Effect of 2″-O-Rhamnosyl Icariside II, Baohuoside I and Baohuoside II in Herba Epimedii on Cytotoxicity Indices in HL-7702 and HepG2 Cells
Abstract
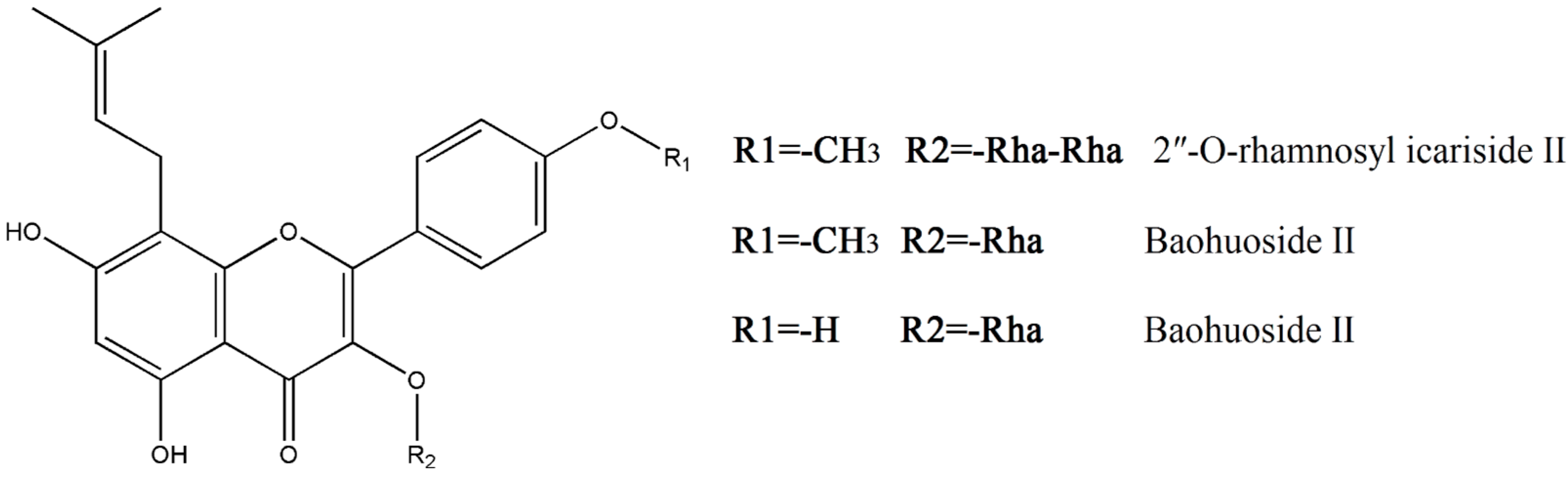
1. Introduction
2. Results
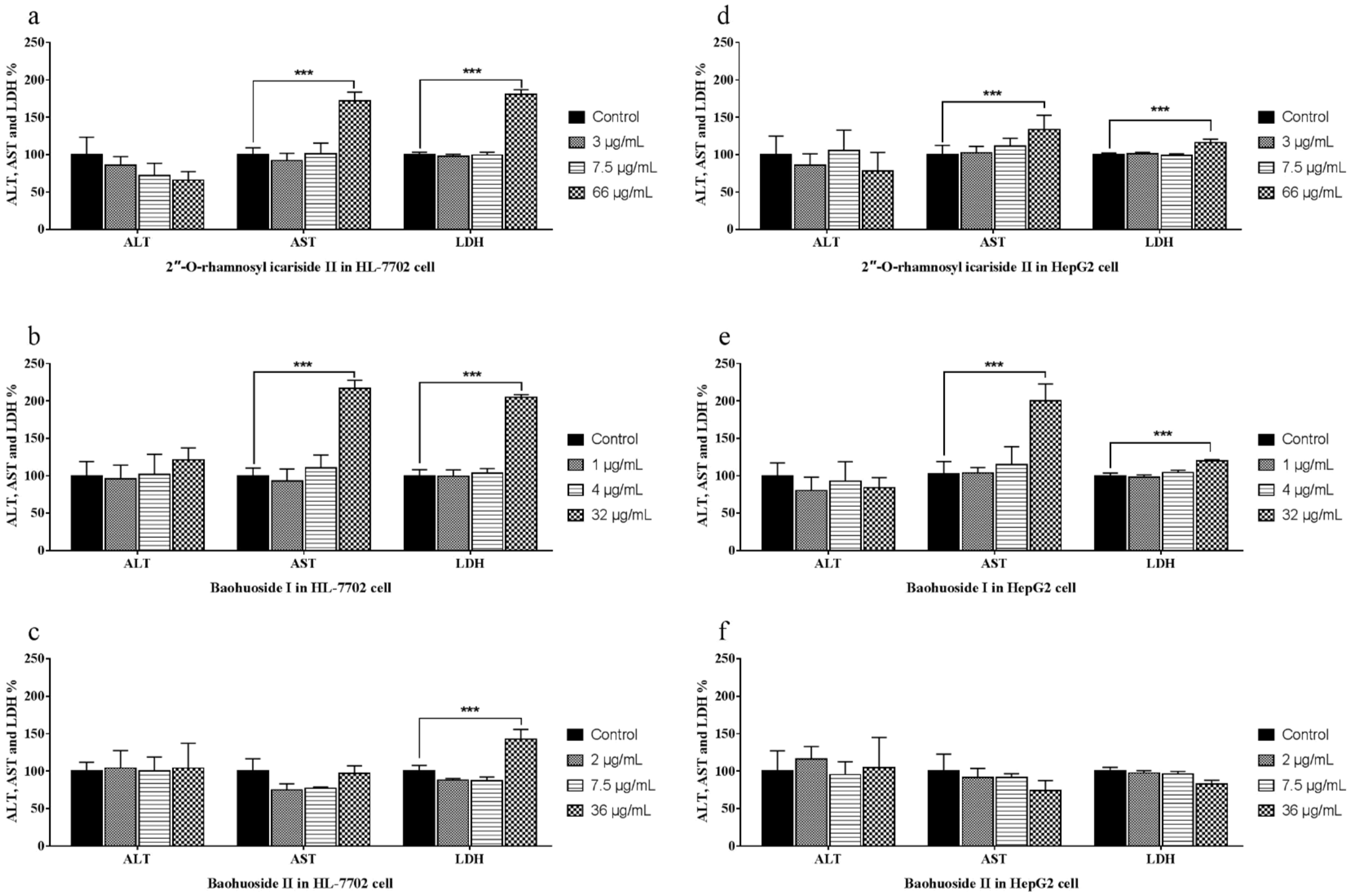
2.1. Effects of the Drugs on the Release of ALT, AST and LDH
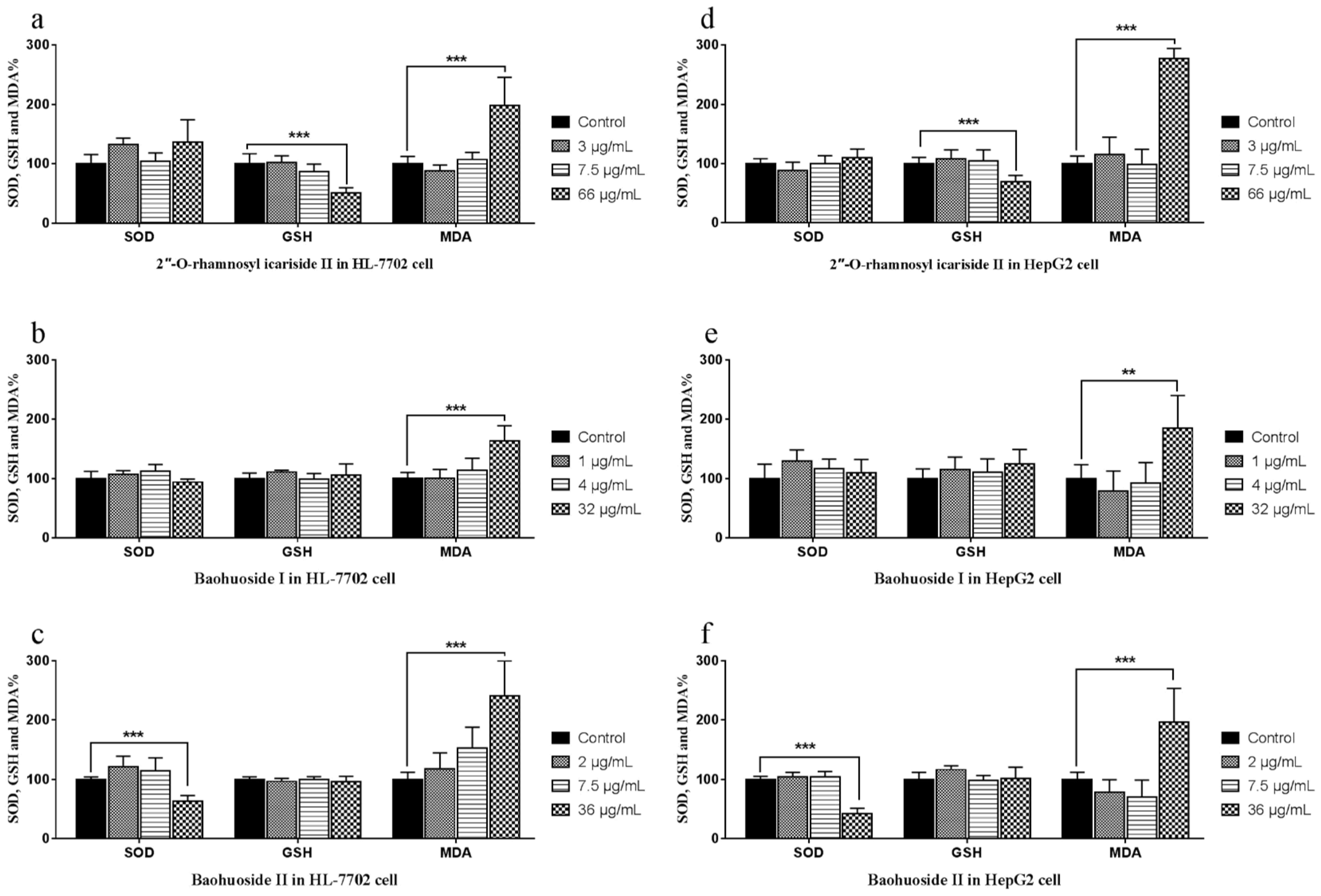
2.2. Effects of the Drugs on the Activities of SOD, GSH and the Level of MDA in Cells
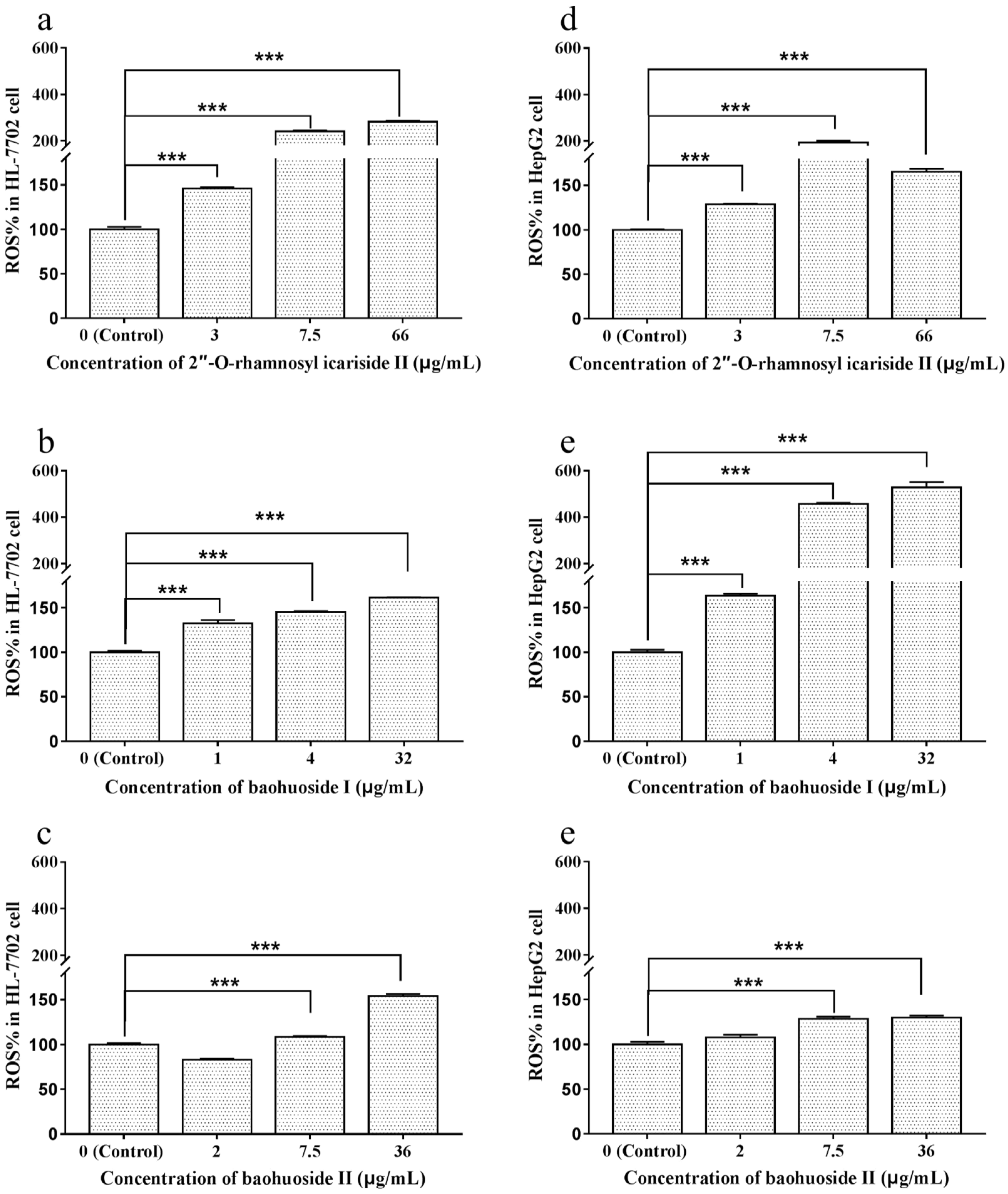
2.3. Effects of Extracts and Compounds on the Generation of ROS
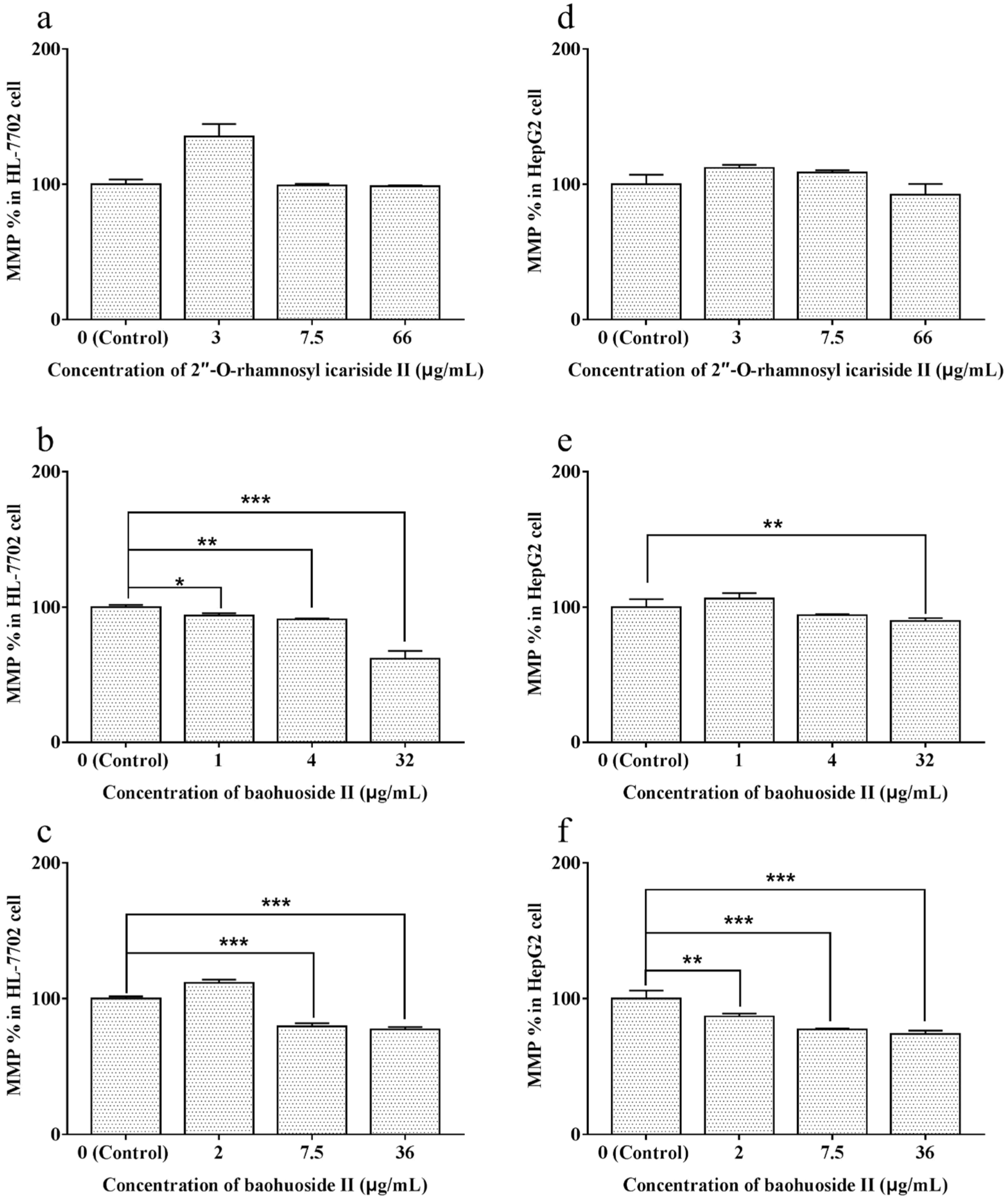
2.4. Effects of Extracts and Compounds on the Change in MMP
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Activity Assessment of ALT, AST and LDH in Cells
4.4. Activity Assessment of SOD, GSH and MDA in Cells
4.5. Measurement of Intracellular ROS Levels
4.6. Assessment of MMP
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bissell, D.M.; Gores, G.J.; Laskin, D.L. Drug-induced liver injury: Mechanisms and test systems. Hepatology 2001, 33, 1009–1013. [Google Scholar] [CrossRef]
- Pessayre, O.; Larrey, D.; Biour, M. Drug-induced liver injury. In Oxford Textbook of Clinical Hepatology; Bircher, J., Benhamou, J., McIntyre, N., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 1261–1315. [Google Scholar]
- Larrey, D. Drug-induced liver diseases. J. Hepatol. 2000, 32, 77–88. [Google Scholar] [CrossRef]
- Holt, M.P.; Ju, C. Mechanisms of drug-induced liver injury. Aaps. J. 2006, 8, E48–E54. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, B.; Kaplowitz, N. Clinical perspectives on xenobiotic-induced hepatotoxicity. Drug Metab. Rev. 2004, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Huang, N.; Chou, Y.J.; Lee, C.H.; Kao, F.Y.; Huang, Y.T. Utilization patterns of Chinese medicine and Western medicine under the National Health Insurance Program in Taiwan, a population-based study from 1997 to 2003. BMC Health Serv. Res. 2008, 8, 170. [Google Scholar] [CrossRef]
- Stickel, F.; Patsenker, E.; Schuppan, D. Herbal hepatotoxicity. J. Hepatol. 2005, 43, 901–910. [Google Scholar] [CrossRef]
- Shaw, D. Toxicological risks of Chinese Herbs. Planta Med. 2010, 76, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Traditional Chinese medicine induced liver injury. J. Clin. Transl. Hepatol. 2014, 2, 80–94. [Google Scholar]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Wu, H.; Lien, E.J.; Lien, L.L. Chemical and pharmacological investigations of Epimedium species: A survey. Prog. Drug Res. 2003, 60, 1–57. [Google Scholar]
- Zhang, Y.J.; Dang, H.S.; Meng, A.P.; Li, J.Q.; Li, X.D. Karyomorphology of Epimedium (Berberidaceae) and its phylogenetic implications. Caryologia 2008, 61, 283–293. [Google Scholar]
- Zhang, Y.J.; Dang, H.S.; Wang, Y.; Li, X.W.; Li, J.Q. A taxonomic revision of unifoliolate Chinese Epimedium L. (Berberidaceae). Kew. Bull. 2011, 66, 253–262. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume I. [Google Scholar]
- He, Q.; Tan, H. Herba Epimedii induced liver injury of a HBV carrier. Chin. J. Liver Dis. 2015, 7, 113–114. [Google Scholar]
- Wang, Q.; Zhang, P.; Yuan, X.; Bi, Y.; Zhou, K.; Zhang, Y. Long-term toxicity of different extracts of Epimedium brevicornu maxim in mice. Chin. J. Pharmacovigil. 2018, 15, 65–69. [Google Scholar]
- Lin, W.; Peng, D. Analysis of adverse reactions cases of Xianlinggubao capsule. Chin. J. Pharmacovigil. 2011, 8, 555–556. [Google Scholar]
- Cheng, J.; Cai, H. Adverse reactions to Zhuangguguanjie Wan and cause analysis. Adv. Drug React. 2000, 2, 15–19. [Google Scholar]
- Zhang, L.; Zhang, J.; Fan, Q.; Su, Z.; Chen, C.; Peng, L.; Wang, T. Hepatoxicity of Epimedii Folium in rat model based on uniform design and regression analysis. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 189–197. [Google Scholar]
- Jin, Y.; Wu, C.S.; Zhang, J.L.; Li, Y.F. A new strategy for the discovery of epimedium metabolites using high-performance liquid chromatography with high resolution mass spectrometry. Anal. Chim. Acta 2013, 768, 111–117. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Xu, W.; Li, N.; Li, H.Y.; Shen, Z.Y.; Zhang, X.M.; Zhang, C. LC-MS-MS method for simultaneous determination of icariin and its active metabolite icariside II in human plasma. Chromatographia 2008, 68, 245–250. [Google Scholar] [CrossRef]
- Liu, J.; Lou, Y.J. Determination of icariin and metabolites in rat serum by capillary zone electrophoresis: Rat pharmacokinetic studies after administration of icariin. J. Pharm. Biomed. Anal. 2004, 36, 365–370. [Google Scholar] [CrossRef]
- Han, H.; Shan, Q.; Zhou, F.-J. Research progress in metabolites of active constitutes from Epimedii Herba. Drugs Clin. 2013, 1, 78–82. [Google Scholar]
- Teschke, R.; Larrey, D.; Melchart, D.; Danan, G. Traditional chinese medicine (TCM) and herbal hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as microRNAs. Medicines 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Zhang, L.; Long, H.; Schwarzenboeck, A.; Schmidt-Taenzer, W.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Traditional Chinese Medicine and herbal hepatotoxicity: A tabular compilation of reported cases. ANN. Hepatol. 2015, 14, 7–19. [Google Scholar] [CrossRef]
- Teschke, R.; Wolff, A.; Frenzel, C.; Schulze, J. Review article: Herbal hepatotoxicity—An update on traditional Chinese medicine preparations. Aliment Pharmacol. Ther. 2014, 40, 32–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Liao, Z.; He, X.; Zhou, Y.; Guo, H. Epidemiology of drug-induced liver injury in China: A systematic analysis of the Chinese literature including 21,789 patients. Eur. J. Gastroenterol. Hepatol. 2013, 25, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Teschke, R. Traditional Chinese Medicine and Herb-induced Liver Injury: Comparison with Drug-induced Liver Injury. J. Clin. Transl. Hepatol. 2018, 6, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J. Gastroenterol. 2008, 14, 6774–6785. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.L. A study on toxicity and adverse reactions caused by Lei Gong Teng. Pharmacol. Clin. Chin. Mater. Med. 2001, 17, 42–47. [Google Scholar]
- Stickel, F.; Shouval, D. Hepatotoxicity of herbal and dietary supplements: An update. Arch Toxicol. 2015, 89, 851–865. [Google Scholar] [CrossRef]
- Efferth, T.; Kaina, B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr. Drug Metab. 2011, 12, 989–996. [Google Scholar] [CrossRef]
- Tu, C.; Jiang, B.Q.; Zhao, Y.L.; Li, C.Y.; Li, N.; Li, X.F. Comparison of processed and crude Polygoni Multiflori Radix induced rat liver injury and screening for sensitive indicators. China J. Chin. Mater. Med. 2015, 40, 654–660. [Google Scholar]
- Huang, S.H.; Tung, C.W.; Fülöp, F.; Li, J.H. Developing a QSAR model for hepatotoxicity screening of the active compounds in traditional Chinese medicines. Food Chem. Toxicol. 2015, 78, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qi, X.; Yoshida, E.M.; Méndez-Sánchez, N.; Teschke, R.; Sun, M.; Hou, F. Clinical characteristics and outcomes of traditional Chinese medicine-induced liver injury: A systematic review. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cui, Y.-H.; Luo, K. Pyruvate oxidase and its applications of clinic biochemical determination. J. Gansu Sci. 2003, 15, 95–100. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, T.; Zhao, B.-S.; Zhang, J.-X.; Yang, S.; Fan, C.-L.; Li, P. Effect of 2″-O-Rhamnosyl Icariside II, Baohuoside I and Baohuoside II in Herba Epimedii on Cytotoxicity Indices in HL-7702 and HepG2 Cells. Molecules 2019, 24, 1263. https://doi.org/10.3390/molecules24071263
Zhang L, Wang T, Zhao B-S, Zhang J-X, Yang S, Fan C-L, Li P. Effect of 2″-O-Rhamnosyl Icariside II, Baohuoside I and Baohuoside II in Herba Epimedii on Cytotoxicity Indices in HL-7702 and HepG2 Cells. Molecules. 2019; 24(7):1263. https://doi.org/10.3390/molecules24071263
Chicago/Turabian StyleZhang, Lin, Ting Wang, Bao-Sheng Zhao, Jing-Xuan Zhang, Song Yang, Chun-Lan Fan, and Pin Li. 2019. "Effect of 2″-O-Rhamnosyl Icariside II, Baohuoside I and Baohuoside II in Herba Epimedii on Cytotoxicity Indices in HL-7702 and HepG2 Cells" Molecules 24, no. 7: 1263. https://doi.org/10.3390/molecules24071263
APA StyleZhang, L., Wang, T., Zhao, B.-S., Zhang, J.-X., Yang, S., Fan, C.-L., & Li, P. (2019). Effect of 2″-O-Rhamnosyl Icariside II, Baohuoside I and Baohuoside II in Herba Epimedii on Cytotoxicity Indices in HL-7702 and HepG2 Cells. Molecules, 24(7), 1263. https://doi.org/10.3390/molecules24071263




