Investigating Stability and Tautomerization of Gossypol—A Spectroscopy Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. 1H NMR
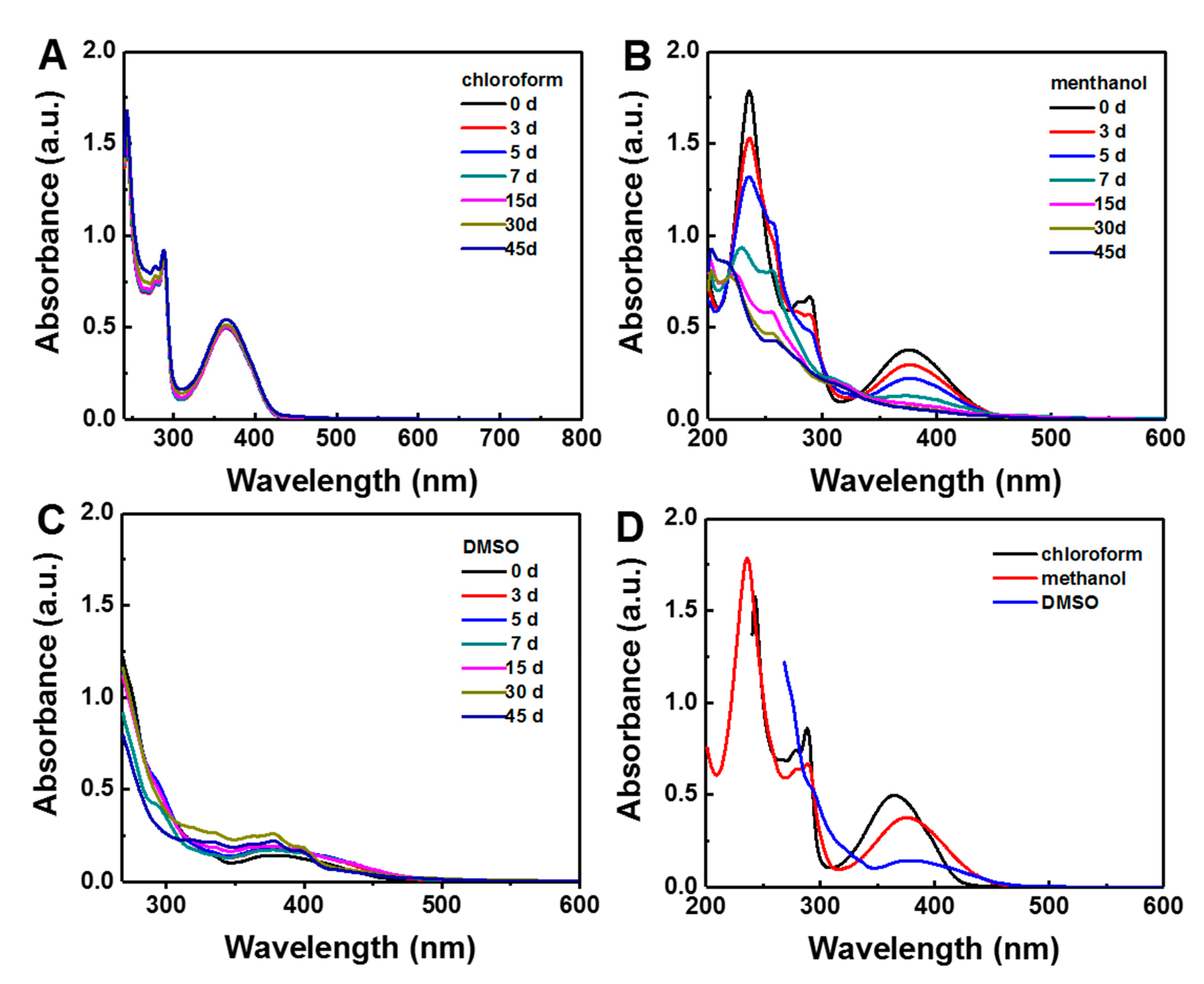
2.2. Gossypol Solution Analyzed by UV-vis Measurement
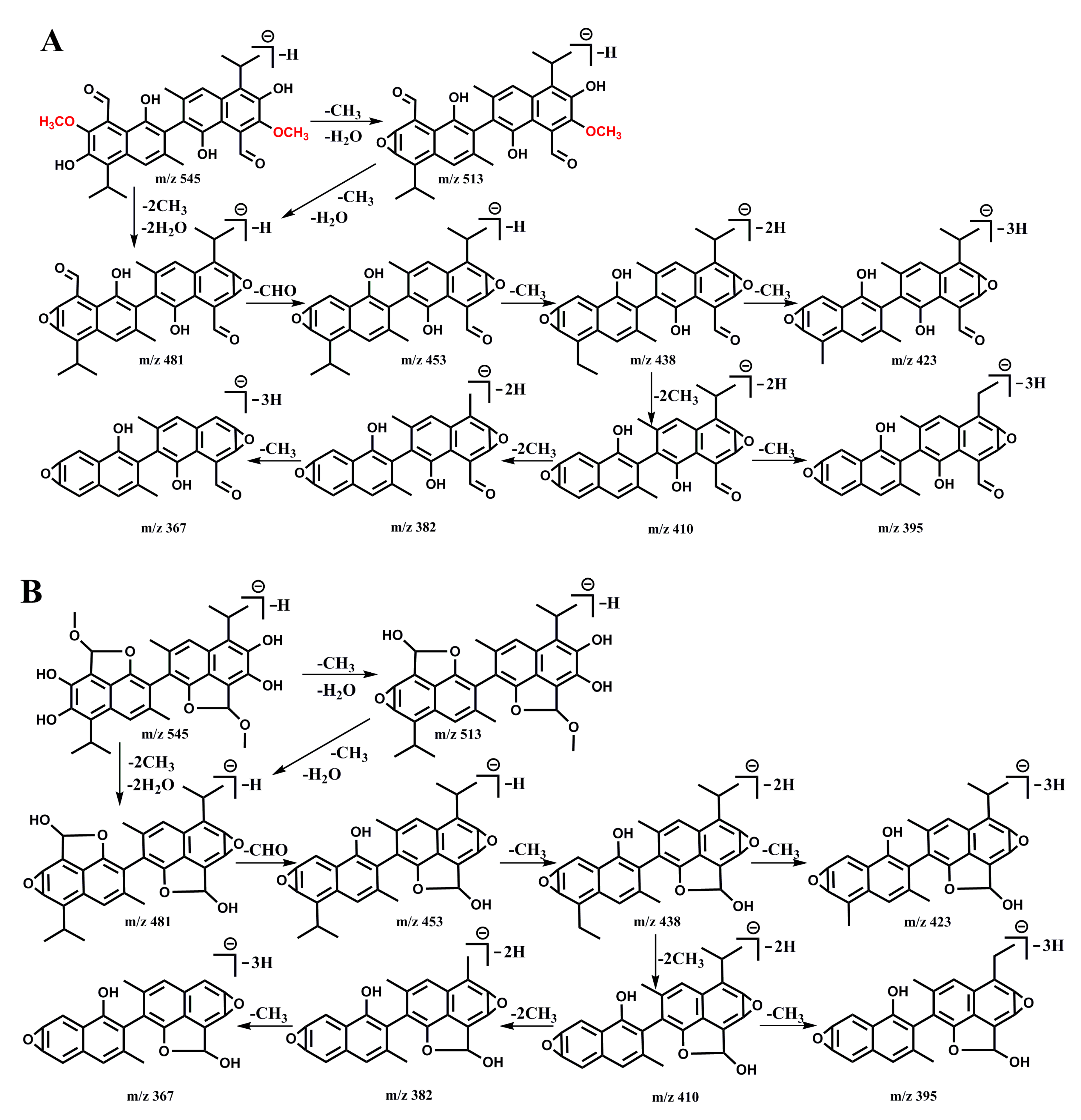
2.3. Gossypol Solution Analyzed by HPLC-QTOF-MS Measurements
3. Materials and Methods
3.1. Sample Preparation for Spectroscopy Analysis
3.2. 1H NMR Measurements
3.3. UV-vis Measurements
3.4. HPLC-QTOF-MS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchlewski, L. Gossypol, ein Bestandtheil der Baumwollsamen. J. Prakt. Chem. 1899, 60, 84–94. [Google Scholar] [CrossRef]
- Longmore, J. Cotton-Seed Oil: Its Colouring Matter and Mucilage and Description of a New Method of Recovering the Loss Occurring in the Refining Process. J. Soc. Chem. Ind. 1886, 5, 200–206. [Google Scholar]
- Adams, R.; Geissman, T.A. Structure of Gossypol. VII. Gossypol Dimethyl Ether. J. Am. Chem. Soc. 1938, 60, 2163–2166. [Google Scholar] [CrossRef]
- Adams, R.; Morris, R.C.; Kirkpatrick, E.C. Structure of Gossypol. IX.1 Oxidation and Degradation of Gossypol Hexamethyl Ether; Gossic Acid. J. Am. Chem. Soc. 1938, 60, 2170–2174. [Google Scholar] [CrossRef]
- Adams, R.; Geissman, T.A.; Morris, R.C. Structure of Gossypol. XVI. Reduction Products of Gossypolone Tetramethyl Ether and Gossypolonic Acid Tetramethyl Ether1. J. Am. Chem. Soc. 1938, 60, 2967–2970. [Google Scholar] [CrossRef]
- Adams, R.; Dial, W.R. Structure of Gossypol. XXII. Gossypol Ethers and their Reduction Products. J. Am. Chem. Soc. 1939, 61, 2077–2082. [Google Scholar] [CrossRef]
- Adams, R.; Geissman, T.A.; Edwards, J.D. Gossypol, a Pigment of Cottonseed. Chem. Rev. 1960, 60, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Withers, W.A.; Carruth, F.E. gossypol-a toxic substance in cottonseed. A preliminary note. Science 1915, 41, 324. [Google Scholar] [CrossRef]
- Ong, T.H.; Kissick, D.J.; Jansson, E.T.; Comi, T.J.; Romanova, E.V.; Rubakhin, S.S.; Sweedler, J.V. Classification of Large Cellular Populations and Discovery of Rare Cells Using Single Cell Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Anal. Chem. 2015, 87, 7036–7042. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Puckhaber, L.S.; Bell, A.A. Ratios of (+)- and (−)-Gossypol in Leaves, Stems, and Roots of Selected Accessions of Gossypium hirsutumVar.marie galante(Watt) Hutchinson. J. Agric. Food Chem. 2006, 54, 1633–1637. [Google Scholar] [CrossRef]
- Liang, X.S.; Rogers, A.J.; Webber, C.L.; Ormsby, T.J.; Tiritan, M.E.; Matlin, S.A.; Benz, C.C. Developing gossypol derivatives with enhanced antitumor activit. Investig. New Drugs 1995, 13, 181–186. [Google Scholar] [CrossRef]
- Maugh, T.H. Male”pill” blocks sperm enzyme. Science 1981, 212, 314. [Google Scholar] [CrossRef] [PubMed]
- Montamat, E.E.; Burgos, C.; De Burgos, N.G.; Rovai, L.E.; Blanco, A.; Segura, E.L. Inhibitory action of gossypol on enzymes and growth of Trypanosoma cruzi. Science 1982, 218, 288–289. [Google Scholar] [CrossRef]
- Vander Jagt, D.L.; Deck, L.M.; Royer, R.E. Gossypol Prototype of Inhibitors Targeted to Dinucleotide Folds. Curr. Med. Chem. 2000, 7, 479–498. [Google Scholar] [CrossRef]
- Taylor, G.T.; Griffin, M.G.; Bardgett, M. Search for a male contraceptive: The effect of gossypol on sexual motivation and epididymal sperm. J. Med. 1991, 22, 29–44. [Google Scholar] [PubMed]
- Admasu, A.; Chandravanshi, B.S. Spectrophotometric determination of total gossypol in cottonseeds and cottonseed meals. Anal. Chem. 1984, 56, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, D.; Sastry, V.R.B.; Agrawal, D.K. Detoxification of undecorticated cottonseed meal by various physical and chemical methods. Anim. Nutr. Feed Technol. 2002, 2, 117–126. [Google Scholar]
- Jingzhi, Y.; Yanhua, S.; Zhongxiang, N.; Weide, Z.; Lianhong, X. The screening of shedding gossypol toxicity and toxicant-shedding resuits. J. Shandong Agric. Univ. 1999, 30, 26–30. [Google Scholar]
- Abou-Donia, S.A.; Lasker, J.M.; Abou-Donia, M.B. High-performance liquid chromatographic analysis of gossypol. J. Chromatogr. A 1981, 206, 606–610. [Google Scholar] [CrossRef]
- Jaroszewski, J.W.; Thorbjørn, S.H.; Lars, L. Hansen. Optical stability of gossypol. Chirality 1992, 4, 216–221. [Google Scholar] [CrossRef]
- Nomeir, A.A.; Abou-Donia, M.B. High-performance liquid chromatographic analysis and stability in various solvents. J. Am. Oil Chem. Soc. 1982, 59, 546. [Google Scholar] [CrossRef]
- Nomeir, A.A.; Abou-Donia, M.B. Photodecomposition of gossypol by ultraviolet irradiation. J. Am. Oil Chem. Soc. 1985, 62, 87. [Google Scholar] [CrossRef]
- Marciniak, B.; Schroeder, G.; Kozubek, H.; Brzezinski, B. Spectroscopic and kinetic studies of the aldehyde–lactol tautomerization of gossypol in solution. J. Chem. Soc. Perkin Trans. 1991, 2, 1359–1362. [Google Scholar] [CrossRef]
- Marciniak, B.; Kozubek, H.; Brzezinski, B. UV-visible absorption studies of gossypol-metal cation complexes in acetonitrile solution. Spectrosc. Lett. 1991, 24, 1265–1273. [Google Scholar] [CrossRef]
- Przybylski, P.; Kwit, M.; Pyta, K.; Pankiewicz, R.; Schroeder, G.; Gawroński, J.; Brzezinski, B. Structure and atropisomerisation of new diastereomeric gossypol Schiff bases with (R)-(+)-2-amino-3-benzyloxy-1-propanol studied by NMR, ECD and DFT methods. Tetrahedron Asymmetry 2010, 21, 973–981. [Google Scholar] [CrossRef]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological properties of Schiff bases and azo derivatives of phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Brycki, B.; Brzezinski, B.; Marciniak, B.; Paszyc, S. 1H AND 13C NMR Studies of Tetrabutylammonium Salts of Gossypol in Chloroform Solution. Spectrosc. Lett. 1991, 24, 509–518. [Google Scholar] [CrossRef]
- Brzezinski, B.; Olejnik, J.; Paszyc, S.; Aripov, T.F. 1H NMR studies of gossypol and its complexes with some organic compounds. J. Mol. Struct. 1990, 220, 261–268. [Google Scholar] [CrossRef]
- Reyes, J.; Wyrick, S.D.; Borriero, L.; Benos, D.J. Membrane actions of male contraceptive gossypol tautomers. Biochem. Biophys. Acta 1986, 863, 101. [Google Scholar] [CrossRef]
- Brzezinski, B.; Olejnik, J.; Paszyc, S. Fourier transform infrared study on the identification of gossypol tautomers. J. Mol. Struct. 1990, 239, 23–31. [Google Scholar] [CrossRef]
- Datta, S.C.; Murti, V.V.S.; Seshadri, T.R. Isolation & study of (+)-gossypol from Thespesia populnea. Indian J. Chem. 1972, 10, 263. [Google Scholar]
- Nazarova, I.P.; Ul’chenko, N.T.; Zaborskaya, I.N.; Glushenkova, A.I. Products of tansformation of gossypol in methanol. Chem. Nat. Compd. 1988, 24, 504–505. [Google Scholar] [CrossRef]
- Abdullaev, N.D.; Tyshchenko, A.A.; Nazarova, I.P.; UI’chenko, N.T.; Yagudaev, M.R.; Glushenkova, A.I. 1H and13C NMR spectra of transformation products of gossypol in solutions. Chem. Nat. Compd. 1990, 26, 129–138. [Google Scholar] [CrossRef]
- Matlin, S.A.; Zhou, R.H. Antifertility Activity of (−)-Gossypol. Future Asp. Contracept. 1985, 12, 237–241. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Bell, A.A.; Homcu, C.R. Spectral identification of the ketol tautomer of gossypol. J. Am. Oil Chem. Soc. 1973, 50, 462–463. [Google Scholar] [CrossRef]
- Baram, N.I.; Ismailov, A.I.; Ziyaev, K.L.; Rezhepov, K.Z. Biological activity of gossypol and its derivatives. Chem. Nat. Compd. 2004, 199–205. [Google Scholar] [CrossRef]
- Kimsey, I.J.; Petzold, K.; Sathyamoorthy, B.; Stein, Z.W.; Al-Hashimi, H.M. Visualizing transient Watson–Crick-like mispairs in DNA and RNA duplexes. Nature 2015, 519, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Bray, W.C.; Branch, G.E.K. Valence and tautomerism. J. Am. Chem. Soc. 1913, 35, 1440–1447. [Google Scholar] [CrossRef]
- Schönberg, A.; Asker, W. Some Adsorption Colors and their Significance for Tautomeric and Thermochromic Effects. Science 1959, 113, 56–57. [Google Scholar] [CrossRef]
- Cleland, W.W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef]
- Dabkowska, I.; Gutowski, M.; Rak, J. Interaction with glycine increases stability of a mutagenic tautomer of uracil. A density functional theory study. J. Am. Chem. Soc. 2005, 127, 2238–2248. [Google Scholar] [CrossRef]
- Nosenko, Y.; Wiosna-Salyga, G.; Kunitski, M.; Petkova, I.; Singh, A.; Buma, W.J.; Thummel, R.P.; Brutsehy, B.; Waluk, J. Proton transfer with a twist? Femtosecond Dynamics of 7-(2-pyridyl)indole in Condensed Phase and in Supersonic Jets. Angew. Chem. 2008, 120, 6126–6129. [Google Scholar] [CrossRef]
- Przybylski, P.; Bejcar, G.; Huczyński, A.; Schroeder, G.; Brzezinski, B.; Bartl, F. 1 H- and 13C-NMR, FTIR, UV-VIS, ESI-MS, and PM5 Studies as Well as Emission Properties of a New Schiff Base of Gossypol with 5-Methoxytryptamine and a New Hydrazone of Gossypol with Dansylhydrazine. Biopolymers 2006, 82, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Liu, H.; Sun, H.; Lu, D.; Zhang, Y.; Zhang, X.; Ma, Z.; Wu, B. Identification of glucuronidation and biliary excretion as the main mechanisms for gossypol clearance: In vivo and in vitro evidence. Xenobiotica 2014, 44, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Stipanovic, R.D.; Bell, A.A.; O’Brien, D.H.; Lukefahr, M.J. Heliocide H a new insecticidal C25 terpenoid from cotton (Gossypium hirsutum). J. Agric. Food Chem. 1978, 26, 115. [Google Scholar] [CrossRef]
- Przybylski, P.; Pospieszny, T.; Huczynski, A.; Brzezinski, B. EI MS and ESI MS studies of the bisesquiterpene from cotton seeds—Gossypol and its Aza-derivatives. J. Mass Spectrom. 2008, 43, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Stipanovic, R.D.; Bell, A.A.; O’Brien, D.H.; Lukefahr, M.J. Heliocide H2: An insecticidal sesterterpenoid from cotton (Gossypium). Tetrahedron Lett. 1977, 6, 567–570. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Altman, D.W.; Begin, D.L.; Greenblatt, G.S.; Benedict, J.H. Terpenoid aldehydes in upland cottons: Analysis by aniline and HPLC methods. J. Agric. Food Chem. 1988, 36, 509–515. [Google Scholar] [CrossRef]
- Matlin, S.A.; Zhou, R.H.; Games, D.E.; Jones, A.; Ramsey, E.D. HPLC, mass spectrometry, and LC/MS of gossypol and its derivatives. J. High Resolut. Chromatogr. 1984, 7, 196–202. [Google Scholar] [CrossRef]
- Gray, R.; Mabry, T.; Bell, A.A.; Stipanovic, R.D.; Lukefahr, M.J. para-Hemigossypolone: A sequiterpenoid aldehyde quinone from Gossypium hirsutum. J. Chem. Soc. Chem. Commun. 1976, 3, 109–110. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Bell, A.A.; Mace, M.E.; Howell, C.R. Antimicrobial terpenoids of Gossypium: 6-methoxygossypol and 6, 6′-dimethoxygossypol. Phytochemistry 1975, 14, 1077–1081. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
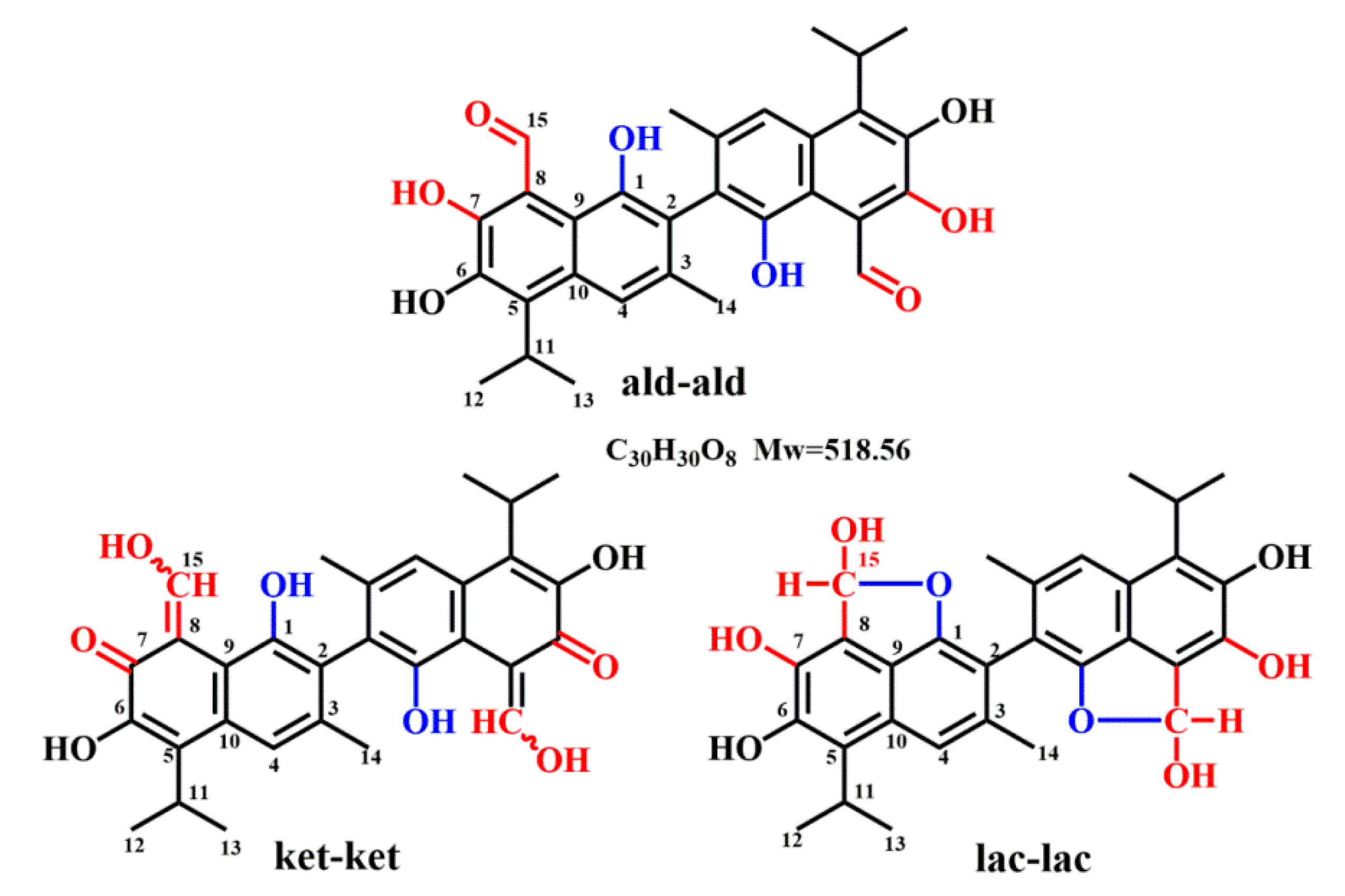
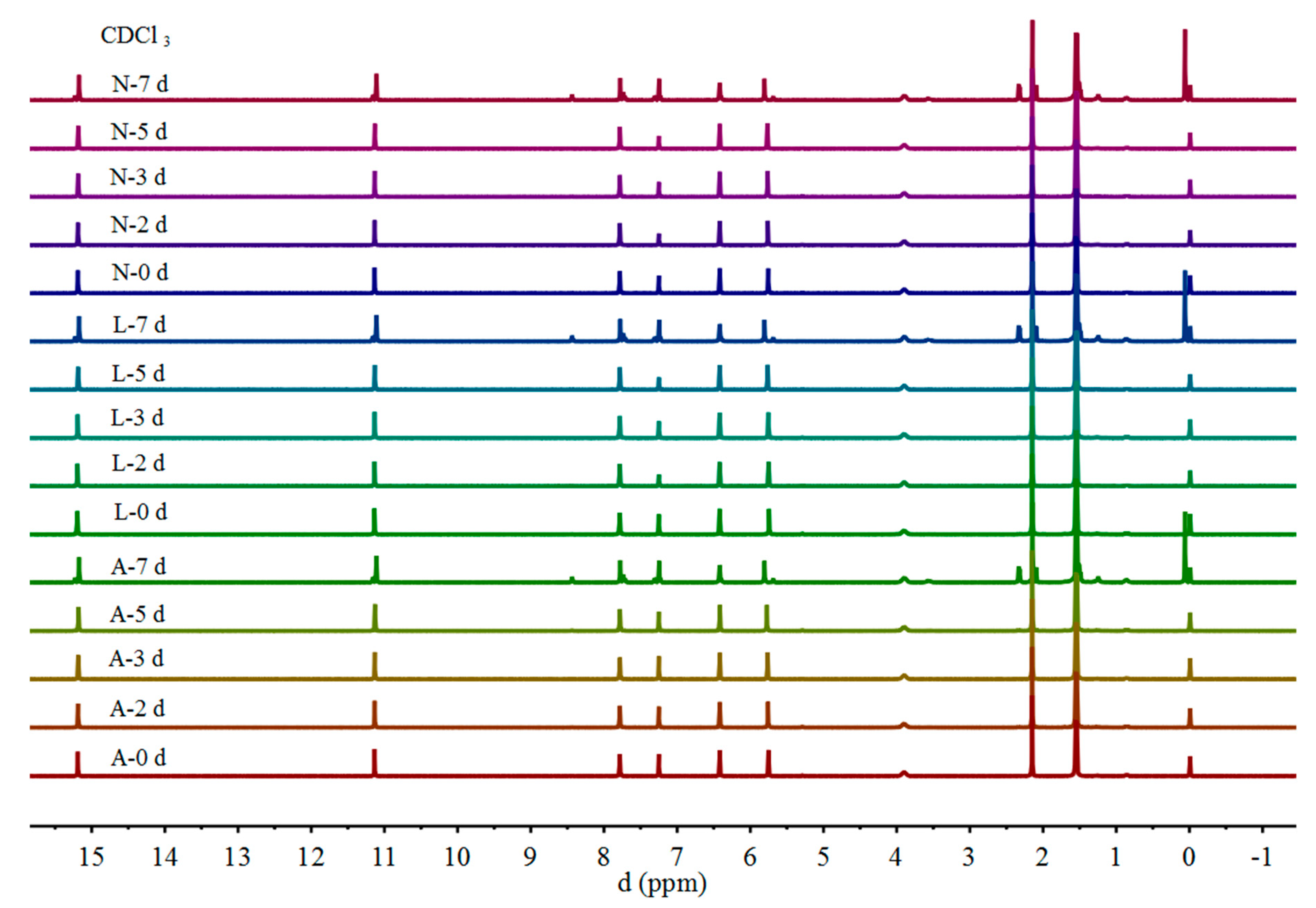
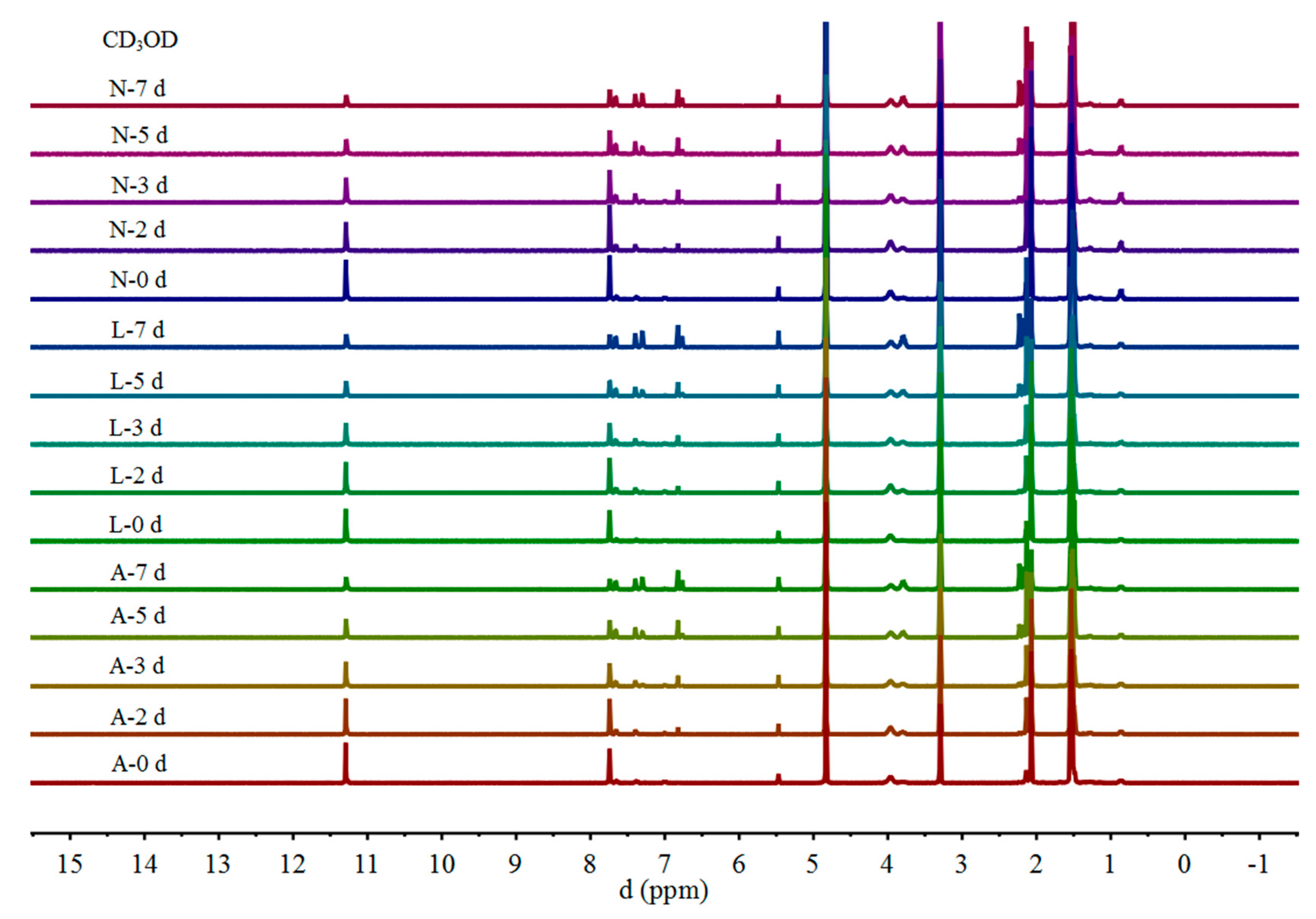



| Proton | Gossypol-CDCl3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 days | 1/3 days | 2 days | 3 days | 5 days | 7 days | 15 days | 30 days | 45 days | |
| H12,12’; H13,13’ (lac) | 1.53 | 1.53 | 1.53 | 1.53 | 1.53 | 1.53 | 1.53 | 1.53 | |
| H12,12’; H13,13’ (ald) | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 |
| H14, H14’ (lac) | 2.34 | 2.34 | 2.34 | 2.34 | 2.34 | 2.34 | 2.34 | 2.34 | |
| H14, H14 (ald) | 2.16 | 2.16 | 2.16 | 2.16 | 2.16 | 2.11, 2.16 | 2.11, 2.16 | 2.11, 2.16 | 2.11, 2.16 |
| H11, H11′ (lac) | 3.58 | 3.58 | 3.58 | 3.58 | 3.58 | 3.57 | 3.57 | 3.57 | |
| H11, H11′ (ald) | 3.91 | 3.91 | 3.91 | 3.91 | 3.91 | 3.91 | 3.91 | 3.91 | 3.91 |
| 1-OH, 1′-OH | 5.77 | 5.77 | 5.77 | 5.77 | 5.77 | 5.70, 5.82 | 5.64, 5.79 | 5.61, 5.79 | 5.64, 5.79 |
| 6-OH, 6′-OH | 6.43 | 6.43 | 6.43 | 6.43 | 6.43 | 6.41 6.42 | 6.41, 6.42 | 6.41, 6.42 | 6.41, 6.44 |
| H4, H4′ (lac) | 7.32 | 7.32 | 7.32 | 7.32 | 7.32 | 7.33 | 7.28, 7.33 | 7.28, 7.33 | |
| H4, H4′ (ald) | 7.79 | 7.79 | 7.79 | 7.79 | 7.79 | 7.74, 7.79 | 7.74, 7.79 | 7.74, 7.79 | 7.74, 7.79 |
| 15-CH 15′-CH (lac) | 8.45 | 8.45 | 8.45 | 8.45 | 8.45 | 8.45 | 8.45 | 8.45 | |
| 15-CHO | 11.14 | 11.14 | 11.14 | 11.14 | 11.14 | 11.12 11.17 | 11.12, 11.17 | 11.12, 11.17 | 11.12, 11.17 |
| 7-OH, 7′-OH | 15.20 | 15.20 | 15.20 | 15.20 | 15.20 | 15.19 15.24 | 15.19, 15.24 | 15.19, 15.24 | 15.19, 15.24 |
| Proton | Gossypol-CD3OD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 day | 1/3 day | 2 days | 3 days | 5 days | 7 days | 15 days | 30 days | 45 days | |
| H12; H13 (lac) | 1.53 | 1.52 | 1.52 | ||||||
| H12; H13 (ald) | 1.57 | 1.57 | 1.56 | 1.56 | 1.56 | 1.55 | 1.57 | ||
| H14, H14’ (lac) | 2.24 | 2.22, 2.26 | 2.24 | 2.24 | 2.24 | 2.22 | 2.22 | ||
| H14, H14’ (ald) | 2.14 | 2.14 | 2.14 | 2.12, 2.17 | 2.14 | 2.13 | 2.14 | ||
| H11, H11′ (lac) | 3.84 | 3.84 | 3.83 | 3.84 | 3.83 | 3.84 | 3.82 | 3.81 | |
| H11, H11′ (ald) | 4.01 | 4.01 | 4.00 | 4.00 | 4.00 | 3.99 | 4.00 | ||
| H4, H4′ (lac) | 7.42 | 7.42 | 7.43 | 7.34, 7.43 | 7.34, 7.43 | 7.34, 7.43 | 7.32 | 7.32 | |
| H4, H4′ (ald) | 7.69, 7.78 | 7.69, 7.78 | 7.70, 7.78 | 7.70, 7.78 | 7.70, 7.78 | 7.69, 7.77 | 7.70, 7.78 | ||
| H4, H4′ (ket) | 7.01 6.99 | 7.01, 6.99 | 7.01, 6.99 | 7.01, 6.99 | 6.99 | 6.99 | |||
| 15,15′-CH (lac) | 6.86 | 6.86 | 6.81, 6.86 | 6.81, 6.86 | 6.81, 6.85 | 6.81, 6.86 | 6.79, 6.85 | 6.79, 6.85 | |
| 15-CHO | 11.32 | 11.33 | 11.32 | 11.32 | 11.32 | 11.31 | 11.32 | ||
| 15-CH=OH | 5.51 | 5.51 | 5.51 | 5.51 | 5.51 | 5.51 | |||
| Proton | Gossypol-DMSO-d6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 days | 1/3 days | 2 days | 3 days | 5 days | 7 days | 15 days | 30 days | 45 days | |
| H12,12’; H13,13’ (lac) | 1.45 | 1.45 | 1.45 | 1.45 | 1.45 | 1.45 | 1.45 | 1.45 | 1.45 |
| H12,12’; H13,13’ (ald) | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 | 1.43 |
| H14, H14’ (lac) | 2.31 | 2.31 | 2.31 | 2.25, 2.32 | 2.25, 2.32 | 2.25, 2.32 | 2.32 | 2.32 | |
| H14, H14’ (ald) | 2.17 | 2.17 | 2.17 | 2.17 | 2.08, 2.18 | 2.08, 2.18 | 2.08, 2.18 | 2.08, 2.18 | 2.06, 2.18 |
| H14, H14’ (ket) | 2.08 | 2.04 | 2.04 | 2.04 | 1.98 | 1.98 | 2.08 | 1.98 | 2.08 |
| H11, H11′ (lac) | 3.59 | 3.59 | 3.59 | 3.59 | 3.59 | 3.59 | 3.59 | ||
| H11, H11′ (ald) | 3.74 | 3.74 | 3.74 | 3.74 | 3.74 | 3.74 | 3.74 | 3.74 | 3.74 |
| H11, H11′ (ket) | 3.93 | 3.93 | 3.93 | 3.93 | 3.93 | 3.93 | 3.93 | 3.93 | 3.93 |
| 1,1′-OH | 7.47 | 7.46 | 7.46 | 7.46 | 7.46 | 7.46 | 7.49 | 7.47 | 7.49 |
| 6-OH,6′-OH | 8.43, 8.47 | 8.43, 8.47 | 8.43, 8.47 | 8.43, 8.47 | 8.43, 8.58 | 8.43, 8.58 | 8.44, 8.56 | 8.45, 8.55 | 8.44, 8.56 |
| H4, H4′ (lac) | 7.22, 7.30 | 7.22, 7.30 | 7.22, 7.30 | 7.22, 7.30 | 7.22, 7.30 | 7.22, 7.30 | 7.22, 7.29 | 7.22, 7.30 | 7.22, 7.29 |
| H4, H4′ (ald) | 7.64, 7.80 | 7.64, 7.77 | 7.64, 7.77 | 7.64, 7.77 | 7.64, 7.77 | 7.64, 7.77 | 7.67, 7.78 | 7.67, 7.78 | 7.67, 7.78 |
| 15-CH,15′-CH(lactol) | 6.88, 6.96 | 6.87, 6.96 | 6.88, 6.96 | 6.88, 6.96 | 6.88, 6.96 | 6.88, 6.96 | 6.89, 6.97 | 6.89, 6.97 | 6.89, 6.97 |
| 15-CHO | 11.16, 11.13 | 11.16, 11.13 | 11.16, 11.13 | 11.16, 11.13 | 11.16, 11.13 | 11.16, 11.13 | 11.11, 11.17 | 11.11, 11.17 | 11.11, 11.17 |
| 7-OH,7′-OH | 9.98, 10.00 | 9.98, 10.00 | 9.98, 10.00 | 9.98, 10.11 | 9.98, 10.16 | 9.98, 10.16 | 9.98, 10.16 | 9.98, 10.16 | 9.98, 10.16 |
| 15-CH=OH | 5.75 | 5.75 | 5.75 | 5.75 | 5.75 | 5.75 | |||
| (15-OH-CH)(lac) | 9.06, 9.16 | 9.06, 9.16 | 9.06, 9.16 | 9.02, 9.10 | 9.02, 9.10 | 9.02, 9.10 | 9.02, 9.09 | 9.02, 9.13 | 9.02, 9.09 |
| 0 day | 2 days | 3 days | 5 days | 7 days | 15 days | 30 days | 45 days | |
|---|---|---|---|---|---|---|---|---|
| CDCl3 | ald | ald:lac 98%:2% | ald:lac 98%:2% | ald:lac 97%:3% | ald:lac 91%:9% | ald:lac 89%:11% | ald:lac 85%:15% | ald:lac 80%:20% |
| CD3OD | ald:ket 92%:8% | ald:lac:ket 79%:16%:5% | ald:lac:ket 72%:18%:10% | ald:lac:ket 66%:31%:3% | ald:lac:ket 55%:41%:4% | ald:lac 22%:78% | lac | lac |
| DMSO-d6 | ald:lac:ket 20%:74%:6% | ald:lac:ket 29%:65%:6% | ald:lac:ket 30%:66%:4% | ald:lac:ket 26%:69%:5% | ald:lac:ket 34%:64%:2% | ald:lac 39%:61% | ald:lac 26%:74% | ald:lac 28%:72% |
| Gossypol in CHCl3 | |||
|---|---|---|---|
| Time | MS1 (m/z) | tR (min) | MS2 (m/z) |
| 0 day | 533.20 | 8.65 | |
| 517.21 | 11.32 | ||
| 3 days | 533.22 | 8.67, 9.34 | 515.21, 487.23, 472.17, 462.17, 459.22, 444.19, 434.17, 429.18, 417.18, 401.16, 385.13, 275.11, 257.10, 232.11, 231.12, 203.05, 188.07 |
| 531.21 | 9.86,10.6 | 503.22, 485.20, 460.15, 457.20, 443.15, 430.58, 401.15, 274.10, 272.09, 259.12, 244.09, 232.12, 231.12, 203.05, 188.06, 179.08 | |
| 517.23 | 11.32 | 471.22, 461.23, 259.12, 231.12, 232.12, 201.11 | |
| 5 days | 531.28 | 10.33 | 485.25, 460.28, 273.12, 257.10, 245.13, 231.14, 179.13 |
| 517.24 | 11.31 | 489.33, 471.22, 461.27, 443.31, 428.28, 259.11, 232.09, 231.13, 215, 201, 188.08 | |
| 7 days | 531.16 | 10.66, 12.08 | 485.17, 471.21, 459.23, 273.07, 257.05, 245.09, 231.10, 203.09, 179.10 |
| 517.14 | 11.41 | 489.20, 471.15, 461.18, 443.20, 428.14, 260, 10, 259.09, 231.09, 201.09, 188.05 | |
| 15 days | 531.20 | 10.47, 12.02 | 485.20, 459.25, 273.09, 257.07, 245.10, 231.11, 179.16 |
| 517.21 | 11.3 | 489.24, 471.20, 461.22, 443.25, 428.20, 259.11, 232.12, 231.11, 201.10, 188.06 | |
| 30 days | 531.21 | 9.87, 10.54 | 503.20, 485.20, 459.21, 401.13, 273.10, 257.16, 245.22, 231.12 |
| 517.20 | 11.42 | 471.20, 461.23, 259.11, 232.11, 231.11, 201.11, 188.07 | |
| Gossypol in CH3OH | |||
|---|---|---|---|
| Time | MS1 (m/z) | tR (min) | MS2 (m/z) |
| 0 day | 545.28 | 9.68 | |
| 531.21 | 10.69 | ||
| 517.22 | 11.61 | ||
| 3 days | 545.20 | 9.72 | 513.20, 481.14, 453.15, 438.14, 423.13, 410.11, 395.12, 382.14 |
| 531.19 | 10.83,12.09 | 499.21, 471.15, 443.18, 428.13, 400.19, 273.09, 257.07, 245.11, 231.10 | |
| 517.14 | 11.06, | 489.20, 471.15, 461.18, 443.19, 428.14, 259.08, 231.09, 232.09, 215.07, 188.04 | |
| 5 days | 545.30 | 9.75 | 481.21, 453.24, 438.22, 423.23, 410.20, 395.21, 382.26 |
| 531.19 | 10.87 | 471.23, 443.30, 428.22 | |
| 517.26 | 11.67 | 489.32, 471.22, 461.27, 443.31, 428.27, 259.12, 232.13, 230.12 | |
| 7 days | 545.19 | 9.68,9.87 | 513.21, 481.14, 438.12, 423.12, 410.11, 395.12, 382.14, 367.16 |
| 531.18 | 10.78, 11.00 | 499.22, 471.23, 443.30, 428.24 | |
| 517.14 | 11.53,12.01 | 501.00, 499.00 | |
| 15 days | 545.25 | 9.75 | 513.25, 481.19, 453.20, 438.18, 423.16, 410.17, 395.16, 382.18, 367.16 |
| 531.24 | 10.86 | 485.35, 471.21, 443.25, 428.20, 273.11, 257.11, 245.13, 231.12 | |
| 517.20 | 11.59 | 471.21, 461.24, 259.11, 232.12, 231.11, 215.10, 201.11, 188.08 | |
| 30 days | 545.23 | 9.74,11.3 | 481.18, 453.19, 438.16, 410.16, 395.19 |
| 531.24 | 10.92, | 503.24, 491.24 | |
| 517.23 | 11.55 | 441.18, 413.21 | |
| 577.00 | 6.74,8.73 | 485.33, 469.22, 453.18 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, Y.; Zhang, Y.; Yasin, A.; Zhang, L. Investigating Stability and Tautomerization of Gossypol—A Spectroscopy Study. Molecules 2019, 24, 1286. https://doi.org/10.3390/molecules24071286
Wang L, Liu Y, Zhang Y, Yasin A, Zhang L. Investigating Stability and Tautomerization of Gossypol—A Spectroscopy Study. Molecules. 2019; 24(7):1286. https://doi.org/10.3390/molecules24071286
Chicago/Turabian StyleWang, Lulu, Yanxia Liu, Yagang Zhang, Akram Yasin, and Letao Zhang. 2019. "Investigating Stability and Tautomerization of Gossypol—A Spectroscopy Study" Molecules 24, no. 7: 1286. https://doi.org/10.3390/molecules24071286
APA StyleWang, L., Liu, Y., Zhang, Y., Yasin, A., & Zhang, L. (2019). Investigating Stability and Tautomerization of Gossypol—A Spectroscopy Study. Molecules, 24(7), 1286. https://doi.org/10.3390/molecules24071286







