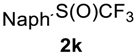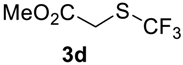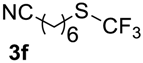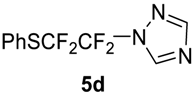Abstract
A simple and efficient protocol for the oxidation of trifluoromethyl, mono- and difluoromethyl sulfides to the corresponding sulfoxides without over-oxidation to sulfones, using TFPAA prepared in situ from trifluoroacetic acid and 15% H2O2 aqueous solution was developed. The methodology is suitable for a wide range of aromatic and aliphatic substrates in milligram and multigram scales.
1. Introduction
The synthesis of trifluoromethylsulfinyl containing compounds is a recent trend in current organic chemistry due to the practical significance of such compounds [1]. In particular, their biological activity is promising. They are also key intermediates for trifluoromethyl-containing sulfoximine synthesis and are excellent chiral auxiliaries [2,3,4,5]. Synthetic approaches dedicated to trifluoromethyl sulfoxides include (1) trifluoromethylation of the corresponding sulfinyl halides or sulfinic esters using TMSCF3 [6,7,8], (2) reaction of aromatic compounds with triflinate salts [9], (3) rearrangement of aryl triflinates in the presence of AlCl3 [10], (4) trifluoromethanesulfinilation using Langlois’ system (CF3SO2Na/POCl3) [11] or with 1-(trifluoromethylsulfinyl)-pyrrolidine-2,5-dione [12]. However, the most important and common method for the synthesis of trifluoromethylsulfoxides is the oxidation of the corresponding trifluoromethylsulfides using various oxidants, mainly peroxyacids, e.g. mCPBA [1]. It is noteworthy to mention that oxidation with mCPBA is sensitive to temperature. As a result, over-oxidation frequently occurs and non-negligible amounts of trifluoromethylsulfone are formed [13]. Furthermore, this reagent converts into m-chlorobenzoic acid, which is sometimes not easy to separate from the sulfoxide. Oxidation of an aliphatic sulfide was also described with Oxone® as oxidant [14]. Waste by-products formed during the oxidation reaction as well as the necessity to use silica as a component of reaction medium cause difficulties in scaling up. Trifluoroperacetic acid (TFPAA) was shown to be a convenient reagent for the oxidation of sulfides to sulfoxides, as it reacts more rapidly at low temperature than other peroxy acids [15]. To the best of our knowledge, TFPAA has not been widely applied to the oxidation of perfluoroalkyl sulfides and only a few articles [14,16,17,18,19,20,21] and patents [22,23,24,25,26,27] documented this procedure. TFPAA solution can be prepared from 30% H2O2 and trifluoroacetic acid or 80–90% H2O2 and trifluoroacetic anhydride [28,29]. On the basis of literature data and our experience in oxidation reactions of trifluoromethyl sulfides, the use of TFPAA, obtained by both methods, has one common limitation as for mCPBA: over-oxidation readily occurs to form difficult-to-separate mixtures of trifluoromethyl sulfide, trifluoromethyl sulfoxide, and trifluoromethyl sulfone [17,18,19]. On the other hand, regarding the advantages of this method (easy to prepare and a cheap reagent, trifluoroacetic acid as side product), we believed there was an urgent need to exploit it. The description of a general and selective method of oxidation is especially important in the context of an exponential growth of molecules bearing a SCF3 group [1].
Herein we propose an easy-to-handle and high-yielding procedure for the oxidation of alkyl and aryl perfluoroalkyl sulfides to the corresponding sulfoxides without over-oxidation thanks to the reappraisal of oxidation by TFPAA.
2. Results and Discussion
We postulated that a decrease in concentration, associated with a rigorous measurement of the latter, could dramatically reduce the formation of unwanted sulfone. Indeed, the use of TFPAA obtained in situ from trifluoroacetic acid and 15% H2O2 aqueous solution proved to be successful (see experimental part). This method was firstly applied to aryl trifluoromethyl sulfides 1 bearing both electrons donating and electron-withdrawing substituents in various positions on the aromatic ring (Scheme 1, Table 1). Corresponding aryl trifluoromethyl sulfoxides 2 were obtained in high yields whatever the nature of the substituents on the aromatic ring. In all experiments, the crude product contained less than 7% of non-reacted trifluoromethyl sulfide; at the same time, trifluoromethyl sulfone was detected in negligible amounts in only two reactions (Table 1, Entry 4, 10).
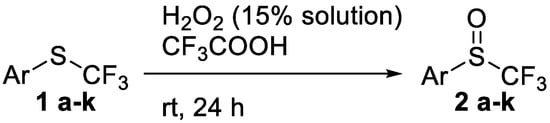
Scheme 1.
Oxidation of aryl trifluoromethyl sulfides 1.

Table 1.
Oxidation of aryl trifluoromethyl sulfides 1.
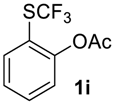
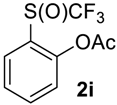
In most cases the crude product needs no further purification. If needed, compounds 2 were easily separated from impurities by standard methods (see experimental part for details). It should be noted that during the oxidation of acylated phenol 1i partial hydrolysis occurred and the crude product contained ~15% of o-trifluoromethylsulfinyl phenol, which we failed to separate from 2i by column chromatography.
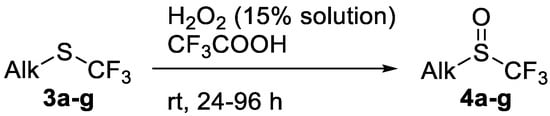
Scheme 2.
Oxidation of alkyl trifluoromethyl sulfides 3.

Table 2.
Oxidation of alkyl trifluoromethyl sulfides 3.
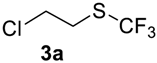
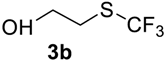
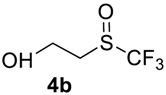
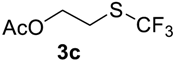
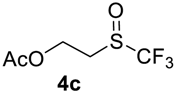
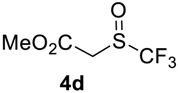
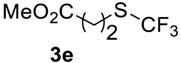
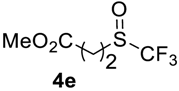
The thioether 3a was oxidized by TFPAA in high yield (83%) in 24 h. This simple procedure can be favorably compared to our previous method which employed Oxone® [14]. Compound 4b was isolated in low yield, most probably due to the high solubility of the product in water. Attempts to separate 4b from 3b by distillation failed. Use of an acyl protective group (Table 2, Entry 3) led to a higher yield of sulfoxide 4c. In this case, as well as in the previous one, a small amount (<3%) of trifluoromethyl sulfone was detected in the reaction mixture and the crude product. As in the experiment with acylated phenol 1i (Table 1, Entry 9), product 4c contains ~15% of the corresponding alcohol (Table 2, Entry 3), which we failed to separate from the ester by distillation. Oxidation of sulfide 3d by 15% H2O2 solution in TFA proceeded with 90% conversion. The use of H2O2 in excess (1.1 equiv.) led to the same results and the sulfone was not observed in this reaction mixture. Two other examples demonstrated the efficiency of this transformation (entries 6–7).
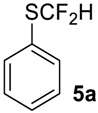
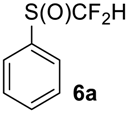
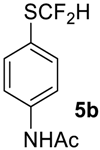
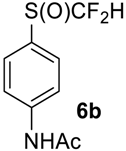
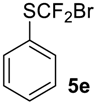
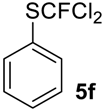
To show the scope of our oxidation procedure a number of sulfides bearing difluoromethyl 5a–c, bromodifluoromethyl 5e, dichlorofluoromethyl 5f, and 1,1,2,2-tetrafluoro-2-((1,2,4-triazol)-1-yl)-ethyl 5d groups were converted into sulfoxides (Scheme 3,Table 3).
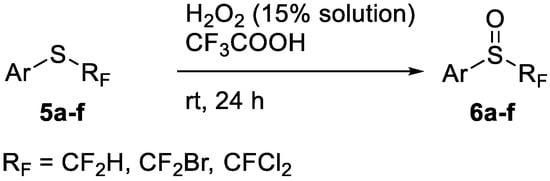
Scheme 3.
Oxidation of fluoromethyl and difluoromethyl sulfides 5.

Table 3.
Oxidation of fluoromethyl and difluoromethyl sulfides 5.
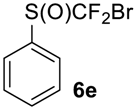
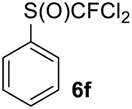
As it is evident from Table 3, all compounds 6a–f were obtained in high yields and the crude products were pure enough to be used without further purification. The oxidation of 5a was strongly exothermic and the reaction was complete within 4 h at room temperature yielding pure sulfoxide 6a, which contained neither difluoromethyl sulfide 5a nor difluoromethyl sulfone. If this reaction was performed at −15 °C for 6 h, with further stirring at ~10 °C for 20 h, the crude product contained ~2% of unreacted sulfide 5a.
It should be emphasized that the oxidation reactions of sulfides 1, 3, and 5 could be performed on a 0.05 to 50 g scale.
The application of this method to heterocyclic thioethers is highly challenging, as demonstrated by the two following preliminary results. Thus, 2-trifluoromethylsulfenyl-5-acetylamino-pyridine 7 was oxidized to sulfoxide 8 with low conversion after seven days stirring, and we failed to separate the product from the starting sulfide (Scheme 4). A study devoted to the oxidation of heterocyclic substrates is needed but falls outside the scope of the present study.
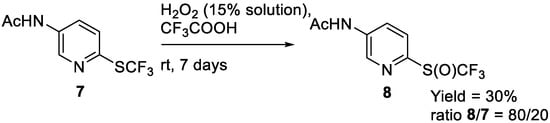
Scheme 4.
Oxidation of heterocyclic trifluoromethyl sulfides.
3. Materials and Methods
3.1. General Information
The purification of products by column chromatography (CC) was performed on Silica gel, 70–230 mesh 60A (Aldrich, Saint Louis, MI, USA) or by preparative TLC chromatography. 1H NMR spectra were recorded at 500 MHz with Bruker AVANCE DRX 500 instrument, or at 200 MHz or 300 MHz with Bruker AC-200 or AC-300, or at 400 MHz with Varian UNITY–Plus 400 spectrometer. 19F NMR spectra were recorded on Varian UNITY–Plus 400 spectrometer at 376.5 MHz or at 470 MHz with Bruker AVANCE DRX 500 instrument or at 188 MHz with Bruker AC-200 (Bruker, Billerica, MA, USA). Chemical shifts are given in ppm relative to Me4Si and CCl3F, respectively, as internal or external standards. 13C NMR-spectra (proton decoupled) were recorded on a Bruker AVANCE DRX 500 instrument at 125.7 MHz, or on Varian UNITY–Plus 400 spectrometer at 100.6 MHz, or at 75 or 50 MHz with Bruker AC-300 or AC-200. IR spectra were recorded with a Vertex 70 (Bruker) instrument (in KBr tablet). Melting points were determined in open capillaries using SMP3 instrument (Stuart Scientific Bibby Sterlin Ltd, Stone, Staffordshire, UK). Elemental analysis was performed in the Analytical Laboratory of the Institute of Organic Chemistry, NAS of Ukraine, Kyiv.
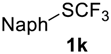
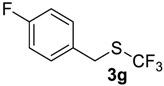
In all experiments trifluoroacetic acid from Sigma-Aldrich, 99% (CAS 76-05-1) was used. Difluoromethyl sulfides 5a–c were synthesized by standard method [30]. Compound 5d was synthesized as was described in the article [31]. Fluoromethylsulfides 1k, 3f,g, and 5e,f were prepared according to the described procedures [32,33,34]. Trifluoromethylsulfides 1a–j, 3a–e, 7, and 9 were purchased from Enamine Ltd (www.enamine.net).
3.2. Important Information
It is critical to know the exact concentration of H2O2 used in order to avoid over dosage of reagent that led to over oxidation. A solution of 15% H2O2 (mass concentration) was prepared from commercial ~30–35% H2O2 solution by dilution with water ~1:1 w/w. The concentration of H2O2 solution was measured using densimetry or titration (see Supplementary Materials).
3.3. General Procedure for Oxidation of Polyfluoroalkyl Sulfides to Polyfluoroalkyl Sulfoxides
To the solution of sulfide 1, 3, or 5 (10 mmol) in CF3COOH (15–20 mL) 15 mass% aqueous solution of H2O2 (containing 10 mmol of H2O2) was added dropwise very slowly (during 40–90 min) at room temperature. Reaction is strongly exothermic; H2O2 was added at such a rate that the temperature was kept in the range 25–28 °C inside the flask (20 °C for compounds 1k, 3f,g, and 0 °C for compounds 5e,f). Reaction mixture was stirred overnight at r.t. (for compound 3d − 96 h, for 7–7 days), poured into water, neutralized with solid NaHCO3 to pH=6–7, then extracted with ether or ethyl acetate (4 × 30 mL). Compounds 2k, 4f,g, and 6e,f were extracted with dichloromethane before neutralizing the TFA with NaHCO3. The organic phase was washed with water (4 × 20 mL), dried with MgSO4 or Na2SO4. Solvent was removed at atmospheric pressure for low-boiling liquids or on a rotary evaporator for solids or high-boiling liquids. Crude product was analyzed by NMR and purified if necessary.
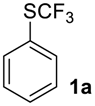
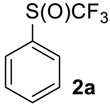
Phenyl Trifluoromethyl sulfoxide2a [13,35,36]. Colorless liquid; yield 1.8 g (95%). 1H NMR (CDCl3, 400 MHz): δ = 7.56–7.63 (m, 3H, Ar-H), 7.75–7.77 (m, 2H, Ar-H). 19F NMR (DMSO-d6, 376.5 MHz): δ = −76.6 (s). Compound 1a was oxidized on a 50 g scale (in 70 mL of CF3COOH) with the same yield.
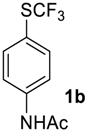
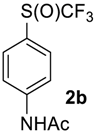
N-(4-((Trifluoromethyl)sulfinyl)phenyl)acetamide2b [37,38]. Crude product was purified by column chromatography, eluent CH2Cl2 (Rf = 0), then methyl-tert-butyl ether (Rf = 0.6). Pale yellow solid; yield 2.1 g (85 %); m.p. 140–141 °C (Lit. 141–142 °C). 1H NMR (DMSO-d6, 500 MHz): δ = 2.10 (s, 3H, CH3), 7.80 (d, 3JH-H = 8 Hz, 2H, Ar-H), 7.90 (d, 3JH-H = 8 Hz, 2H, Ar-H), 10.41 (s, 1H, NH). 19F NMR (DMSO-d6, 376.5 MHz): δ = −75.2 (s). 13C NMR (DMSO-d6, 125.7 MHz): δ = 24.5, 119.8, 125.2 (q, 1JC-F = 335.6 Hz, CF3), 127.6, 128.2, 144.8, 169.6. Compound 1b was also oxidized on a 10 g scale (in 30 mL of CF3COOH) with the same yield.
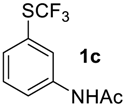
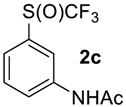
N-(3-((Trifluoromethyl)sulfinyl)phenyl)acetamide2c [37]. White solid; yield 2.4 g (96%); m.p. 101–102 °C (Lit. 104.5–105.5 °C). 1H NMR (DMSO-d6, 500 MHz): δ = 2.08 (s, 3H, CH3), 7.49 (d,3JH-H = 8 Hz, 1H, Ar-H), 7.61 (t, 3JH-H = 8 Hz, 1H, Ar-H), 7.85 (d, 3JH-H = 8 Hz, 1H, Ar-H), 8.24 (s, 1H, Ar-H), 10.38 (s, 1H, NH). 19F NMR (DMSO-d6, 376.5 MHz): δ = −74.8 (s). 13C NMR (DMSO-d6, 125.7 MHz): δ = 24.0, 115.1, 120.2, 123.6, 124.7 (q, 1JC-F = 335.6 Hz, CF3), 130.2, 135.8, 140.7, 168.9.
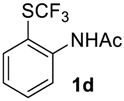
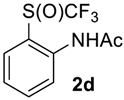
N-(2-((Trifluoromethyl)sulfinyl)phenyl)acetamide2d. Crude product was purified by column chromatography, eluent methyl-tert-butyl ether (Rf = 0.6). White solid; yield 2.1 g (86%); m.p. 68–69 °C. IR (KBr): 3295, 3103, 2999, 2765, 2387, 2310, 2116, 1685, 1586, 1512, 1474, 1431, 1377, 1304, 1266, 1180, 1136, 1074, 1044, 1007, 889, 853, 776, 670, 603, 576, 549, 522, 476, 455, 431 cm−1. 1H NMR (CDCl3, 500 MHz): δ = 2.17 (s, 3H, CH3), 7.20 (t, 3JH-H = 7.5 Hz, 1H, Ar-H), 7.39 (d, 3JH-H = 7.5 Hz, 1H, Ar-H), 7.61 (t, 3JH-H = 7.5 Hz, 1H, Ar-H), 8.55 (d, 3JH-H = 7.5 Hz, 1H, Ar-H), 10.00 (s, 1H, NH). 19F NMR (CDCl3, 376.5 MHz): δ = -72.3 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 24.9, 118.1, 123.0, 123.6, 125.4 (q, 1JC-F = 337 Hz, CF3), 128.5, 135.1, 142.2, 168.9. Anal. Calcd for C9H8F3NO2S: C, 43.03; H, 3.21; N, 5.58. Found: C, 43.00; H, 3.18; N, 5.57.
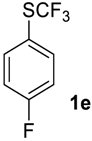
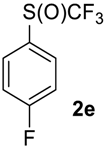
1-Fluoro-4-((trifluoromethyl)sulfinyl)benzene2e [9]. Crude product contains 97 mol% of 2e and 3 mol% of starting sulfide, which may be removed in vacuum (1 mBar) to give pure sulfoxide 2e. Light yellow liquid; yield 1.9 g (96%). 1H NMR (CDCl3, 400 MHz): δ = 7.25–7.29 (m, 2H, Ar-H), 7.76–7.80 (m, 2H, Ar-H). 19F NMR (DMSO-d6, 376.5 MHz): δ = −75.7 (s, 3F, CF3), −104.8 (s, 1F, Ar-F). 13C NMR (CDCl3, 100.6 MHz): δ = 117.2 (d, 2JC-F = 22 Hz), 124.6 (q, 1JC-F = 335 Hz, CF3), 128.5 (d, 3JC-F = 9 Hz), 131.1, 166.0, (d, 1JC-F = 254.5 Hz, C-F). Compound 1e was oxidized on a 50 g scale (in 70 mL of CF3COOH) with the same yield.
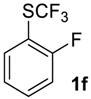
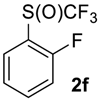
1-Fluoro-2-((trifluoromethyl)sulfinyl)benzene2f. White solid; yield 2.0 g (98%); m.p. 44–45 °C. IR (KBr): 3094, 2124, 1986, 1948, 1820, 1724, 1633, 1598, 1474, 1449, 1261, 1141, 955, 867, 823, 762, 706, 673, 579, 544, 490, 459, 430 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.19–7.23 (m, 1H, Ar-H), 7.42–7.46 (m, 1H, Ar-H), 7.61–7.67 (m, 1H, Ar-H), 7.94–7.98 (m, 1H, Ar-H). 19F NMR (CDCl3, 376.5 MHz): δ = −75.9 (s, 3F, CF3), −114.5 (s, 1F, Ar-F). 13C NMR (CDCl3, 125.7 MHz): δ = 116.4 (d, 2JC-F = 20 Hz), 123.5 (dq, 3JC-F = 15 Hz, 4JC-F 2.5 Hz), 124.9 (qd, 1JC-F = 335.5 Hz, 4JC-F = 3.8 Hz, CF3),125.6 (d, 4JC-F = 3.8 Hz), 127.2, 135.5 (d, 3JC-F = 7.5 Hz), 159.7 (d, 1JC-F = 252.7 Hz, C-F). Anal. Calcd for C7H4F4OS: C, 39.63; H, 1.90; S, 15.11. Found: C, 39.62; H, 1.91; S, 15.11.
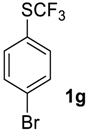
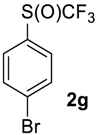
1-Bromo-4-((trifluoromethyl)sulfinyl)benzene2g [30,38]. Crude product contains 97 mol% of 2g and 3 mol% of starting sulfide, which may be removed by washing with pentane to give pure product 2g. White solid; yield 2.6 g (96%); m.p. 57–58 °C (Lit. 56–57 °C). 1H NMR (CDCl3, 500 MHz): δ = 7.66 (d, 3JH-H = 8.5 Hz, 2H, Ar-H), 7.75 (d, 3JH-H = 8.5 Hz, 2H, Ar-H). 19F NMR (CDCl3, 470 MHz): δ = −74.4 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 124.4 (q, 1JC-F = 335.6 Hz, CF3), 127.3, 128.6, 133.0, 134.7. Compound 1g was oxidized on a 15 g scale (in 35 mL of CF3COOH) with the same yield.
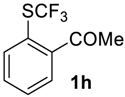
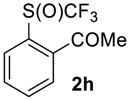
2-((Trifluoromethyl)sulfinyl)-acetophenone2h. Crude product contains 97 mol% of 2h and 3 mol% of starting sulfide, which may be removed by washing with pentane to give pure product 2h. Pale yellow solid; yield 2 g (85 %); m.p. 97–98 °C. IR (KBr): 3080, 2968, 1675, 1585, 1468, 1438, 1359, 1280, 1187, 1130, 1073, 961, 885, 772, 663, 602, 571, 483, 434 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 2.68 (s, 3H, CH3), 7.74 (t, 3JH-H= 7 Hz, 1H, Ar-H), 7.88 (t, J = 7 Hz, 1H, Ar-H), 8.04 (d, 3JH-H = 7 Hz, 1H, Ar-H), 8.36 (d, 3JH-H= 7 Hz, 1H, Ar-H). 19F NMR (CDCl3, 376.5 MHz): δ = −70.0 (s). 13C NMR (CDCl3, 100.6 MHz): δ = 26.2, 125.1 (q, 1JC-F = 340 Hz, CF3), 126.0, 131.0, 132.4, 134.2, 135.4, 140.3, 198.3. Anal. Calcd for C9H7F3O2S: C, 45.76; H, 2.99; S, 13.57. Found: C, 45.78; H, 3.01; S, 13.60.
2-((Trifluoromethyl)sulfinyl)phenyl acetate2i. Crude product was purified by column chromatography, eluent hexane-methyl-tert-butyl ether, 2:1 (Rf = 0.5), but the product contains ~15% of the corresponding phenol. Pale yellow oil; yield 1.8 g (70 %). 1H NMR (CDCl3, 500 MHz): δ = 2.33 (s, 3H, CH3), 7.05 (d, 3JH-H = 8 Hz, 1H, Ar-H), 7.53 (t, 3JH-H = 8 Hz, 1H, Ar-H), 7.68 (t, 3JH-H = 8 Hz, 1H, Ar-H), 8.03 (d, 3JH-H = 8 Hz, 1H, Ar-H). 19F NMR (CDCl3, 470 MHz): δ = −73.5 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 20.5, 123.4, 124.9 (q, 1JC-F = 336 Hz, CF3), 126.8, 127.0, 127.8, 134.5, 148.6, 167.8.
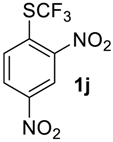
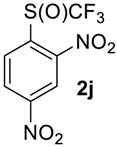
2,4-Dinitro-1-((trifluoromethyl)sulfinyl)benzene2j. Crude product was purified by column chromatography, eluent hexane-methyl-tert-butyl ether, 5:1 (Rf = 0.2). Pale yellow solid; yield 2.4 g (83 %), m.p. 85–87 °C. IR (KBr): 3110, 3039, 2883, 1611, 1544, 1347, 1224, 1186, 1134, 1074, 917, 896, 860, 835, 742, 678, 602, 574, 531, 496, 439 cm−1. 1H NMR (DMSO-d6, 500 MHz): δ = 8.46 (d, 3JH-H = 8 Hz, 1H, Ar-H), 8.92 (d,3JH-H = 8 Hz, 1H, Ar-H), 8.97 (s, 1H, Ar-H). 19F NMR (CDCl3, 376.5 MHz): δ = −68.5 (s). 13C NMR (DMSO-d6, 125.7 MHz): δ = 121.0, 125.7 (q, 1JC-F = 342 Hz, CF3), 128.8, 130.4, 142.1, 146.9, 150.8. Anal. Calcd for C7H3F3N2O5S: C, 29.59; H, 1.06; N, 9.86; S, 11.28. Found: C, 29.58; H, 1.05; N, 9.87; S, 11.29.
2-((Trifluoromethyl)sulfinyl)naphthalene2k. Product was purified by column chromatography, eluent Et2O-petroleum ether, 1:9 (Rf = 0.6). Pale yellow liquid; yield 1.4 g (87%). IR (KBr): 2940, 1171, 1126, 1073, 813, 744,639 cm−1. 1H NMR (300 MHz, CDCl3): δ = 7.53–7.67 (m, 2H, Ar-H), 7.75 (d, 3JH-H = 8.6 Hz, 1H, Ar-H), 7.85–7.96 (m, 2H, Ar-H), 7.99 (d, 3JH-H = 8.7 Hz, 1H, Ar-H), 8.31 (s, 1H, Ar-H). 19F NMR (188 MHz, CDCl3): δ = −74.5 (s). 13C NMR (75 MHz, CDCl3): δ = 120.4, 124.9 (q, 1JC-F = 335 Hz, CF3), 127.7, 127.8, 128.2, 128.8, 129.0, 129.9, 132.4, 132.5, 132.6, 135.5. HRMS: calcd. for C11H7F3SONa 267.0063; found 267.0067 [M − Na]+ (δ = −1.5).
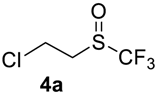
1-Chloro-2-((trifluoromethyl)sulfinyl)ethane4a [14]. Product was purified by distillation. Colorless liquid; yield 1.5 g (83%); b.p. 68–70 °C (10 Torr). 1H NMR (CDCl3, 400 MHz): δ = 3.21–3.24 (m, 1H, CH2), 3.43–3.48 (m, 1H, CH2), 3.95–4.00 (m, 2H, CH2). 19F NMR (CDCl3, 376.5 MHz): δ = −73.6 (s). Compound 3a was oxidized on a 15 g scale (in 35 mL of CF3COOH) with the same yield.
2-(Trifluoromethyl)sulfinyl-ethanol4b. Crude product was purified by distillation, but the product contains 11% of starting sulfide. Colorless liquid; yield 0.25 g (15%); b.p. 108–110 °C (10 Torr). IR (KBr): 3417, 2893, 1396, 1293, 1174, 1138, 1047, 1004, 946, 844, 749, 645, 563, 442 cm−1. 1H NMR (CDCl3, 500 MHz): δ = 3.02–3.20 (m, 2H, CH2), 3.67 (s, 1H, OH), 4.09–4.13 (m, 2H, CH2). 19F NMR (CDCl3, 470 MHz): δ = −73.6 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 51.2, 54.4, 125.5 (q, 1JC-F = 332 Hz, CF3).
2-((Trifluoromethyl)sulfinyl)ethyl acetate4c. Crude product was purified by distillation, but the product contains 15% of the corresponding alcohol. Colorless liquid; yield 1.1 g (54%); b.p. 100–102 °C (10 Torr). 1H NMR (CDCl3, 500 MHz): δ = 2.10 (s, 3H, CH3), 3.20–3.33 (m, 2H, CH2), 4.47–4.64 (m, 2H, CH2). 19F NMR (CDCl3, 470 MHz): δ = −73.7 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 20.4, 48.2, 55.9, 125.3 (q, 1JC-F = 333 Hz, CF3), 170.3.
Methyl 2-((trifluoromethyl)sulfinyl)acetate4d [16]. Product was purified by distillation. Colorless liquid; yield 1.1 g (60%); b.p. 90 °C (10 Torr). 1H NMR (CDCl3, 500 MHz): δ = 3.79 (s, 3H, CH3), 3.87 (d, 2JH-H = 15 Hz, 1H, CH2), 3.94 (d, 2JH-H = 15 Hz, 1H, CH2). 19F NMR (CDCl3, 376.5 MHz): δ = −73.8 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 53.5, 53.6, 125.1 (q, 1JC-F = 334.5 Hz, CF3), 164.2.
Methyl 3-((trifluoromethyl)sulfinyl)propanoate4e. Product was purified by distillation. Colorless liquid; yield 1.25 g (60%); b.p. 96–98 °C (10 Torr). IR (KBr): 2959, 1735, 1440, 1367, 1173, 1136, 1074, 980, 828, 746, 661, 564, 450 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 2.77–2.97 (m, 2H, CH2), 3.17–3.27 (m, 2H, CH2), 3.72 (s, 3H, CH3). 19F NMR (CDCl3, 376.5 MHz): δ = −74.2 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 25.6, 43.2 (q, 3JC-F = 2.5 Hz, CH2-S(O)CF3), 52.4, 125.4 (q, 1JC-F = 334.5 Hz, CF3), 170.8. Anal. Calcd for C5H7F3O3S: C, 29,42; H, 3,46; S, 15.70. Found: C, 29.43; H, 3.44; S, 15.72.
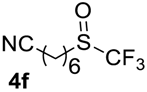
7-((Trifluoromethyl)sulfinyl)heptanenitrile4f. Compound 3f was oxidized on a 0.12 g scale. Product was purified by preparative TLC chromatography, eluent Et2O-petroleum ether, 1:9 (Rf = 0.1). Pale yellow liquid; yield 0.11 g (85%). IR (KBr): 2936, 2864, 2239, 1171, 1143, 1080 cm−1. 1H NMR (CDCl3, 300 MHz): δ = 1.40–1.56 (m, 4H, CH2 -CH2), 1.56–1.76 (m, 2H, CH2), 1.74–1.92 (m, 2H, CH2), 2.31 (t, 3JH-H = 6.9 Hz, 2H, CH2), 2.73–2.90 (m, 1H, CH), 2.93–3.11 (m, 1H, CH). 19F NMR (CDCl3, 188 MHz): δ = −74.1 (s). 13C NMR (CDCl3, 75 MHz): δ = 17.0, 21.5, 24.9, 27.8, 28.1, 48.2, 119.5, 125.3 (q, 1JC-F = 333 Hz, CF3). HRMS: calcd. for C8H12NF3SONa 250.0477; found 250.0489 [M − Na]+ (δ = 0.4).
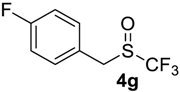
1-Fluoro-4-(((trifluoromethyl)sulfinyl)methyl)benzene4g [39]. Compound 3g was oxidized on a 0.05 g scale. Product was purified by preparative TLC chromatography, eluent Et2O-petroleum ether, 1:9 (Rf = 0.3). Pale yellow solid; yield 0.041 g (85%); m.p. 80 °C (Lit. 81–82 °C). IR (KBr): 2983, 2927, 1600, 1507, 1243, 1178, 1139, 1050, 1011, 842, 745 cm−1. 1H NMR (CDCl3, 300 MHz): δ = 4.20 (AB syst., 2JH-H = 13.2 Hz, 2H, CH2), 7.11 (t, 3JH-H = 3JH-F= 8.4 Hz, 2H, Ar-H), 7.35 (dd, 3JH-H = 8.4 Hz, 4JH-F = 5.3 Hz, 2H, Ar-H). 19F NMR (CDCl3, 188 MHz): δ = −73.11 (s, 3F, CF3), −112.25(m, 1F, Ar-F). 13C NMR (CDCl3, 75 MHz): δ = 54.7 (q, 3JC-F = 3.1 Hz), 116.5 (d, 2JC-F = 21.9 Hz), 123.3 (d, 4JC-F = 3.3 Hz), 125.3 (q, 1JC-F = 335.0 Hz, CF3), 132.3 (d, 3JC-F = 8.5 Hz), 163.4 (d, 1JC-F = 249.3 Hz, C-F).
((Difluoromethyl)sulfinyl)benzene6a [40]. Colorless liquid; yield 1.2 g (70%); b.p. 130 °C (10 Torr). 1H NMR (CDCl3, 400 MHz): δ = 6.02 (t, 2JH-F = 55.6 Hz, 1H, CHF2), 7.55-7.60 (m, 3H, Ar-H), 7.69–7.71 (m, 2H, Ar-H). 19F {H} NMR (CDCl3, 376.5 MHz): δ = −119.4 (d, 2JF-F = 260 Hz, 1F, CHF2), -120.2 (d, 2JF-F = 260 Hz, 1F, CHF2). 13C NMR (CDCl3, 100.6 MHz): δ = 120.8 (dd, 1JC-F= 288 Hz, 287 Hz, CHF2), 125.4, 129.5, 132.8, 136.6 (t, 3JC-F = 4 Hz).
N-(4-((Difluoromethyl)sulfinyl)phenyl)acetamide6b [30]. Pale yellow solid; yield 1.9 g (80%); m.p. 166–167 °C (Lit. 170–171 °C). 1H NMR (DMSO-d6, 500 MHz): δ = 2.09 (s, 3H, CH3), 6.89 (t, 2JH-F = 54 Hz, 1H, CHF2), 7.70 (d, 3JH-H = 8 Hz, 2H, Ar-H), 7.85 (d, 3JH-H = 8 Hz, 2H, Ar-H), 10.34 (s, 1H, NH). 19F NMR (DMSO-d6, 376.5 MHz): δ = -121.4 (dd, 2JF-F = 257 Hz, 2JH-F = 54 Hz, 1F, CHF2), -125.5 (dd, 2JF-F = 257 Hz, 2JH-F = 54 Hz, 1F, CHF2). 19F {H} NMR (CDCl3, 376.5 MHz): δ = −121.5 (d, 2JF-F = 257 Hz, 1F, CHF2), -123.3 (d, 2JF-F = 257 Hz, 1F, CHF2). 13C NMR (DMSO-d6, 125.7 MHz): δ = 24.6, 119.8, 120.6 (t, 1JC-F = 292 Hz, CHF2), 120.9 (t, 1JC-F = 280 Hz, CHF2), 127.2, 127.3, 129.9, 143.8, 169.5.
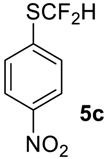
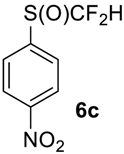
1-((Difluoromethyl)sulfinyl)-4-nitrobenzene6c [30]. Pale yellow solid; yield 1.7 g (75%); m.p. 84–85 °C (Lit. 81–83 °C). 1H NMR (DMSO-d6, 500 MHz): δ = 7.08 (t, 2JH-F = 56 Hz, 1H, CHF2), 8.04 (d, 3JH-H = 6.5 Hz, 2H, Ar-H), 8.46 (d, 3JH-H = 6.5 Hz, 2H, Ar-H). 19F NMR (DMSO-d6, 376.5 MHz): δ = −120.8 (dd, 2JF-F = 254 Hz, 2JH-F = 56 Hz, 1F, CHF2), -125.9 (dd, 2JF-F = 254 Hz, 2JH-F = 56 Hz, 1F, CHF2). 19F {H} NMR (DMSO-d6, 376.5 MHz): δ = −120.8 (d, 2JF-F = 254 Hz, 1F, CHF2), −125.9 (d, 2JF-F = 254 Hz, 1F, CHF2). 13C NMR (DMSO-d6, 125.7 MHz): δ = 120.4 (t, 1JC-F = 284 Hz, CHF2), 120.6 (t, 1JC-F = 287 Hz, CHF2), 124.7, 124.9, 127.4, 127.5, 133.9, 144.3, 150.4.
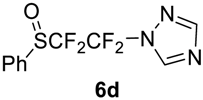
(1,1,2,2-Tetrafluoro-2-((1,2,4-triazol)-1-yl)-ethyl)-sulfinylbenzene6d. Crude product was purified by column chromatography, eluent CH2Cl2, then methyl-tert-butyl ether (Rf = 0.7). Pale yellow solid; yield 2.7 g (92%); m.p. 77–78 °C. IR (KBr): 3105, 2872, 1819, 1688, 1582, 1512, 1477, 1453, 1403, 1378, 1294, 1262, 1221, 1191, 1140, 1111, 1070, 981, 903, 830, 756, 691, 667, 629, 591, 514, 482, 449 cm−1. 1H NMR (CDCl3, 500 MHz): δ = 7.56–7.65 (m, 3H, Ar-H), 7.77 (d, 2H, Ar-H), 8.15 (s, 1H, Het-H), 8.59 (s, 1H, Het-H). 19F NMR (CDCl3, 376.5 MHz): δ = −94.0 (d, 2JF-F = 226 Hz, 1F, CF2), −94.6 (d, 2JF-F = 226 Hz, 1F, CF2), −112.5 (d, 2JF-F = 237 Hz, 1F, CF2), 124.3 (d, 2JF-F = 237 Hz, 1F, CF2). 13C NMR (CDCl3, 125.7 MHz): δ = 113.0 (tt, 1JC-F = 271 Hz, 2JC-F = 31 Hz, CF2), 116.9 (ddt, 1JC-F = 318 Hz, 1JC-F = 308 Hz, 2JC-F 38 Hz, CF2), 126.7, 129.5, 133.8, 135.0, 143.4, 154.1. Anal. Calcd for C12H12F4N3OS: C, 44.72; H, 3.75; N, 13.04; S, 9.95. Found: C, 44.74; H, 3.76; N, 13.02; S, 9.98.
((Bromodifluoromethyl)sulfinyl)benzene6e [41]. Compound 5e was oxidized only on a 50 mmol scale. Crude product was purified by column chromatography, eluent petroleum ether-diethyl ether, 8:2 (Rf = 0.6). Yellow oil; yield 8.1 g (91%). IR (KBr):1443, 1114, 1073, 1056, 854, 827, 742, 687 cm−1. 1H NMR (CDCl3, 200 MHz): δ= 7.52 (m, 3H, ArH), 7.72 (m, 2H, ArH). 19F NMR (CDCl3, 188 MHz): δ = -53.49 and -55.56 (2F, CF2Br, syst. AB 2JC-F = 145 Hz). 13C NMR (CDCl3, 50 MHz): δ = 126.2, 128.4 (dd, 1JC-F = 355 Hz, 350.5Hz), 129.1, 133.4, 136.7 (dd, 3JC-F = 3 Hz, 1 Hz).
((Dichlorofluoromethyl)sulfinyl)benzene6f [42]. Compound 5f was oxidized only on a 50 mmol scale. Crude product was purified by column chromatography, eluent petroleum ether-diethyl ether, 8:2 (Rf = 0.5). Yellow oil; yield 10.6 g (69%). IR (KBr): 1445, 1104, 1061, 840, 792, 744, 684 cm−1. 1H NMR (CDCl3, 200 MHz): δ = 7.61 (m, 3H, Ar-H), 7.82 (m, 2H, Ar-H). 19F NMR (CDCl3, 188 MHz): δ = −62.8 (s). 13C NMR (CDCl3, 50 MHz): δ = 126.9 (d, 1JC-F = 340 Hz), 126.9 (d, 4JC-F = 1.5 Hz), 128.9, 133.5, 137.4 (d, 3JC-F = 1.5 Hz).
N-(6-((Trifluoromethyl)sulfinyl)pyridin-3-yl)acetamide8. Crude product was purified by column chromatography, eluent ethyl acetate (Rf = 0.5) to give compound 8 with 80 % purity (contains ~20% of starting sulfide). Yellow solid; yield 0.75 g (~30%). 1H NMR (DMSO-d6, 400 MHz): δ = 2.12 (s, 3H, CH3), 8.02 (d, 3JH-H = 8.8 Hz, 1H, Het-H), 8.41 (d, 3JH-H = 8.8 Hz, 1H, Het-H), 10.6 (s, 1H, NH), 8.88 (s, 1H, Het-H). 19F {H} NMR (DMSO-d6, 376.5 MHz): δ = −72.7 (s). 13C NMR (CDCl3, 125.7 MHz): δ = 24.4, 122.5, 125.3 (q, 1JC-F = 338 Hz, CF3), 127.9, 139.8, 141.3, 149.3, 170.1.
4. Conclusions
In summary, a simple, convenient, and high-yielding procedure for the oxidation of fluoroalkyl sulfides to fluoroalkyl sulfoxides was developed. It was shown that the new protocol is suitable for a wide range of aliphatic and aromatic substrates on a milligram and multigram scales. In almost all cases, over-oxidation did not occur, and the crude products contained only a small quantity of starting sulfides.
Supplementary Materials
The following are available online: titration of H2O2 solution, NMRs and IRs of the new synthesized compounds.
Author Contributions
Y.L.Y. conceived idea of the article; L.V.S., R.K.O., A.F., B.P., P.D. performed the experiments; L.V.S. wrote the paper, Y.L.Y. and E.M. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank Enamine Ltd (www.enamine.net) and CNRS for generous support of our research. We thank Karen Wright for the improvement of the English Manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, X.-H.; Matsuzaki, K.; Shibata, N. Synthetic Methods for Compounds Having CF3-S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731–764. [Google Scholar] [CrossRef] [PubMed]
- Bizet, V.; Kowalczyk, R.; Bolm, C. Fluorinated sulfoximines: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 2426–2438. [Google Scholar] [CrossRef]
- Shen, X.; Hu, J. Fluorinated Sulfoximines: Preparation, Reactions and Applications. Eur. J. Org. Chem. 2014, 4437–4451. [Google Scholar] [CrossRef]
- Barthelemy, A.L.; Magnier, E. Recent trends in perfluorinated sulfoximines. C. R. Chim. 2018, 21, 711–722. [Google Scholar] [CrossRef]
- Batisse, C.; Panossian, A.; Hanquet, G.; Leroux, F. Access towards enantiopure α,α-difluoromethyl alcohols by means of sulfoxides as traceless chiral auxiliaries. Chem. Comm. 2018, 54, 10423–10426. [Google Scholar] [CrossRef]
- Movchun, V.N.; Kolomeitsev, A.A.; Yagupolskii, Y.L. Nucleophilic trifluoromethylation of organic substrates using (trifluoromethyl)trimethylsilane in the presence of a fluoride anion II. A convenient route to aryltrifluoromethyl-sulfides, -sulfoxides and -sulfones. J. Fluorine. Chem. 1995, 70, 255–257. [Google Scholar] [CrossRef]
- Patel, N.R.; Kirchmeier, R.L. Trifluoromethylation and pentafluorophenylation of sulfur and carbon centers using (trifluoromethyl)- and (pentafluorophenyl)trimethylsilane. Inorg. Chem. 1992, 31, 2537–2540. [Google Scholar] [CrossRef]
- Singh, R.P.; Cao, G.; Kirchmeier, R.L.; Shreeve, J.M. Cesium Fluoride Catalyzed Trifluoromethylation of Esters, Aldehydes, and Ketones with (Trifluoromethyl)trimethylsilane. J. Org. Chem. 1999, 64, 2873–2876. [Google Scholar] [CrossRef] [PubMed]
- Wakselman, C.; Tordeux, M.; Freslon, C.; Saint-Jalmes, L. Aryltrifluoromethylsulfoxides: Sulfinylation of Aromatics by Triflinate Salts in Acid Medium. Synlett 2001, 4, 550–552. [Google Scholar] [CrossRef]
- Chen, X.; Tordeux, M.; Desmurs, J.-R.; Wakselman, C. Thia-Fries rearrangement of aryl triflinates to trifluoromethanesulfinylphenols. J. Fluor. Chem. 2003, 123, 51–56. [Google Scholar] [CrossRef]
- Billard, T.; Greiner, A.; Langlois, B.R. A new equivalent of the CF3S(O)+ cation. Synthesis of trifluoromethanesulfinates and trifluoromethanesulfinamides. Tetrahedron 1999, 55, 7243–7250. [Google Scholar] [CrossRef]
- Romanenko, V.D.; Thoumazet, C.; Lavallo, V.; Tham, F.S.; Bertrand, G. Synthesis and reactivity of a stable crystalline diastereomerically pure trifluoromethanesulfinic acid derivative: (S)-(−)-1-trifluoromethylsulfinyl-(R)-4-phenyloxazolidin-2-one. Chem. Commun. 2003, 14, 1680–1681. [Google Scholar] [CrossRef]
- Yang, J.-J.; Kirchmeier, R.L.; Shreeve, J.M. New Electrophilic Trifluoromethylating Agents. J. Org. Chem. 1998, 63, 2656–2660. [Google Scholar] [CrossRef] [PubMed]
- Sokolenko, L.V.; Maletina, I.I.; Yagupolskii, L.M.; Yagupolskii, Yu. L. A Convenient and Efficient Synthesis of Trifluoromethyl Vinyl Sulfoxide and its Reactivity in Addition Reactions. Synlett 2010, 14, 2075–2078. [Google Scholar] [CrossRef]
- Venier, C.G.; Squires, T.G.; Chen, Y.-Y.; Hussmann, G.P.; Shei, J.C.; Smith, B.F. Peroxytrifluoroacetic acid oxidation of sulfides to sulfoxides and sulfones. J. Org. Chem. 1982, 47, 3773–3774. [Google Scholar] [CrossRef]
- Sokolenko, L.V.; Yagupolskii, Yu. L.; Kumanetska, L.S.; Marrot, J.; Magnier, E.; Lipetskij, V.O.; Kalinin, I.V. CF3S(O)n-Containing Enaminones as Precursors for syntheses of Pyrimidine-4(3H)-ones. Tetrahedron Lett. 2017, 58, 1308–1311. [Google Scholar] [CrossRef]
- Tang, R.-Y.; Zhong, P.; Lin, Q.-L. A convenient conversion of pyrazolyl disulfide to sulfides by sodium dithionite and synthesis of sulfoxides. J. Fluorine. Chem. 2006, 127, 948–953. [Google Scholar] [CrossRef]
- Scribner, R.M. Some New Sulfonyl- and Trifluoromethylthio-p-benzoquinones. Their Reactions, Polarographic Reduction Potentials, and π Acid Strengths. J. Org. Chem. 1966, 31, 3671–3682. [Google Scholar] [CrossRef]
- Pazenok, S.V.; Kondratenko, N.V.; Popov, V.I.; Troitskaya, V.I.; Il’chenko, A.Y.; Al’perovich, M.A.; Yagupolskii, L.M. Indo- and benzindocyanine dyes with fluorine-containing substituents. Chemistry of Heterocyclic Compounds 1983, 19, 1182–1187. [Google Scholar] [CrossRef]
- Magnier, E.; Tordeux, M.; Goumont, R.; Magder, K.; Wakselman, C. Perfluoroalkylation of 2-mercaptoethanol as a key step for a new synthesis of perfluoroalkyl vinyl sulfides, sulfoxides and sulfones. J. Fluorine. Chem. 2003, 124, 55–59. [Google Scholar] [CrossRef]
- Moise, J.; Goumont, R.; Magnier, E.; Wakselman, C. Synthesis of Fluoroalkyl Vinyl Sulfoxides and their Use in Diels-Alder Reactions. Synthesis 2004, 14, 2297–2302. [Google Scholar] [CrossRef]
- Meng, C.Q.; Le Hir de Fallois, L.P.; Lee, H.I.; Zhan, X.; Labrosse, J.R.; Mulhauser, M. Processes for the Preparation of 1-Aryl-5-Alkyl Pyrazole Compounds. U.S. Patent 2013/281710, 24 October 2013. [Google Scholar]
- Lee, H.I.; Le Hir de Fallois, L.P.; Timmons, Ph.R.; Cawthorne, W.G.; de Leon, A.P. 1-Aryl-5-Alkyl Pyrazole Derivative Compounds, Processes of Making and Methods of Using Thereof. U.S. Patent 2008/031902, 7 February 2008. [Google Scholar]
- Chou, D.T.; Knauf, W.; Maier, M.; Malaska, M.J.; Mcintyre, D.; Lochhaas, F.; Huber, S. K Pesticidal Agents on the Basis of 1-Aryl-Aminopyrrol. WO Patent 2006/000315, 5 January 2006. [Google Scholar]
- Wu, T.-T.; Sinodis, D.N.; Timmons, P.R.; Powell, G.S.; Chou, D.T.; Newsome, P.W.; Hall, L.S. Pesticidal 1-Arylimidazoles. U.S. Patent 5223525, 29 June 1993. [Google Scholar]
- Hatton, L.R.; Buntain, I.G.; Hawkins, D.W.; Parnell, E.W.; Pearson, C.J.; Roberts, D.A. Derivatives of N-phenylpyrazoles. U.S. Patent 5232940, 3 August 1993. [Google Scholar]
- Huang, J.; Lowder, P.D.; Ray, N.C.; Hawkins, D.W. Pesticidal 1-Arylpyrazole Derivatives. U.S. Patent 5817688, 6 October 1998. [Google Scholar]
- Caster, K.C.; Rao, A.S.; Mohan, H.R. Trifluoroperacetic acid. In Handbook of Reagents for Organic Synthesis. Fluorine-Containing Reagents; Paquette, L.A., Ed.; Wiley: Chichester, England, 2007; pp. 619–624. [Google Scholar]
- Emmons, W.D. Peroxytrifluoroacetic Acid. I. The Oxidation of Nitrosamines to Nitramines1. J. Am. Chem. Soc. 1954, 76, 3468–3470. [Google Scholar] [CrossRef]
- Sedova, L.N.; Gandel’sman, L.Z.; Alekseeva, L.A.; Yagupol’skii, L.M. Derivatives of phenyldifluoromethyl sulfide, sulfoxide, and sulfone. Zh. Obshch. Khim. 1969, 39, 2057–2062. [Google Scholar]
- Petko, K.I.; Sokolenko, T.M.; Bezdudny, A.V.; Yagupolskii, L.M. N-(2-Bromotetrafluoroethyl) derivatives of five-membered nitrogen-containing heterocycles. J. Fluorine. Chem. 2005, 126, 1342–1346. [Google Scholar] [CrossRef]
- Anselmi, E.; Blazejewski, J.-C.; Tordeux, M.; Wakselman, C. Perfluoroalkylation of aliphatic thiols in the presence of sodium hydroxymethanesulfinate. J. Fluorine Chem. 2000, 105, 41–44. [Google Scholar] [CrossRef]
- Anselmi, E.; Simon, C.; Marrot, J.; Bernardelli, P.; Schio, L.; Pégot, B.; Magnier, E. Ionic Liquids for Fast and Solvent-Free Nucleophilic Trifluoromethylthiolation of Alkyl Halides and Alcohols. Eur. J. Org. Chem. 2017, 2017, 6319–6326. [Google Scholar] [CrossRef]
- Pégot, B.; Urban, C.; Bourne, A.; Le, T.-N.; Bouvet, S.; Marrot, J.; Diter, P.; Magnier, E. Difluoromethyl and Chlorofluoromethyl Sulfoximines: Synthesis and Evaluation as Electrophilic Perfluoroalkylating Reagents. Eur. J. Org. Chem. 2015, 2015, 3069–3075. [Google Scholar] [CrossRef]
- Bzhezovsky, V.M.; Penkovsky, V.V.; Rozhenko, A.B.; Iksanova, S.V.; Kondratenko, N.V.; Yagupolskii, L.M. Multinuclear NMR spectroscopy and semi-empirical MNDO-PM3 quantum chemical investigations of the compounds C6H5XY (X=S, SO, SO2; Y=CF3, CH3). J. Fluor. Chem. 1994, 69, 41–49. [Google Scholar] [CrossRef]
- Tordeux, M.; Magnier, E.; Guidotti, J.; Diter, P.; Wakselman, C. 29/28, 30/28Silicon, 34/32sulfur and 80/77selenium isotope-induced fluorine chemical shifts through two bonds. Magn. Reson. Chem. 2004, 42, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Yagupol’skii, L.M.; Marenets, M.S.; Kondratenko, N.V. The orienting action of trifluoromethyl sulfoxide group. Zh. Obshch. Khim. 1965, 62, 377, Chem. Abstr.1965, 62, 14551a. [Google Scholar]
- Yagupol’skii, L.M.; Troitskaya, V.I.; Gruz, B.E.; Kondratenko, N.V. Cyanine dyes containing fluorine. XII. Cyanine dyes from derivatives of 5-trifluoromethylthio-2-methylbenzimidazole. Zh. Obshch. Khim. 1966, 64, 1644–1650, Chem. Abstr.1966, 64, 3731c. [Google Scholar]
- Orda, V.V.; Yagupol’skii, L.M.; Bystrov, V.F.; Stepanyants, A.U. Transmission of inductive effect of substituents SCF3, SOCF3, and SO2CF3, through a methylene group. Zh. Obshch. Khim. 1965, 63, 1628–1636, Chem. Abstr.1965, 63, 17861c. [Google Scholar]
- Surya Prakash, G.K.; Weber, C.; Chacko, S.; Olah, G.A. New Electrophilic Difluoromethylating Reagent. Org. Lett. 2007, 9, 1863–1866. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.; Wiemers, D.M. Synthesis of bromodifluoromethyl phenyl sulfide, sulfoxide and sulfone. J. Fluorine Chem. 1981, 18, 573–582. [Google Scholar] [CrossRef]
- Saikia, A.K.; Tsuboi, S. Chemistry of Trichlorofluoromethane: Synthesis of Chlorofluoromethyl Phenyl Sulfone and Fluoromethyl Phenyl Sulfone and some of Their Reactions. J. Org. Chem. 2001, 66, 643–647. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–6 are available from the corresponding authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).