. | 16.4 ± 2.3b |
| 5 | Octanal | C8H16O | 1284 | 1003 | MS, LRI, O | Orange peel | 0.7 | 33.2 ± 2.3a | 21.2 ± 1.8c | 25.1 ± 2.2b | 31.9 ± 0.2a |
| 6 | Nonanal | C9H18O | 1389 | 1104 | MS, LRI, O | Citrus, fatty | 1 | 166.6 ± 4.6a | 122.4 ± 9.5c | 68.6 ± 4.9d | 135.9 ± 3.2b |
| 7 | 2-Octenal isomer | C8H14O | 1426 | N.A. | MS, LRI, O | Fatty, green | 3 | 3.7 ± 1.1 | N.D. | N.D. | N.D. |
| 8 | Benzaldehyde | C7H6O | 1520 | 961 | MS, LRI, O | Bitter almond | 350 | 29.2 ± 1.8c | 102.9 ± 1.3b | N.D. | 156.2 ± 1.7a |
| 9 | 2-Nonenal isomer | C9H16O | 1533 | N.A. | MS, LRI, O | Fatty, cucumber | 0.19 | 4.2 ± 1.1 | N.D. | N.D. | N.D. |
| 10 | Anisaldehyde | C8H8O2 | 1683 | N.A. | MS, LRI, O | Mint, sweet | 27 | 13.8 ± 2.1a | 12.7 ± 2.0a | N.D. | N.D. |
| 11 | 2,4-Decadienal isomer | C10H16O | 1719 | N.A. | MS, LRI, O | Fatty, deep-fried | 0.07 | 15.8 ± 1.2 | N.D. | N.D. | N.D. |
| 12 | Pentadecanal | C15H30O | N.A. | 1712 | MS, LRI | Fresh | N.A. | N.D. | 0.2 ± 0.1 | N.D. | N.D. |
| 13 | Hexadecanal | C16H30O | N.A. | 1793 | MS, LRI | Cardboard | N.A. | 0.5 ± 0.1c | 1.1 ± 0.1b | 1.8 ± 0.3a | 1.1 ± 0.3b |
| Alcohols (9) | 127.4 ± 0.6c | 107.5 ± 7.4d | 249.8 ± 13.4a | 202.8 ± 15.2b | |||||||
| 14 | 1,8-Cineole | C10H18O | 1204 | 1034 | MS, LRI, O | Mint, sweet | 1 | 38.1 ± 1.3c | 36.4 ± 0.3c | 113.3 ± 12.1b | 133.3 ± 13.4a |
| 15 | 1-Hexanol | C6H14O | 1349 | N.A. | MS, LRI | Flower, green | 2500 | N.D. | N.D. | 18.9 ± 2.6 | N.D. |
| 16 | 1-Octen-3-ol | C8H16O | 1445 | 981 | MS, LRI, O | Mushroom | 2 | 10.6 ± 0.3b | N.D. | 16.6 ± 1.8a | 14.3 ± 1.3a |
| 17 | cis-4-Thujanol | C10H18O | 1462 | 1071 | MS, LRI | Balsamic | N.A. | 15.7 ± 0.2 | N.D. | N.D. | N.D. |
| 18 | Linalool | C10H18O | 1541 | 1101 | MS, LRI, O | Aniseed, citrus | 6 | 13.4 ± 0.7b | 9.7 ± 1.8c | 18.9 ± 1.5a | 12.7 ± 1.3b |
| 19 | 1-Octanol | C8H18O | 1553 | N.A. | MS, LRI, O | Herbal, green | 110 | N.D. | N.D. | 9.2 ± 0.7 | N.D. |
| 20 | Terpinen-4-ol | C10H18O | 1601 | 1183 | MS, LRI, O | Musty, terpene | 340 | 38.4 ± 0.6b | 31.4 ± 4.3bc | 72.9 ± 3.9a | 34.1 ± 1.0c |
| 21 | α-Terpineol | C10H18O | 1695 | 1196 | MS, LRI, O | Oil, anise, mint | 350 | 11.1 ± 0a | 5.7 ± 0.2c | N.D. | 8.4 ± 0.8b |
| 22 | 2-Phenylethanol | C8H10O | 1908 | N.A. | MS, LRI | Perfumy, rose | 1100 | N.D. | 24.3 ± 1.2 | N.D. | N.D. |
| Ketones (5) | 0.6 ± 0.1d | 9.6 ± 1.6c | 40.7 ± 4.5a | 15.0 ± 1.2b | |||||||
| 23 | 2-Butanone | C4H8O | 885 | N.A. | MS, LRI | Ethereal, cheesy | 35400 | N.D. | N.D. | N.D. | 11.7 ± 0.9 |
| 24 | 2-Heptanone | C7H14O | 1179 | 890 | MS, LRI, O | Blue cheese | 140 | N.D. | N.D. | 34.9 ± 3.6 | N.D. |
| 25 | 6-Methyl-5-hepten-2-one | C8H14O | N.A. | 987 | MS, LRI, O | Pepper, rubber | 50 | 0.6 ± 0.1 | N.D. | N.D. | N.D. |
| 26 | 2-Nonanone | C9H18O | 1386 | N.A. | MS, LRI, O | Hot milk, green | 200 | N.D. | N.D. | 5.9 ± 1.0 | N.D. |
| 27 | Piperitone | C10H16O | 1583 | 1260 | MS, LRI, O | Mint, fresh | N.A. | N.D. | 9.6 ± 1.6 | N.D. | 3.4 ± 0.2 |
| Esters (3) | 47.6 ± 0.8b | 92.9 ± 4.7a | 16.2 ± 3.9c | 10.7 ± 1.6c | |||||||
| 28 | Ethyl acetate | C4H8O2 | 869 | N.A. | MS, LRI | Fruity, sweet | 5 | 15.5 ± 0.2b | 92.9 ± 4.7a | N.D. | 10.7 ± 1.6c |
| 29 | Ethyl hexanoate | C8H16O2 | 1229 | N.A. | MS, LRI, O | Apple, sweet | 30 | 32.1 ± 0.6 | N.D. | N.D. | N.D. |
| 30 | Terpinyl acetate | C12H20O2 | 1695 | 1354 | MS, LRI | Fruity, mint | N.A. | N.D. | N.D. | 16.2 ± 3.9 | N.D. |
| Hydrocarbons (14) | 34.8 ± 1.8c | 99.6 ± 3.2b | 591.0 ± 18.4a | 46.9 ± 4.7c | |||||||
| 31 | Decane | C10H22 | 993 | 1206 | MS, LRI | Irritant | 741 | N.D. | N.D. | 20.8 ± 2.4 | N.D. |
| 32 | Toluene | C7H8 | 1031 | 765 | MS, LRI, O | Rubber, pungent | 1550 | 10.0 ± 1.0c | 16.7 ± 1.4b | N.D. | 23.9 ± 2.8a |
| 33 | β-Pinene | C10H16 | N.A. | 975 | MS, LRI | Benzene-like | 140 | N.D. | N.D. | 22.6 ± 0.9 | N.D. |
| 34 | Ethylbenzene | C8H10 | 1122 | N.A. | MS, LRI, O | Ethereal, floral | 2205.3 | N.D. | N.D. | 5.0 ± 1.5 | N.D. |
| 35 | p-Xylene | C8H10 | 1133 | 870 | MS, LRI, | N.A. | 490 | N.D. | 6.6 ± 0.2b | 9.5 ± 0.4a | N.D. |
| 36 | 3-Carene | C10H16 | 1144 | 1012 | MS, LRI, O | Resin, lemon | 0.4 | N.D. | N.D. | 45.2 ± 3.3 | N.D. |
| 37 | α-Terpinene | C10H16 | 1172 | 1018 | MS, LRI | N.A. | 200 | 11.3 ± 0.4b | 7.6 ± 1.7c | 16.8 ± 3.7a | N.D. |
| 38 | Dodecane | C12H26 | 1076 | 1200 | MS, LRI | Irritant | 2040 | N.D. | 17.7 ± 0.6a | N.D. | 16.4 ± 1.1a |
| 39 | d-Limonene | C10H16 | 1186 | 1031 | MS, LRI, O | Fresh | 10 | N.D. | 16.8 ± 1.9b | 263.9 ± 13.6a | N.D. |
| 40 | β-Phellandrene | C10H16 | 1202 | N.A. | MS, LRI, O | Turpentine, mint | 8 | N.D. | 11.8 ± 2.2b | 95.7 ± 6.4a | N.D. |
| 41 | trans-β-Ocimene | C10H16 | 1231 | 1039 | MS, LRI | Sweet, herb | N.A. | N.D. | N.D. | 67.5 ± 4.4 | N.D. |
| 42 | p-Cymene | C10H14 | 1265 | 1027 | MS, LRI, O | Fruity, herbal | 13 | 12.3 ± 0.5b | 11.5 ± 1.3b | 36.4 ± 6.1a | 6.6 ± 1.7b |
| 43 | 1,3,5-Trimethylbenzene | C9H12 | 1277 | N.A. | MS, LRI | Peculiar | 229 | N.D. | 8.4 ± 1.8 | N.D. | N.D. |
| 44 | Naphthalene | C10H8 | 1741 | N.A. | MS, LRI, O | Camphoric | 60 | 1.2 ± 0.1c | 4.6 ± 1.0b | 7.6 ± 0.8a | N.D. |
| Ethers (2) | 529.3 ± 24.6a | 127.9 ± 3.1c | 398.5 ± 2.3b | 93.9 ± 6.9d | |||||||
| 45 | Estragole | C10H12O | 1667 | 1201 | MS, LRI, O | Liquorice-like | 6 | 105.3 ± 1.6a | 5.1 ± 0.4c | 21.8 ± 1.5b | 4.6 ± 0.6c |
| 46 | Anethol | C10H12O | 1824 | 1290 | MS, LRI, O | Aniseed-like | 15 | 424.0 ± 22.9a | 122.8 ± 3.5c | 376.8 ± 3.7b | 89.2 ± 6.7d |
| Phenols (2) | 10.6 ± 2.6c | 32.1 ± 1.1b | 117.8 ± 11.3a | N.D. | |||||||
| 47 | Eugenol | C10H12O2 | 2164 | 1364 | MS, LRI, O | Spicy, clove | 7.1 | 6.6 ± 1.5c | 32.1 ± 1.1b | 117.8 ± 11.3a | N.D. |
| 48 | Isoeugenol | C10H12O2 | 2255 | N.A. | MS, LRI | Floral, spicy | N.A. | 4.0 ± 1.1 | N.D. | N.D. | N.D. |
| Furans (7) | 71.3 ± 2.9d | 269.1 ± 13.3b | 230.0 ± 13.6a | 63.3 ± 2.8c | |||||||
| 49 | 2-Methylfuran | C5H6O | 847 | N.A. | MS, LRI | Chocolate | 3500 | N.D. | 21.0 ± 1.4a | N.D. | 12.2 ± 2.5b |
| 50 | 2-Ethylfuran | C6H8O | 952 | N.A. | MS, LRI, O | Rubber, pungent | 2.3 | N.D. | N.D. | 11.9 ± 2.5 | N.D. |
| 51 | 2-Pentylfuran | C9H14O | 1225 | 992 | MS, LRI, O | Fruity, sweet | 6 | 21.0 ± 2.7d | 28.3 ± 5.8c | 178.2 ± 3.7a | 40.2 ± 1.2b |
| 52 | Furfural | C5H4O2 | 1457 | 829 | MS, LRI, O | Almond, sweet | 3000 | 26.6 ± 0.4b | 62.8 ± 3.4a | 16.7 ± 4.4c | 5.1 ± 0.3d |
| 53 | 2-Acetylfuran | C6H6O2 | 1500 | 912 | MS, LRI, O | Sweet, smoky | 80000 | 12.6 ± 0b | 30.7 ± 1.5a | 12.7 ± 2.9b | N.D. |
| 54 | 5-Methylfurfural | C6H6O2 | 1568 | N.A. | MS, LRI, O | Almond, sweet | 1100 | 4.6 ± 0.4b | 92.9 ± 2.5a | N.D. | N.D. |
| 55 | 2-Furanmethanol | C5H6O2 | 1652 | 863 | MS, LRI, O | Sweet, honey | 2000 | 6.5 ± 0.2bc | 33.4 ± 3.7a | 11.5 ± 3.9b | 5.8 ± 0.6c |
| N-containing compounds (3) | 17.8 ± 1.8a | 8.5 ± 0.4b | N.D. | 5.5 ± 0.3c | |||||||
| 56 | 2-Methylpyrazine | C5H6N2 | 1262 | N.A. | MS, LRI, O | Popcorn | 250 | N.D. | N.D. | N.D. | 5.5 ± 0.3 |
| 57 | 1-Vinylimidazole | C5H6N2 | 1263 | N.A. | MS, LRI | N.A. | N.A. | N.D. | 8.5 ± 0.4 | N.D. | N.D. |
| 58 | 2-Acetylpyrazine | C6H6N2O | 1624 | N.A. | MS, LRI, O | Nutty, roast | 62 | 17.8 ± 1.8 | N.D. | N.D. | N.D. |
| S-containing compounds (4) | 12.9 ± 1.2a | 4.3 ± 1.2c | 4.2 ± 1.0c | 7.5 ± 0.8b | |||||||
| 59 | 3-Methylthiophene | C5H6S | 1083 | N.A. | MS, LRI, O | Fatty, wine | 5000 | N.D. | 4.3 ± 1.2b | N.D. | 7.5 ± 0.8a |
| 60 | 2-Methylthiophene | C5H6S | 1075 | N.A. | MS, LRI, O | Gasoline, green | 3000 | N.D. | N.D. | 4.2 ± 1.0 | N.D. |
| 61 | 2-Acetylthiazole | C5H5NOS | 1644 | N.A. | MS, LRI, O | Caramel, sweet | 10 | 10.7 ± 1.5 | N.D. | N.D. | N.D. |
| 62 | 3-(Methylthio)propanol | C4H10OS | 1712 | N.A. | MS, LRI | Sweet, potato | 123 | 2.2 ± 0.8 | N.D. | N.D. | N.D. |
| Total | 1224.2 ± 34.7b | 1073.4 ± 35.3c | 1791.8 ± 64.8a | 822.7 ± 26.2d | |||||||
| Code | Compounds | Odour Activity Values (OAVs) | p Value | VIP Value | |||
|---|---|---|---|---|---|---|---|
| DHM | DXC | HHT | TFH | ||||
| 1 | 2-Methylbutanal | 0.0b | 10.0 ± 1.2a | 0.0b | 0.0b | 0.000 | 0.827 |
| 2 | 3-Methylbutanal | 0.0b | 10.6 ± 1.6a | 0.0b | 0.0b | 0.000 | 0.824 |
| 3 | Hexanal | 20.3 ± 0.9a | 3.6 ± 0.4d | 11.7 ± 1.2b | 8.9 ± 0.3c | 0.000 | 0.817 |
| 4 | Heptanal | 8.0 ± 0.5a | 8.5 ± 1.0a | 0.0c | 5.5 ± 0.8b | 0.000 | 1.068 |
| 5 | Octanal | 47.5 ± 3.2a | 30.3 ± 2.6c | 35.9 ± 3.1b | 45.6 ± 0.2a | 0.000 | 0.867 |
| 6 | Nonanal | 166.6 ± 4.6a | 122.4 ± 9.5c | 68.6 ± 4.9d | 135.9 ± 3.2b | 0.000 | 1.078 |
| 7 | 2-Octenal isomer | 1.2 ± 0.4a | 0.0b | 0.0b | 0.0b | 0.000 | 0.946 |
| 9 | 2-Nonenal isomer | 22.2 ± 5.7a | 0.0b | 0.0b | 0.0b | 0.000 | 0.956 |
| 11 | 2,4-Decadienal isomer | 225.5 ± 17.7a | 0.0b | 0.0b | 0.0b | 0.000 | 0.980 |
| 14 | 1,8-Cineole | 38.1 ± 1.3c | 36.4 ± 0.3c | 113.3 ± 12.1b | 133.3 ± 13.4a | 0.000 | 0.954 |
| 15 | 1-Octen-3-ol | 5.3 ± 0.2c | 0.0d | 8.3 ± 0.9a | 7.2 ± 0.7b | 0.000 | 0.943 |
| 16 | Linalool | 2.2 ± 0.1b | 1.6 ± 0.3c | 3.1 ± 0.2a | 2.1 ± 0.2b | 0.000 | 0.978 |
| 28 | Ethyl acetate | 3.1 ± 0b | 18.6 ± 0.9a | 0.0d | 2.1 ± 0.3c | 0.000 | 0.892 |
| 29 | Ethyl hexanoate | 1.1 ± 0.0a | 0.0b | 0.0b | 0.0b | 0.000 | 0.983 |
| 36 | 3-Carene | 0.0b | 0.0b | 113.0 ± 8.3a | 0.0b | 0.000 | 1.078 |
| 39 | d-Limonene | 0.0c | 1.7 ± 0.2b | 26.4 ± 1.4a | 0.0c | 0.000 | 1.082 |
| 40 | β-Phellandrene | 0.0c | 1.5 ± 0.3b | 12.0 ± 0.8a | 0.0c | 0.000 | 1.081 |
| 42 | p-Cymene | 0.9 ± 0.0b | 0.9 ± 0.1b | 2.8 ± 0.5a | 0.5 ± 0.1b | 0.000 | 1.039 |
| 45 | Estragole | 17.5 ± 0.3a | 0.9 ± 0.1c | 3.6 ± 0.2b | 0.8 ± 0.1c | 0.000 | 0.934 |
| 46 | Anethol | 28.3 ± 1.5a | 8.2 ± 0.2c | 25.1 ± 0.2b | 5.9 ± 0.4d | 0.000 | 0.791 |
| 47 | Eugenol | 0.9 ± 0.2c | 4.5 ± 0.2b | 16.6 ± 1.6a | 0.0d | 0.000 | 1.073 |
| 50 | 2-Ethylfuran | 0.0b | 0.0b | 5.2 ± 1.1a | 0.0b | 0.000 | 1.060 |
| 51 | 2-Pentylfuran | 3.5 ± 0.5d | 4.7 ± 0.1c | 29.7 ± 0.6a | 6.7 ± 0.2b | 0.000 | 1.085 |
| 61 | 2-Acetylthiazole | 1.1 ± 0.2a | 0.0b | 0.0b | 0.0b | 0.000 | 0.974 |
| Products | Ingredients |
|---|---|
| DHM | Pork hock, salt, soy sauce, white granulated sugar, flavour liquor, soy protein, monosodium glutamate. |
| DXC | Pork hock, salt, soy sauce, white granulated sugar, glucose, rice wine, soy protein, spices, monosodium glutamate, pork seasoning. |
| HHT | Pork hock, salt, soy sauce, white granulated sugar, glucose, soy protein, spices. |
| TFH | Pork hock, salt, sugar, baijiu, spices. |
| Odour Attributes | Definitions | References (Intensity) |
|---|---|---|
| Fatty | The smell associated with lard oil | Lard oil at 25 °C (6.0) |
| Meaty | The smell associated with cooked pork | 20.0 g of defatted pork in 60.0 mL of water was boiled for 1 h (8.0) |
| Soy sauce | The smell associated with soy sauce | 3.0 g of soy sauce in 50.0 mL of water (7.0) |
| Fruity | The smell associated with fresh fruit | Newly cut orange peel or apple peel (8.0) |
| Caramel | The smell associated with burning white sugar | 5.0 g of burning white sugar in 50.0 mL water (6.0) |
| Roasted | The smell associated with roasted pork | 1.0 kg pork was roasted by charcoal fires for 1 h (6.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Mi, S.; Zhang, C.-H.; Li, J.; Song, H.-L.; Fauconnier, M.-L.; Tyteca, E. Characterization and Discrimination of Chinese Marinated Pork Hocks by Volatile Compound Profiling Using Solid Phase Microextraction Gas Chromatography-Mass Spectrometry/Olfactometry, Electronic Nose and Chemometrics. Molecules 2019, 24, 1385. https://doi.org/10.3390/molecules24071385
Han D, Mi S, Zhang C-H, Li J, Song H-L, Fauconnier M-L, Tyteca E. Characterization and Discrimination of Chinese Marinated Pork Hocks by Volatile Compound Profiling Using Solid Phase Microextraction Gas Chromatography-Mass Spectrometry/Olfactometry, Electronic Nose and Chemometrics. Molecules. 2019; 24(7):1385. https://doi.org/10.3390/molecules24071385
Chicago/Turabian StyleHan, Dong, Si Mi, Chun-Hui Zhang, Juan Li, Huan-Lu Song, Marie-Laure Fauconnier, and Eva Tyteca. 2019. "Characterization and Discrimination of Chinese Marinated Pork Hocks by Volatile Compound Profiling Using Solid Phase Microextraction Gas Chromatography-Mass Spectrometry/Olfactometry, Electronic Nose and Chemometrics" Molecules 24, no. 7: 1385. https://doi.org/10.3390/molecules24071385
APA StyleHan, D., Mi, S., Zhang, C.-H., Li, J., Song, H.-L., Fauconnier, M.-L., & Tyteca, E. (2019). Characterization and Discrimination of Chinese Marinated Pork Hocks by Volatile Compound Profiling Using Solid Phase Microextraction Gas Chromatography-Mass Spectrometry/Olfactometry, Electronic Nose and Chemometrics. Molecules, 24(7), 1385. https://doi.org/10.3390/molecules24071385