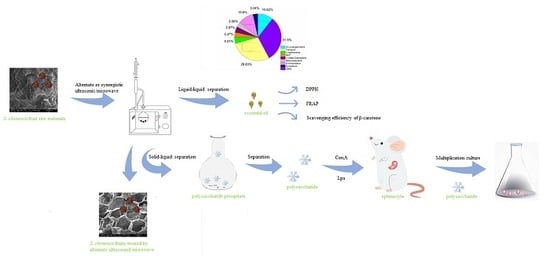
Alternate Ultrasound/Microwave Digestion for Deep Eutectic Hydro-distillation Extraction of Essential Oil and Polysaccharide from Schisandra chinensis (Turcz.) Baill
Abstract
:1. Introduction
2. Results and Discussion
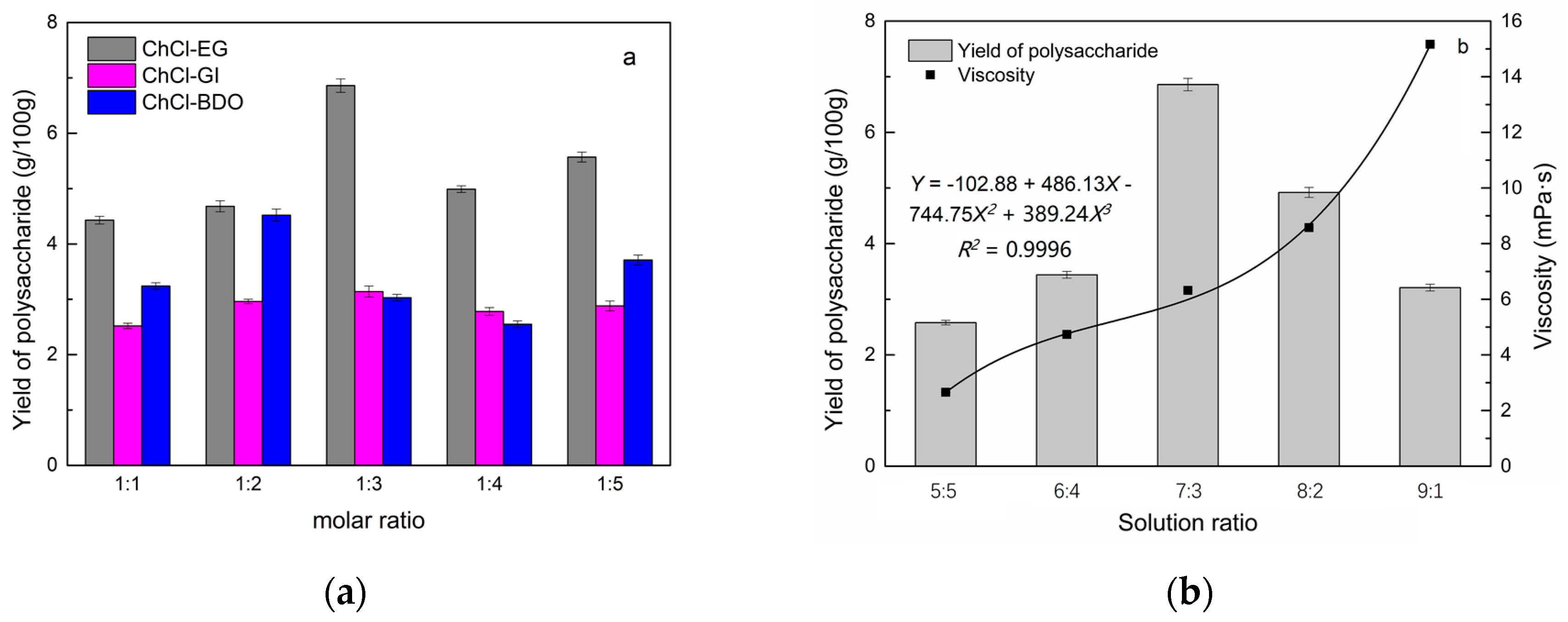
2.1. Screening of DES
2.2. Single Factor Analysis of Polysaccharide Extraction
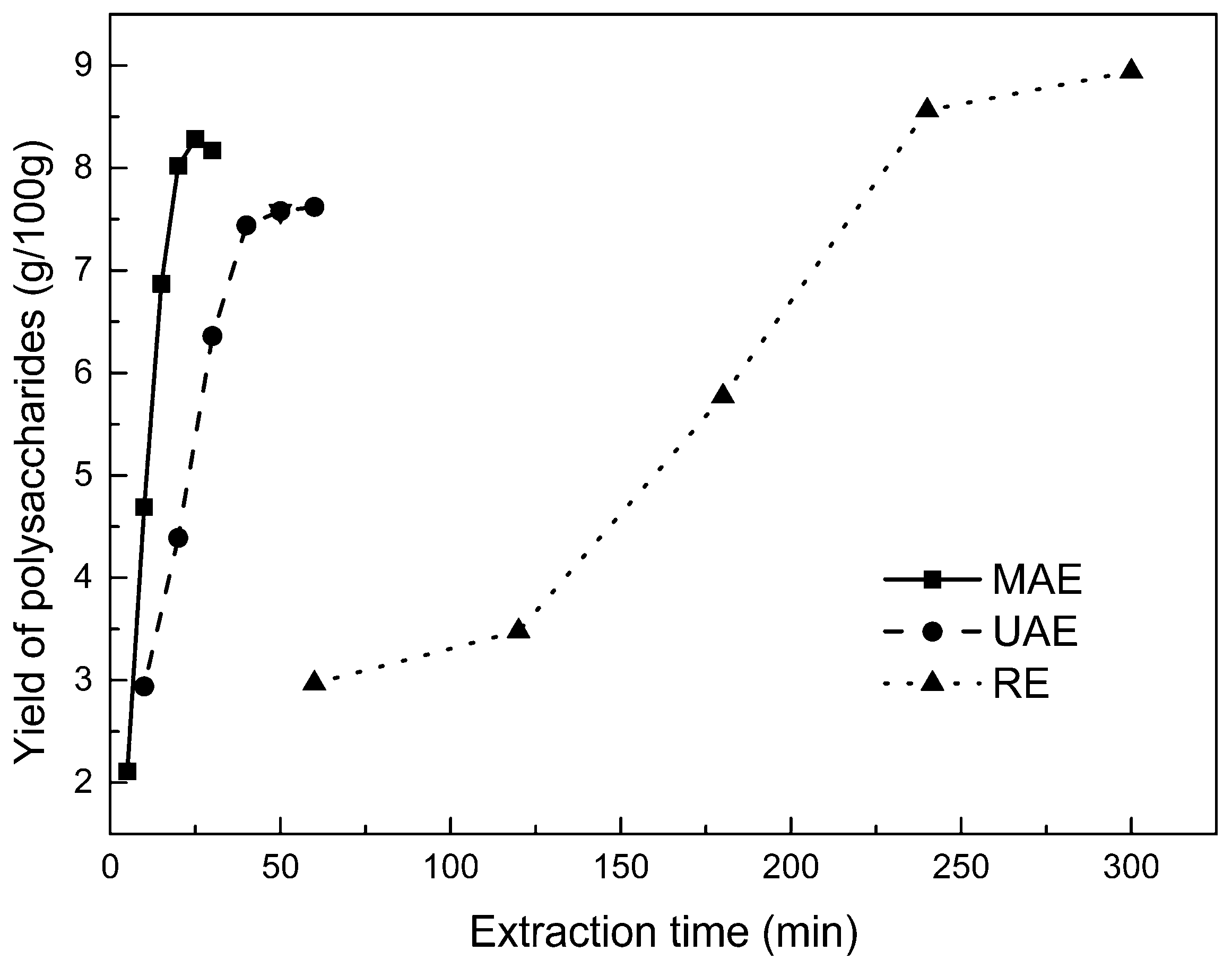
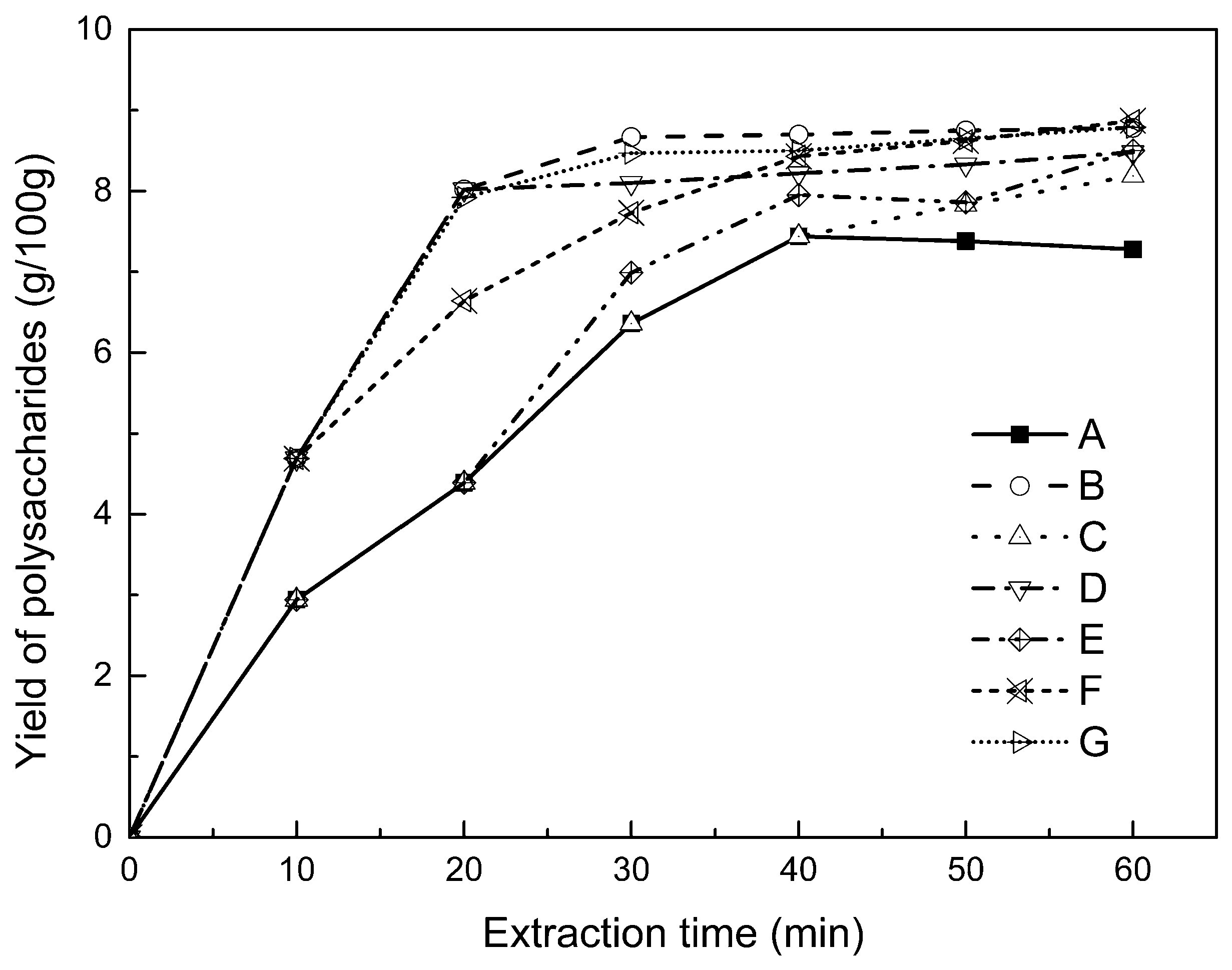
2.2.1. Kinetic Study of Polysaccharide Extraction
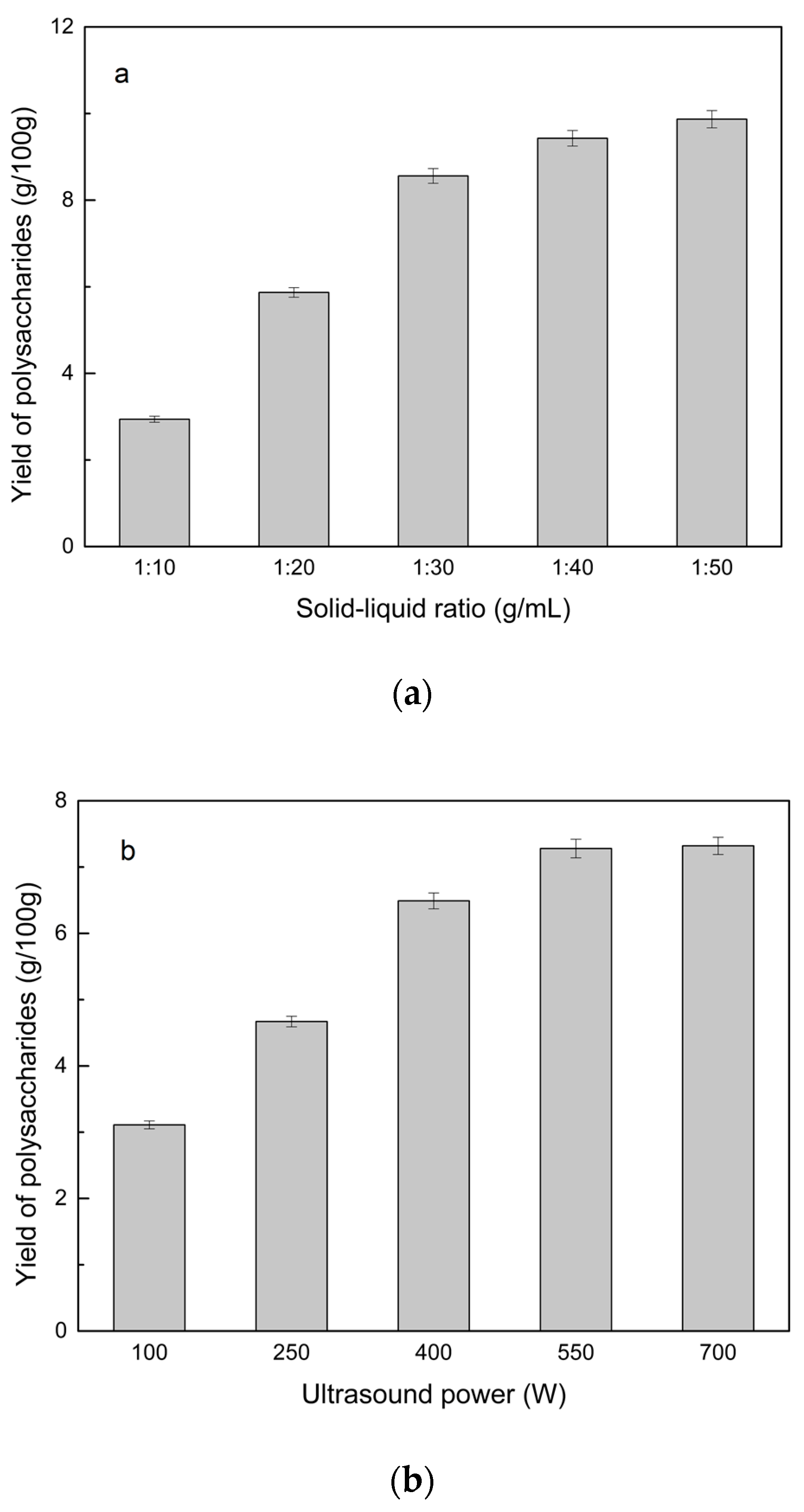
2.2.2. Solid–Liquid Ratio for Extracting Polysaccharides
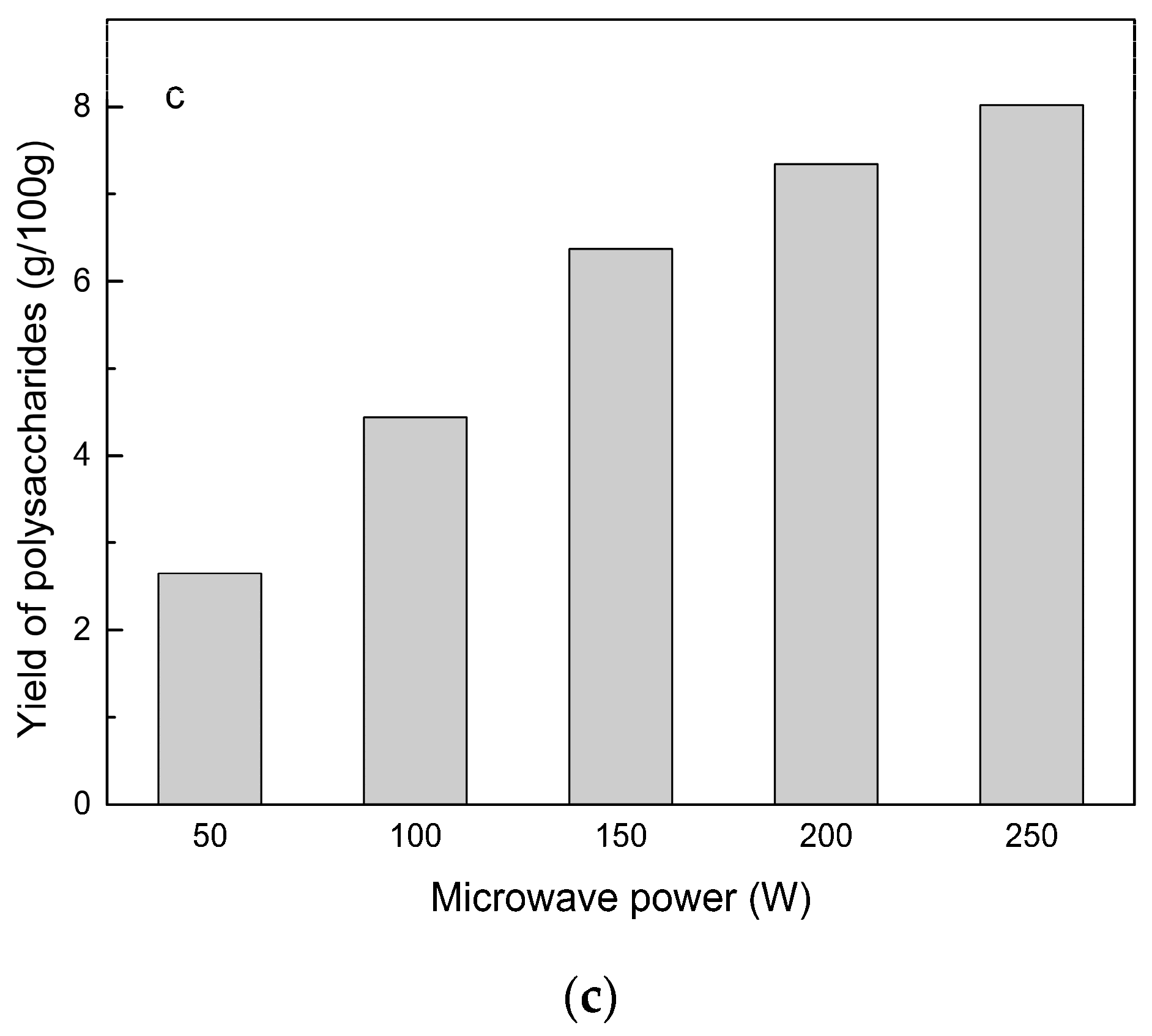
2.2.3. Power of Ultrasound and Microwave Treatments for Polysaccharide Extraction
2.3. Optimization Extraction Parameters of Polysaccharides by Response Surface Method (RSM)
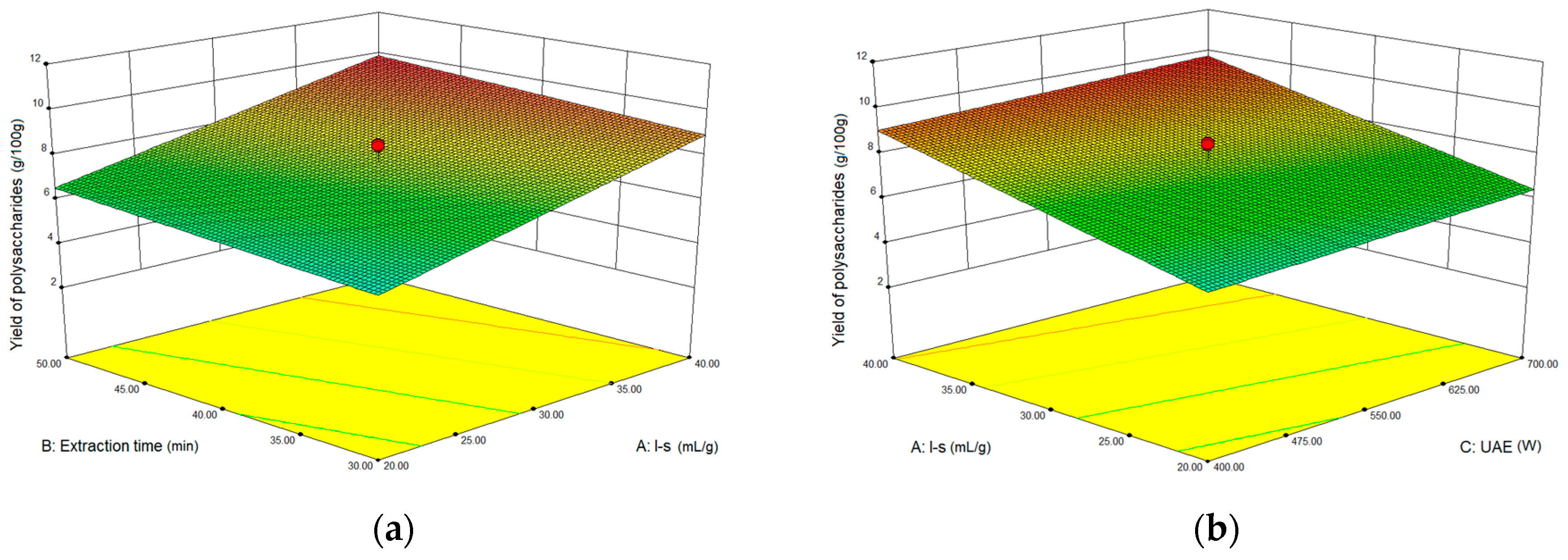
2.3.1. Optimization Parameters for Ultrasound-assisted Extraction (UAE)
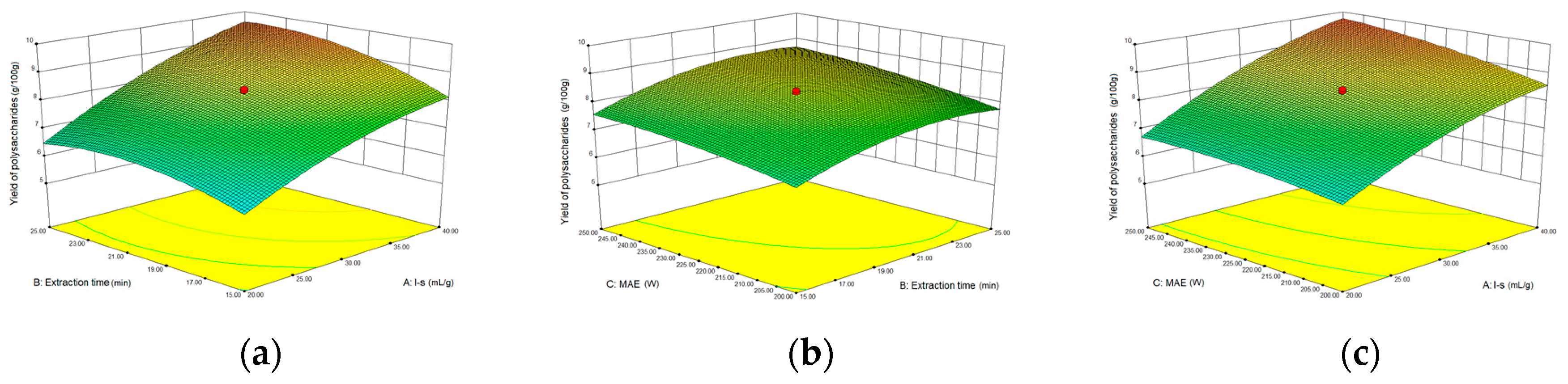
2.3.2. Optimization Parameters for Microwave-assisted Extraction (MAE)
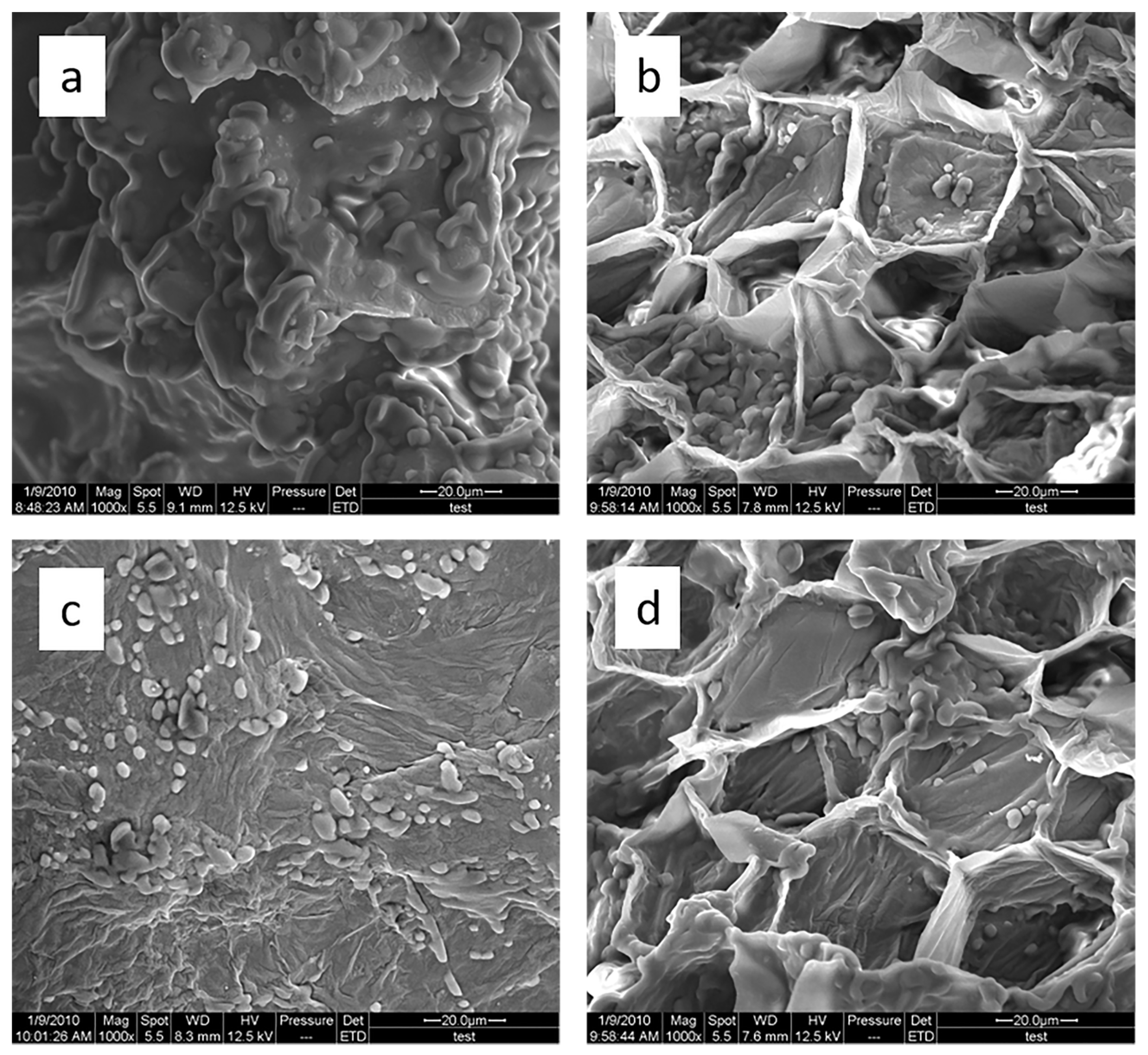
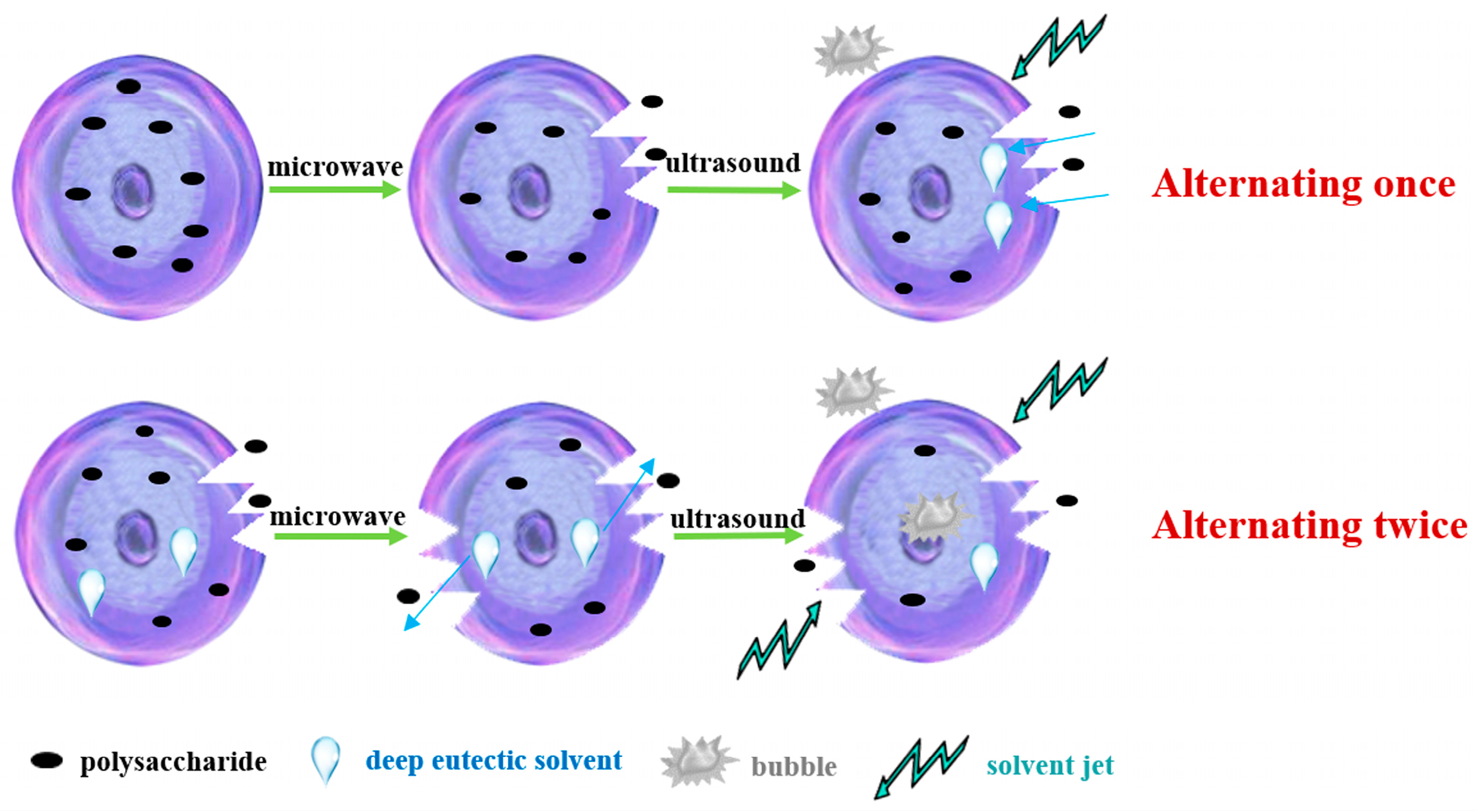
2.4. Alternate Ultrasound/Microwave Extraction Design
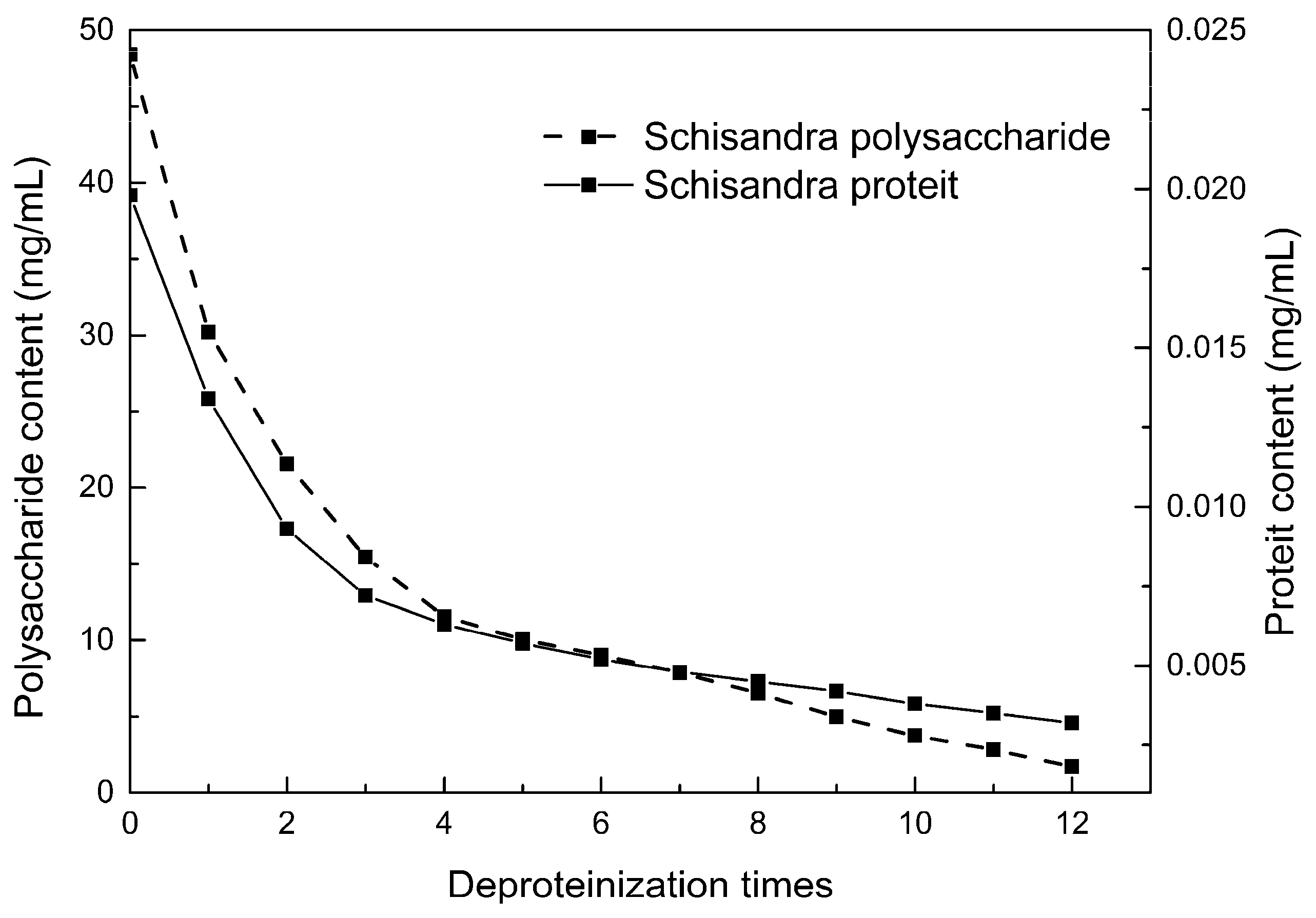
2.5. Polysaccharide Deproteinization
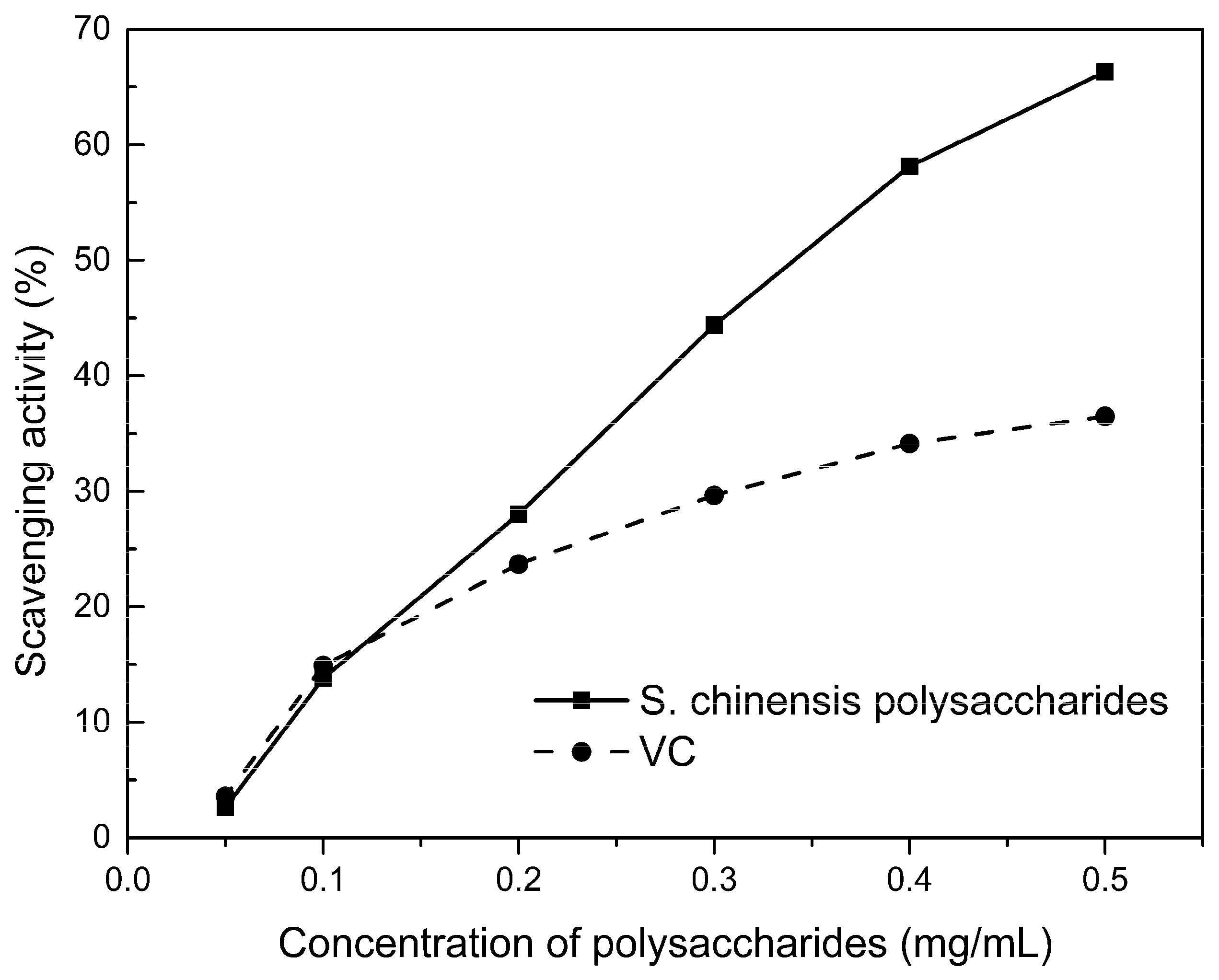
2.6. Free Radical Scavenging Activity of Polysaccharides
2.7. In vitro MTT Activity Test of Polysaccharides
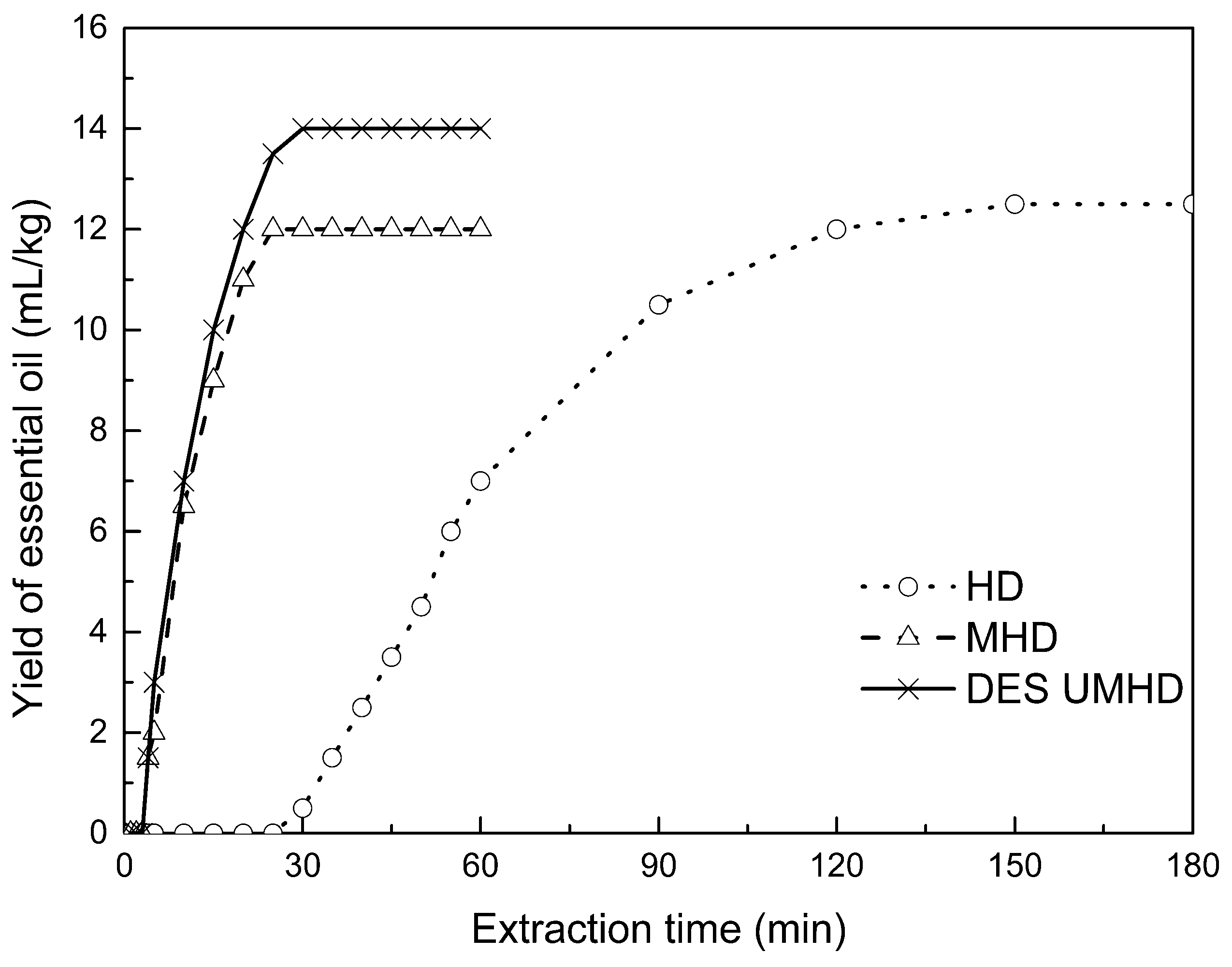
2.8. Kinetic Study of Essential Oils
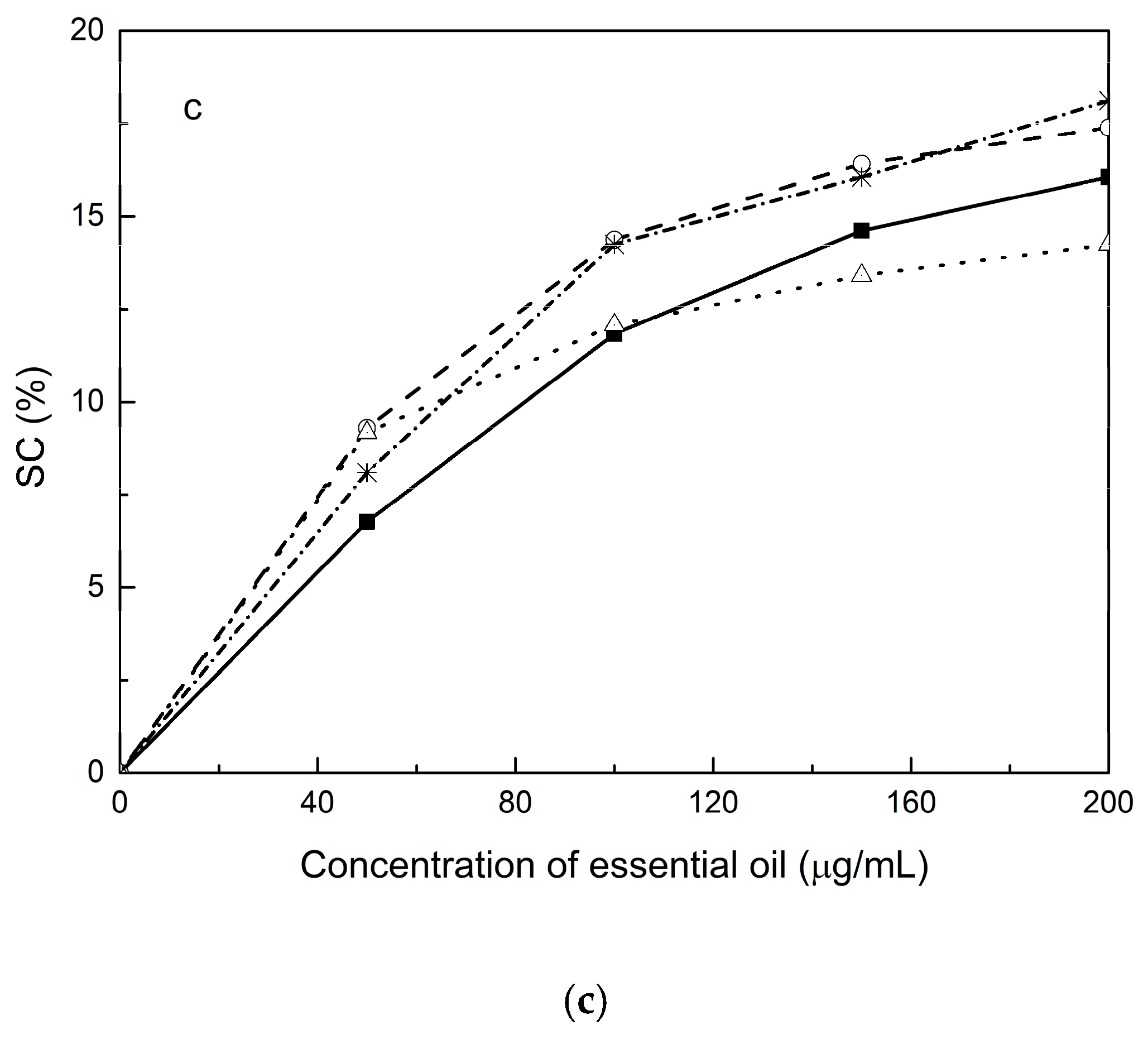
2.9. Antioxidant Analysis of Essential Oils
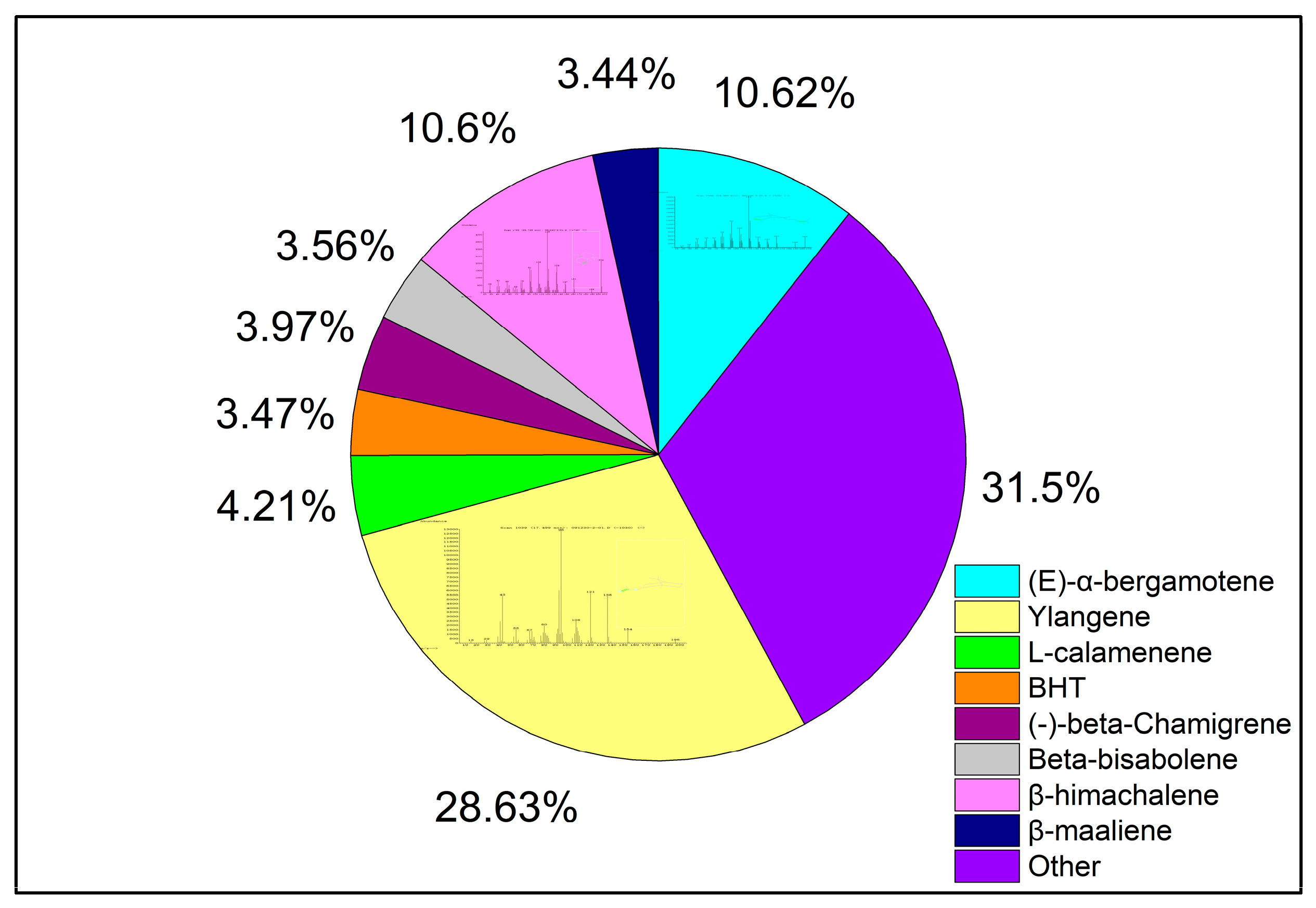
2.10. Chemical Composition of S. chinensis Essential Oil by Different Extraction Methods
3. Materials and Methods
3.1. Raw Materials and Chemical Reagents
3.2. Preparation of DES
3.3. Methods
3.3.1. Polysaccharide Extraction Methods
Determination Method of Polysaccharide
Determination Method of Protein
Compared Extraction Method of Polysaccharides
Ultrasound-assisted Extraction (UE) of Polysaccharides
Microwave-assisted Extraction (ME) of Polysaccharides
Alternate Ultrasound/Microwave Extraction of Polysaccharides
RSM Optimization Parameters of Polysaccharides
3.3.2. Sevag Deproteinization Method for Polysaccharides
3.3.3. Hydroxyl Free Radical Scavenging Method for Polysaccharides
3.3.4. In vitro MTT Activity Test Method for Polysaccharides
3.3.5. Essential Oil Extraction Methods
Hydro-distillation (HD) Extraction of Essential Oils
Microwave-assisted Hydro-distillation Extraction (MHD) of Essential Oils
Ultrasound/Microwave-assisted Hydro-distillation (UMHD) Extraction of Essential Oils
3.3.6. Chemical Composition Analysis of Essential Oil
3.3.7. Scanning Electron Microscopy (SEM)
3.3.8. Antioxidant Analysis Methods for Essential Oils
Free Radical Scavenging Activity
Ferric Reducing/Antioxidant Power (FRAP) Assay
Scavenging efficiency of β-carotene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeong, K.M.; Yang, M.; Jin, Y.; Kim, E.M.; Ko, J.; Lee, J. Identification of major flavone C-glycosides and their optimized extraction from Cymbidium kanran using deep eutectic solvents. Molecules 2017, 22, 2006. [Google Scholar] [CrossRef]
- Cao, Q.; Li, J.H.; Xia, Y.; Li, W.; Luo, S.; Ma, C.; Liu, S.X. Green Extraction of Six Phenolic Compounds from Rattan (Calamoideae faberii) with Deep Eutectic Solvent by Homogenate-Assisted Vacuum-Cavitation Method. Molecules 2019, 24, 113. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Saravana, P.S.; Ho, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J.; Chun, B.S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohyd. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef]
- Sun, J.X.; Mei, Z.X.; Tang, Y.J.; Ding, L.J.; Jiang, G.C.; Zhang, C.; Sun, A.D.; Bai, W.B. Stability, Antioxidant capacity and degradation kinetics of pelargonidin-3-glucoside exposed to ultrasound power at low temperature. Molecules 2016, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Vauchel, P.; Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of ultrasound-assisted extraction of polyphenols from chicory grounds under different operational conditions. J. Clean. Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]
- Otu, P.N.Y.; Jiang, H.N.; Zhou, C.S.; Yang, H.P. Sorghum Bicolor L. leaf sheath polysaccharides: Dual frequency ultrasound-assisted extraction and desalination. Ind. Crop. Prod. 2018, 126, 368–379. [Google Scholar] [CrossRef]
- Ma, C.H.; Yang, L.; Wang, W.J.; Yang, F.J.; Zhao, C.J.; Zu, Y.G. Extraction of dihydroquercetin from Larix gmelinii with ultrasound-assisted and microwave-assisted alternant digestion. Int. J. Mol. Sci. 2012, 13, 8789–8804. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, X.; Jing, C.L.; Zou, P.; Zhang, C.S.; Li, Y.Q. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohyd. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Albalawi, A.H.; El-Sayed, W.S.; Aljuhani, A.; Almutairi, S.M.; Rezki, N.; Aouad, M.R.; Messali, M. Microwave-assisted synthesis of some potential bioactive imidazolium-based room-temperature ionic liquids. Molecules 2018, 23, 1727. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Deng, B.Q.; Li, S.L.; Zou, Z.R. Optimization of solvent-free microwave assisted extraction of essential oil from Cinnamomum camphora leaves. Ind. Crop. Prod. 2018, 124, 353–362. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Raone, A.; Cassanelli, A.; Scheggi, S.; Rauggi, R.; Danielli, B.; Montis, M.G.D. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience 2007, 146, 1734–1742. [Google Scholar] [CrossRef]
- Ma, C.H.; Yang, L.; Zu, Y.G.; Liu, T.T. Optimization of conditions of solvent-free microwave extraction and study on antioxidant capacity of essential oil from Schisandra chinensis (Turcz.) Baill. Food. Chem. 2012, 134, 2532–2539. [Google Scholar] [CrossRef]
- Zeng, H.L.; Zhang, Y.; Lin, S.; Jian, Y.Y.; Miao, S.; Zheng, B.D. Ultrasonic–microwave synergistic extraction (UMSE) and molecular weight distribution of polysaccharides from Fortunella margarita (Lour.) Swingle. Sep. Purif. Technol. 2015, 144, 97–106. [Google Scholar] [CrossRef]
- Yue, C.J.; Chen, J.; Hou, R.R.; Tian, W.J.; Liu, K.H.; Wang, D.Y.; Lu, Y.; Liu, J.G.; Wu, Y.; Hu, Y.L. The antioxidant action and mechanism of selenizing Schisandra chinensis polysaccharide in chicken embryo hepatocyte. Int. J. Biol. Macromol. 2017, 98, 506–514. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Yang, Y.J.; Liu, Y.; Liu, Z.G.; Zhou, H.L.; Hu, H.B. Two-steps extraction of essential oil, polysaccharides and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Pharmaceut. Biomed. 2014, 96, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, K.N.; Channarayappa; Venkatarangana, M. Exemplified screening standardization of potent antioxidant nutraceuticals by principles of design of experiments. J. Funct. Foods. 2015, 17, 260–270. [Google Scholar]
- Gao, X.X.; Meng, X.J.; Li, J.H. Hypoglycemic effects of a water-soluble polysaccharide isolated from Schisandra chinensis (Turcz.) Bail. in alloxan-induced diabetic mice. J. Biotechnol. 2008, 136, 725. [Google Scholar]
- Baj, T.; Baryluk, A.; Sieniawska, E. Application of mixture design for optimum antioxidant activity of mixtures of essential oils from Ocimum basilicum L., Origanum majorana L. and Rosmarinus officinalis L. Ind. Crop. Prod. 2018, 115, 52–61. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, X.M.; Zhao, M.X.; Qi, H.M. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformis. Carbohyd. Polym. 2014, 112, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Huang, G.L.; Huang, H.L. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int. J. Biol. Macromol. 2019, 126, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Hou, X.D.; Lin, K.P.; Zhang, X.; Fu, M.H. Rice straw pretreatment using deep eutectic solvents with different constituents molar ratios: Biomass fractionation, polysaccharides enzymatic digestion and solvent reuse. J. Biosci. Bioeng. 2018, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Hasheminya, S.M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food. Chem. 2019, 18. [Google Scholar] [CrossRef]
- Cao, D.Q.; Song, X.; Hao, X.D.; Yang, W.Y.; Iritanib, E.; Katagiri, N. Ca2+-aided separation of polysaccharides and proteins by microfiltration: Implications for sludge processing. Sep. Purif. Technol. 2018, 202, 318–325. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.M.; Zhao, M.M. Effects of ultrasonic extraction on the physical and chemical properties of polysaccharides from longan fruit pericarp. Polym. Degrad. Stabil. 2008, 93, 268–272. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloid. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Ma, C.H.; Liu, T.T.; Yang, L.; Zu, Y.G.; Wang, S.Y.; Zhang, R.R. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal. Chim. Acta. 2011, 689, 110–116. [Google Scholar] [CrossRef]
- Xiong, Q.P.; Huang, S.; Chen, J.H.; Wang, B.L.; He, L.; Zhang, L.; Li, S.J.; Wang, J.Z.; Wu, J.G.; Lai, X.P.; et al. A novel green method for deproteinization of polysaccharide from Cipangopaludina chinensis by freeze-thaw treatment. J. Clean. Prod. 2017, 142, 3409–3418. [Google Scholar] [CrossRef]
- Vian, M.A.; Fernandez, X.; Visinoni, F.; Chemat, F. Microwave hydrodiffusion and gravity, a new technique for extraction of essential oils. J. Chromatogr. A. 2008, 1190, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Chen, L.; Li, P.; Zhao, J.Z.; Duan, J.Y. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int. J. Biol. Macromol. 2018, 120, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent-free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale. Food. Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Rostaei, M.; Fallah, S.; Lorigooini, Z.; Surki, A.A. The effect of organic manure and chemical fertilizer on essential oil, chemical compositions and antioxidant activity of dill (Anethum graveolens) in sole and intercropped with soybean (Glycine max). J. Clean. Prod. 2018, 199, 18–26. [Google Scholar] [CrossRef]
- Wang, L.B.; Liu, F.C.; Wang, A.X.; Yu, Z.Y.; Xu, Y.Q.; Yang, Y. Purification, characterization and bioactivity determination of a novel polysaccharide from pumpkin (Cucurbita moschata) seeds. Food Hydrocolloid. 2017, 66, 357–364. [Google Scholar] [CrossRef]
- Muíñoa, I.; Díaz, M.T.; Apeleo, E.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Cañeque, V.; Lauzurica, S.; Fuente, J. Valorisation of an extract from olive oil waste as a natural antioxidant for reducing meat waste resulting from oxidative processes. J. Clean. Prod. 2017, 140, 924–932. [Google Scholar] [CrossRef]
- Liang, R.; Han, R.M.; Fu, L.M.; Ai, X.C.; Zhang, J.P.; Skibsted, L.H. Baicalin in radical scavenging and its synergistic effect with β-Carotene in antilipoxidation. J. Agric. Food Chem. 2009, 57, 7118–7124. [Google Scholar] [CrossRef]
- Kıvrak, S. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crop. Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 43.18 | 3 | 14.39 | 19.51 | <0.0001 |
| A:liquid-solid (mL/g) | 38.00 | 1 | 38.00 | 51.50 | <0.0001 |
| B:Extraction time (min) | 3.31 | 1 | 3.31 | 4.48 | 0.0502 |
| C:UAE power (W) | 1.87 | 1 | 1.87 | 2.53 | 0.1310 |
| Residual | 11.81 | 16 | 0.74 | —— | —— |
| Lack of Fit | 11.78 | 11 | 1.07 | 247.97 | <0.0001 |
| Pure Error | 0.022 | 5 | 4.320 × 10−3 | —— | —— |
| Cor Total | 54.98 | 19 | —— | —— | —— |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 34.91 | 9 | 3.88 | 17.30 | <0.0001 |
| A:liquid-solid (mL/g) | 24.17 | 1 | 24.17 | 107.82 | <0.0001 |
| B:Extraction time (min) | 3.45 | 1 | 3.45 | 15.39 | 0.0029 |
| C:MAE power (W) | 2.27 | 1 | 2.27 | 10.14 | 0.0098 |
| AB | 0.42 | 1 | 0.42 | 1.87 | 0.2017 |
| AC | 0.26 | 1 | 0.26 | 1.17 | 0.3043 |
| BC | 0.023 | 1 | 0.023 | 0.10 | 0.7548 |
| A2 | 1.73 | 1 | 1.73 | 7.70 | 0.0196 |
| B2 | 2.90 | 1 | 2.90 | 12.93 | 0.0049 |
| C2 | 0.33 | 1 | 0.33 | 1.48 | 0.2523 |
| Residual | 2.24 | 10 | 0.22 | —— | —— |
| Lack of Fit | 2.20 | 5 | 0.44 | 47.86 | 0.0003 |
| Pure Error | 0.046 | 5 | 9.177 × 10−3 | —— | —— |
| Cor Total | 37.16 | 19 | —— | —— | —— |
| Item | Experimental Program | Essential Oil Yield (mL/kg) | Polysaccharide Yield (g/100g) | Environmental Impact (g CO2 Rejected) |
|---|---|---|---|---|
| A | UAE 60 min | 10.6 | 7.02 ± 0.11 | 440 |
| B | MAE 60 min | 10.9 | 7.83 ± 0.12 | 200 |
| C | UAE 40 min + MAE 20 min | 11.2 | 8.19 ± 0.20 | 360 |
| D | MAE 20 min + UAE 40 min | 11.5 | 8.48 ± 0.33 | 360 |
| E | UAE 20 min + MAE 10 min + UAE 20 min + MAE 10 min | 11.6 | 8.50 ± 0.31 | 360 |
| F | MAE 10 min + UAE 20 min + MAE 10 min + UAE 20 min | 12.2 | 8.87 ± 0.29 | 360 |
| G | UAE & MAE 60 min | 11.9 | 8.79 ± 0.26 | 640 |
| H | Reflux extraction 4 h | 11.7 | 8.56 ± 0.30 | 3200 |
| Concentration | Parallel Sample 1 | Parallel Sample 2 | Parallel Sample 3 | |||
|---|---|---|---|---|---|---|
| (mg/mL) | PR/% | A | PR/% | A | PR/% | A |
| 0.80 | 1.54 | 0.7268 | 2.53 | 0.7339 | 4.62 | 0.7489 |
| 0.40 | 24.35 | 0.8914 | 24.36 | 0.8902 | 25.44 | 0.8979 |
| 0.20 | 29.48 | 0.9268 | 30.58 | 0.9347 | 29.30 | 0.9255 |
| 0.10 | 29.66 | 0.9281 | 23.54 | 0.8843 | 26.15 | 0.9030 |
| 0.05 | 20.86 | 0.8651 | 22.63 | 0.8778 | 23.40 | 0.8833 |
| Control group | —— | 0.7194 | —— | 0.7118 | —— | 0.7162 |
| Concentration | Parallel Sample 1 | Parallel Sample 2 | Parallel Sample 3 | |||
|---|---|---|---|---|---|---|
| (mg/mL) | PR/% | A | PR/% | A | PR/% | A |
| 0.20 | 29.48 | 0.9268 | 30.58 | 0.9347 | 29.30 | 0.9255 |
| 0.20 + ConA | 108.76 | 1.4943 | 107.11 | 1.4825 | 108.97 | 1.4958 |
| 0.20 + Lps | 146.76 | 1.7663 | 143.15 | 1.7405 | 150.42 | 1.7925 |
| ConA | 79.04 | 1.2816 | 80.33 | 1.2908 | 80.53 | 1.2922 |
| Lps | 79.06 | 1.2817 | 79.23 | 1.2829 | 78.85 | 1.2802 |
| Control group | —— | 0.7194 | —— | 0.7118 | —— | 0.7162 |
| No. | Retention Time (min) | Compounds | Alias Name | CAS Number | Molecular Formular | RA % | ||
|---|---|---|---|---|---|---|---|---|
| DES UMHD | MHD | HD | ||||||
| 1 | 4.94 | α-Pinene | α-Pinene | 7785-70-8 | C10H16 | 0.82 | —— | —— |
| 2 | 5.68 | Camphene | Camphene | 79-92-5 | C10H16 | 0.78 | —— | —— |
| 3 | 8.02 | Bicyclo[4.1.0]hept-2-ene, 3,7,7-trimethyl | 2-Carene | 554-61-0 | C10H16 | 0.61 | —— | —— |
| 4 | 8.40 | D-Limonene | D-Limonene | 5989-27-5 | C10H16 | 0.68 | —— | —— |
| 5 | 9.22 | Benzene, 1-methyl-2-(1-methylethyl) | o-Cymene | 527-84-4 | C10H14 | 0.39 | —— | —— |
| 6 | 9.61 | 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl) | gamma-Terpinene | 99-85-4 | C10H16 | 0.34 | —— | —— |
| 7 | 16.43 | Benzene, 2-methoxy-4-methyl-1-(1-methylethyl) | Thymol methyl ether | 1076-56-8 | C11H16O | 0.79 | 1.26 | 1.13 |
| 8 | 17.79 | Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate | L-Bornyl acetate | 5655-61-8 | C12H20O2 | 1.53 | 2.04 | 2.18 |
| 9 | 18.22 | Ylangene | Ylangene | 14912-44-8 | C15H24 | 28.63 | 38.57 | 37.60 |
| 10 | 19.16 | Cyclobuta[1,2:3,4]dicyclopentene, decahydro-3a-methyl-6-methylene-1-(1-methylethyl) | β-bourbonene | 5208-59-3 | C15H24 | 0.49 | —— | —— |
| 11 | 19.55 | Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl) | (-)-β-Elemene | 515-13-9 | C15H24 | 0.72 | —— | —— |
| 12 | 21.10 | 1,6-Cyclodecadiene, 1-methyl-5-methylene-8-(1-methylethyl) | Germacrene D | 23986-74-5 | C15H24 | 0.54 | —— | —— |
| 13 | 22.81 | 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene | (E)-(β)-Farnesene | 18794-84-8 | C15H24 | 1.34 | —— | —— |
| 14 | 25.00 | Bicyclo[3.1.1]hept-2-ene, 2,6-dimethyl-6-(4-methyl-3-pentenyl) | (E)-α-bergamotene | 13474-59-4 | C15H24 | 10.62 | 11.20 | 10.57 |
| 15 | 25.32 | Cyclohexane, 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene) | Elixene | 3242-08-8 | C15H24 | 1.13 | —— | —— |
| 16 | 25.64 | Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl) | α-amorphene | 483-75-0 | C15H24 | 1.88 | 2.55 | 2.51 |
| 17 | 26.11 | Spiro[5.5]undec-2-ene, 3,7,7-trimethyl-11-methylene | (-)-β-Chamigrene | 18431-82-8 | C15H24 | 3.97 | 4.33 | 5.19 |
| 18 | 26.35 | Cyclohexene, 1-methyl-4-(5-methyl-1-methylene-4-hexenyl) | β-bisabolene | 495-61-4 | C15H24 | 3.56 | —— | —— |
| 19 | 26.47 | 1H-Cyclopropa[a]naphthalene, 1a,2,3,3a,4,5,6,7b-octahydro-1,1,3a,7-tetramethyl | β-maaliene | 489-29-2 | C15H24 | 3.44 | 2.45 | 3.55 |
| 20 | 27.07 | 1H-Benzocycloheptene, 2,4a,5,6,7,8-hexahydro-3,5,5,9-tetramethyl | β-himachalene | 1461-03-6 | C15H24 | 10.6 | 11.88 | 10.54 |
| 21 | 27.34 | Spiro[5.5]undeca-1,8-diene, 1,5,5,9-tetramethyl | a-Chamigrene | 19912-83-5 | C15H24 | 1.27 | 1.13 | 1.18 |
| 22 | 27.74 | Tricyclo[5.4.0.0(2,8)]undec-9-ene, 2,6,6,9-tetramethyl | (+)-α-Longipinene | 5989-08-2 | C15H24 | 3.05 | 3.10 | 3.12 |
| 23 | 28.25 | Isoledene | 95910-36-4 | C15H24 | 0.74 | 1.04 | 0.96 | |
| 24 | 28.72 | Benzene, 1-methyl-4-(1,2,2-trimethylcyclopentyl) | (+)-Cuparene | 16982-00-6 | C15H22 | 1.37 | 1.75 | 1.95 |
| 25 | 29.85 | Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene | b-Caryophyllene | 87-44-5 | C15H24 | 0.66 | —— | —— |
| 26 | 30.58 | Naphthalene, 1,2-dihydro-1,1,6-trimethyl | 55682-80-9 | C15H16 | 0.47 | —— | —— | |
| 27 | 30.68 | alpha-Calacorene | 21391-99-1 | C15H20 | 0.23 | 0.24 | 0.38 | |
| 28 | 30.86 | Azulene, 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethylidene) | Guaiene | 88-84-6. | C15H24 | 0.55 | 0.40 | 0.55 |
| 29 | 33.63 | Benzenemethanol, 2,4-dimethyl | 16308-92-2 | C9H12O | 3.02 | —— | —— | |
| 30 | 33.80 | Tricyclo[4.4.0.0(2,7)]dec-3-ene-3-methanol, 1-methyl-8-(1-methylethyl) | 41610-69-9 | C15H24O | 3.47 | 3.28 | 3.8 | |
| 31 | 34.66 | 1H-Cycloprop[e]azulene, 1a,2,3,5,6,7,7a,7b-octahydro-1,1,4,7-tetramethyl | (+)-LEDENE | 21747-46-6 | C15H24 | 0.69 | —— | —— |
| 32 | 36.45 | Naphthalene, 1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methylethyl) | L-calamenene | 6617-49-8 | C15H22 | 4.21 | 5.69 | 5.86 |
| 33 | 37.79 | Cyclohexane, 1,2-diethenyl-4-(1-methylethylidene) | C13H20 | 0.76 | —— | —— | ||
| 34 | 38.50 | 4,6,6-Trimethyl-2-(3-methylbuta-1,3-dienyl)-3-oxatricyclo[5.1.0.0(2,4)]octane | C15H22O | 3.34 | 5.22 | 5.08 | ||
| 35 | 39.62 | Benzenemethanol, 3,5-dimethyl | 27129-87-9 | C9H12O | 2.07 | 3.84 | 3.84 | |
| 36 | 43.10 | Dibutyl phthalate | 84-74-2 | C16H22O4 | 1.19 | —— | —— | |
| Total | 99.95 | 99.97 | 99.99 | |||||
| Run | Factor A | Factor B | Factor C | Response |
|---|---|---|---|---|
| L–S (mL/g) | Extraction Time (min) | UAE (W) | Yield of Polysaccharides (g/100g) | |
| 1 | 40.00 | 50.00 | 400.00 | 9.39 |
| 2 | 40.00 | 50.00 | 700.00 | 9.45 |
| 3 | 40.00 | 30.00 | 400.00 | 9.11 |
| 4 | 46.82 | 40.00 | 550.00 | 9.77 |
| 5 | 30.00 | 40.00 | 297.73 | 5.59 |
| 6 | 20.00 | 30.00 | 700.00 | 6.09 |
| 7 | 40.00 | 30.00 | 700.00 | 9.26 |
| 8 | 30.00 | 40.00 | 550.00 | 8.47 |
| 9 | 30.00 | 56.82 | 550.00 | 8.74 |
| 10 | 20.00 | 50.00 | 400.00 | 6.82 |
| 11 | 30.00 | 40.00 | 550.00 | 8.52 |
| 12 | 30.00 | 40.00 | 550.00 | 8.56 |
| 13 | 20.00 | 50.00 | 700.00 | 6.22 |
| 14 | 13.18 | 40.00 | 550.00 | 3.55 |
| 15 | 30.00 | 40.00 | 550.00 | 8.44 |
| 16 | 20.00 | 30.00 | 400.00 | 5.76 |
| 17 | 30.00 | 23.18 | 550.00 | 5.73 |
| 18 | 30.00 | 40.00 | 550.00 | 8.37 |
| 19 | 30.00 | 40.00 | 550.00 | 8.46 |
| 20 | 30.00 | 40.00 | 802.27 | 8.63 |
| Run | Factor A | Factor B | Factor C | Response |
|---|---|---|---|---|
| l-s (mL/g) | Extraction Time (min) | MAE (W) | Yield of Polysaccharides (g/100g) | |
| 1 | 40.00 | 15.00 | 200.00 | 7.33 |
| 2 | 40.00 | 25.00 | 250.00 | 9.56 |
| 3 | 46.82 | 20.00 | 225.00 | 10.11 |
| 4 | 30.00 | 20.00 | 225.00 | 8.34 |
| 5 | 30.00 | 20.00 | 225.00 | 8.43 |
| 6 | 20.00 | 25.00 | 250.00 | 6.84 |
| 7 | 20.00 | 15.00 | 250.00 | 6.33 |
| 8 | 30.00 | 20.00 | 225.00 | 8.21 |
| 9 | 30.00 | 20.00 | 225.00 | 8.44 |
| 10 | 30.00 | 20.00 | 225.00 | 8.24 |
| 11 | 20.00 | 25.00 | 200.00 | 6.02 |
| 12 | 13.18 | 20.00 | 225.00 | 4.45 |
| 13 | 40.00 | 15.00 | 250.00 | 8.66 |
| 14 | 30.00 | 28.41 | 225.00 | 8.32 |
| 15 | 30.00 | 20.00 | 267.04 | 8.52 |
| 16 | 30.00 | 20.00 | 225.00 | 8.37 |
| 17 | 20.00 | 15.00 | 200.00 | 6.25 |
| 18 | 30.00 | 11.59 | 225.00 | 5.66 |
| 19 | 40.00 | 25.00 | 200.00 | 8.54 |
| 20 | 30.00 | 20.00 | 182.96 | 7.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-H.; Li, W.; Luo, S.; Ma, C.-H.; Liu, S.-X. Alternate Ultrasound/Microwave Digestion for Deep Eutectic Hydro-distillation Extraction of Essential Oil and Polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules 2019, 24, 1288. https://doi.org/10.3390/molecules24071288
Li J-H, Li W, Luo S, Ma C-H, Liu S-X. Alternate Ultrasound/Microwave Digestion for Deep Eutectic Hydro-distillation Extraction of Essential Oil and Polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules. 2019; 24(7):1288. https://doi.org/10.3390/molecules24071288
Chicago/Turabian StyleLi, Jun-Han, Wei Li, Sha Luo, Chun-Hui Ma, and Shou-Xin Liu. 2019. "Alternate Ultrasound/Microwave Digestion for Deep Eutectic Hydro-distillation Extraction of Essential Oil and Polysaccharide from Schisandra chinensis (Turcz.) Baill" Molecules 24, no. 7: 1288. https://doi.org/10.3390/molecules24071288
APA StyleLi, J.-H., Li, W., Luo, S., Ma, C.-H., & Liu, S.-X. (2019). Alternate Ultrasound/Microwave Digestion for Deep Eutectic Hydro-distillation Extraction of Essential Oil and Polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules, 24(7), 1288. https://doi.org/10.3390/molecules24071288