Concerted Catalysis by Nanocellulose and Proline in Organocatalytic Michael Additions
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Cellulose Nanofibers on Proline-Catalyzed Michael Additions
2.2. Optimization of Reaction Conditions
2.3. Substrate Scope
3. Materials and Methods
3.1. General
3.2. Preparation of TOCNs with Low Carboxylate Content
3.3. Water Removal Process from Nanocellulose Samples
3.4. Representative Procedure for the Proline-Catalyzed Michael Addition of Cyclohexanone to trans-β-Nitrostyrene in the Presence of TOCNs
Spectroscopic Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials-binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Fujisawa, S.; Saito, T.; Isogai, A. Selective Permeation of Hydrogen Gas Using Cellulose Nanofibril Film. Biomacromolecules 2013, 14, 1705–1709. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultrafine Polysaccharide Nanofibrous Membranes for Water Purification. Biomacromolecules 2011, 12, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Nyström, G.; Mihranyan, A.; Razaq, A.; Lindström, T.; Nyholm, L.; Strømme, M. A nanocellulose polypyrrole composite based on microfibrillated cellulose from wood. J. Phys. Chem. B 2010, 114, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Nogi, M.; Komoda, N.; Nge, T.T.; Sugahara, T.; Suganuma, K. Uniformly connected conductive networks on cellulose nanofiber paper for transparent paper electronics. NPG Asia Mater. 2014, 6, e93. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and High Gas Barrier Films of Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef] [PubMed]
- Hamou, K.B.; Kaddami, H.; Dufresne, A.; Boufi, S.; Magnin, A.; Erchiqui, F. Impact of TEMPO-oxidization strength on the properties of cellulose nanofibril reinforced polyvinyl acetate nanocomposites. Carbohydr. Polym. 2018, 181, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, T.; Sawada, T.; Wada, M. Chirality-specific hydrolysis of amino acid substrates by cellulose nanofibers. Chem. Commun. 2013, 49, 8827–8829. [Google Scholar] [CrossRef]
- Koga, H.; Namba, N.; Takahashi, T.; Nogi, M.; Nishina, Y. Renewable Wood Pulp Paper Reactor with Hierarchical Micro/Nanopores for Continuous-Flow Nanocatalysis. ChemSusChem 2017, 10, 2560–2565. [Google Scholar] [CrossRef]
- Kaushik, M.; Basu, K.; Benoit, C.; Cirtiu, C.M.; Vali, H.; Moores, A. Cellulose nanocrystals as chiral inducers: Enantioselective catalysis and transmission electron microscopy 3D characterization. J. Am. Chem. Soc. 2015, 137, 6124–6127. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef]
- Tamura, Y.; Kanomata, K.; Kitaoka, T. Interfacial Hydrolysis of Acetals on Protonated TEMPO-oxidized Cellulose Nanofibers. Sci. Rep. 2018, 8, 5021. [Google Scholar] [CrossRef] [PubMed]
- Kanomata, K.; Tatebayashi, N.; Habaki, X.; Kitaoka, T. Cooperative catalysis of cellulose nanofiber and organocatalyst in direct aldol reactions. Sci. Rep. 2018, 8, 4098. [Google Scholar] [CrossRef] [PubMed]
- List, B. Proline-catalyzed asymmetric reactions. Tetrahedron 2002, 58, 5573–5590. [Google Scholar] [CrossRef]
- Berner, O.M.; Tedeschi, L.; Enders, D. Asymmetric Michael additions to nitroalkenes. Eur. J. Org. Chem. 2002, 2002, 1877–1894. [Google Scholar] [CrossRef]
- Mase, N.; Watanabe, K.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas, C.F. Organocatalytic Direct Michael Reaction of Ketones and Aldehydes with β-Nitrostyrene in Brine. J. Am. Chem. Soc. 2006, 128, 4966–4967. [Google Scholar] [CrossRef]
- Nising, C.F.; Bräse, S. Recent developments in the field of oxa-michael reactions. Chem. Soc. Rev. 2012, 41, 988–999. [Google Scholar] [CrossRef]
- Enders, D.; Wang, C.; Liebich, J.X. Organocatalytic asymmetric aza-Michael additions. Chem. Eur. J. 2009, 15, 11058–11076. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Gómez, C.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Recent advances in asymmetric organocatalyzed conjugate additions to nitroalkenes. Molecules 2017, 22, 895. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W. Recent advances in organocatalytic asymmetric Michael reactions. Catal. Sci. Technol. 2012, 2, 42–53. [Google Scholar] [CrossRef]
- Hayashi, Y.; Ogasawara, S. Time Economical Total Synthesis of (−)-Oseltamivir. Org. Lett. 2016, 18, 3426–3429. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, A.Y.; Sukhanova, A.A.; Zlotin, S.G. Stereoselective reactions of nitro compounds in the synthesis of natural compound analogs and active pharmaceutical ingredients. Tetrahedron 2016, 72, 6191–6281. [Google Scholar] [CrossRef]
- List, B.; Pojarliev, P.; Martin, H.J. Efficient Proline-Catalyzed Michael Additions of Unmodified Ketones to Nitro Olefins. Org. Lett. 2001, 3, 2423–2425. [Google Scholar] [CrossRef]
- Enders, D.; Seki, A. Proline-Catalyzed Enantioselective Michael Additions of Ketones to Nitrostyrene. Synlett 2002, 2002, 0026–0028. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Wei, S.; Sun, J. l-Proline derived triamine as a highly stereoselective organocatalyst for asymmetric Michael addition of cyclohexanone to nitroolefins. Tetrahedron Asymmetry 2007, 18, 1308–1312. [Google Scholar] [CrossRef]
- Cobb, A.J.A.; Longbottom, D.A.; Shaw, D.M.; Ley, S.V. 5-Pyrrolidin-2-yltetrazole as an asymmetric organocatalyst for the addition of ketones to nitro-olefins. Chem. Commun. 2004, 1808–1809. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.; Zhao, C.G. Modularly designed organocatalytic assemblies for direct nitro-Michael addition reactions. Angew. Chem. Int. Ed. 2008, 47, 7714–7717. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Abe, T.; Wang, X.B.; Kodama, K.; Hirose, T.; Zhang, G.Y. Self-assembled proline-amino thioureas as efficient organocatalysts for the asymmetric Michael addition of aldehydes to nitroolefins. Tetrahedron Asymmetry 2010, 21, 2925–2933. [Google Scholar] [CrossRef]
- Porcar, R.; Burguete, M.I.; Lozano, P.; Garcia-Verdugo, E.; Luis, S.V. Supramolecular Interactions Based on Ionic Liquids for Tuning of the Catalytic Efficiency of (l)-Proline. ACS Sustain. Chem. Eng. 2016, 4, 6062–6071. [Google Scholar] [CrossRef]
- Kotrusz, P.; Toma, S.; Schmalz, H.-G.; Adler, A. Michael Additions of Aldehydes and Ketones to β-Nitrostyrenes in an Ionic Liquid. Eur. J. Org. Chem. 2004, 2004, 1577–1583. [Google Scholar] [CrossRef]
- Benaglia, M.; Cinquinia, M.; Cozzi, F.; Puglisi, A.; Celentano, G. Poly(ethylene-glycol)-supported proline: A recyclable aminocatalyst for the enantioselective synthesis of γ-nitroketones by conjugate addition. J. Mol. Catal. A Chem. 2003, 204–205, 157–163. [Google Scholar] [CrossRef]
- Alza, E.; Cambeiro, X.C.; Jimenez, C.; Pericàs, M.A. Highly enantioselective Michael additions in water catalyzed by a PS-supported pyrrolidine. Org. Lett. 2007, 9, 3717–3720. [Google Scholar] [CrossRef] [PubMed]
- Betancort, J.M.; Sakthivel, K.; Thayumanavan, R.; Tanaka, F.; Barbas III, C.F. Catalytic Direct Asymmetric Michael Reactions: Addition of Unmodified Ketone and Aldehyde Donors to Alkylidene Malonates and Nitro Olefins. Synthesis 2004, 2004, 1509–1521. [Google Scholar] [CrossRef]
- Kaplaneris, N.; Koutoulogenis, G.; Raftopoulou, M.; Kokotos, C.G. 4-Fluoro and 4-Hydroxy Pyrrolidine-thioxotetrahydropyrimidinones: Organocatalysts for Green Asymmetric Transformations in Brine. J. Org. Chem. 2015, 80, 5464–5473. [Google Scholar] [CrossRef]
- Patil, M.P.; Sunoj, R.B. The role of noninnocent solvent molecules in organocatalyzed asymmetric michael addition reactions. Chem. Eur. J. 2008, 14, 10472–10485. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Jalal, S.; Sarkar, S.; Bera, K.; Maiti, S.; Jana, U. Synthesis of nitroalkenes involving a cooperative catalytic action of iron(III) and piperidine: A one-pot synthetic strategy to 3-alkylindoles, 2H-chromenes and N-arylpyrrole. Eur. J. Org. Chem. 2013, 2013, 4823–4828. [Google Scholar] [CrossRef]
- Kondo, T.; Kose, R.; Naito, H.; Kasai, W. Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr. Polym. 2014, 112, 284–290. [Google Scholar] [CrossRef]
- Syu, S.; Kao, T.-T.; Lin, W. A new type of organocatalyst for highly stereoselective Michael addition of ketones to nitroolefins on water. Tetrahedron 2010, 66, 891–897. [Google Scholar] [CrossRef]
Sample Availability: All data generated and/or compound samples analyzed during this study are included in this article and are available from the corresponding author on reasonable request. |
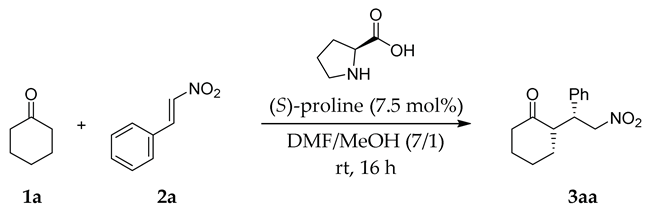
| Entry | Additive | Yield (%) b | syn:antic | ee for syn (%) c |
|---|---|---|---|---|
| 1 | None | 35 | 89:11 | 32 |
| 2 d | TOCN | 78 | 90:10 | 35 |
| 3 | TOCN | 88 | 90:10 | 43 |
| 4 e | TOCN | Trace | - | - |
| 5 f | TOCN | 81 | 95:5 | 33 |
| 6 g | TOCN | 86 | 93:7 | -39 |
| 7 | Sodium acetate | 41 | 89:11 | 6 |
| 8 h | Carboxymethylcellulose | 34 | 90:10 | 27 |
| 9 i | TOCN/low carboxylate | 42 | 82:18 | 42 |
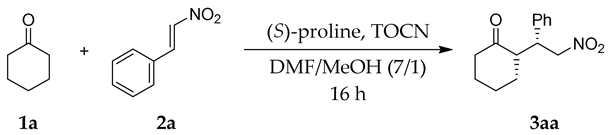
| Entry | Conditions | TOCN | Yield (%) b | syn:antic | ee for syn (%) c |
|---|---|---|---|---|---|
| 1 | 5 mol% catalyst, rt | − | 37 | 87:13 | 29 |
| + | 74 | 93:7 | 39 | ||
| 2 | 15 mol% catalyst, rt | − | 58 | 69:31 | 26 |
| + | 77 | 69:31 | 39 | ||
| 3 | 7.5 mol% catalyst, 0 °C | − | 9 | 77:23 | 19 |
| + | 23 | 74:26 | 39 | ||
| 4 d | 7.5 mol% catalyst, 40 °C | − | 66 | 94:6 | 32 |
| + | 91 | 94:6 | 42 | ||
| 5 | Substrate/TOCN (mg/mg) 74.6/25 | + | 51 | 92:8 | 41 |
| 6 | Substrate/TOCN (mg/mg) 74.6/50 | + | 66 | 94:6 | 43 |
| 7 | Substrate/TOCN (mg/mg) 74.6/100 | + | 88 | 90:10 | 41 |
| 8 | Substrate/TOCN (mg/mg) 74.6/150 | + | 63 | 71:29 | 41 |
| 9 | Substrate/TOCN (mg/mg) 74.6/200 | + | 43 | 83:17 | 43 |
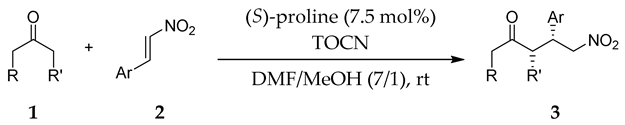
| Entry | Substrates | Product | Time (h) | TOCN | Yield (%) b | syn:anti | ee for syn (%) c |
|---|---|---|---|---|---|---|---|
| 1 |  |  | 48 | − | 26 | 90:10 d | 31 |
| 48 | + | 68 | 93:7 d | 35 | |||
| 2 | 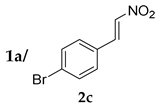 | 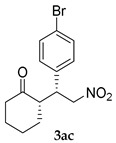 | 24 | − | 59 | 96:4 c | 28 |
| 24 | + | 83 | 95:5 c | 50 | |||
| 3 |  | 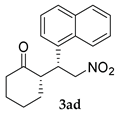 | 48 | − | 75 | 87:13 d | 10 |
| 48 | + | 71 | 87:13 d | 21 | |||
| 4 | 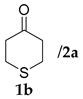 | 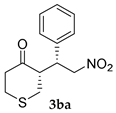 | 24 | − | 18 | 94:6 d | 24 |
| 24 | + | 76 | 97:3 d | 38 | |||
| 5 |  | 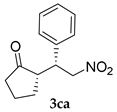 | 24 | − | 43 | 61:39 c | 29 |
| 24 | + | 12 | 72:28 c | 59 | |||
| 6 | 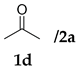 | 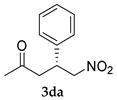 | 24 | − | 32 | - | 10 |
| 24 | + | 70 | - | 23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranaivoarimanana, N.J.; Kanomata, K.; Kitaoka, T. Concerted Catalysis by Nanocellulose and Proline in Organocatalytic Michael Additions. Molecules 2019, 24, 1231. https://doi.org/10.3390/molecules24071231
Ranaivoarimanana NJ, Kanomata K, Kitaoka T. Concerted Catalysis by Nanocellulose and Proline in Organocatalytic Michael Additions. Molecules. 2019; 24(7):1231. https://doi.org/10.3390/molecules24071231
Chicago/Turabian StyleRanaivoarimanana, Naliharifetra Jessica, Kyohei Kanomata, and Takuya Kitaoka. 2019. "Concerted Catalysis by Nanocellulose and Proline in Organocatalytic Michael Additions" Molecules 24, no. 7: 1231. https://doi.org/10.3390/molecules24071231
APA StyleRanaivoarimanana, N. J., Kanomata, K., & Kitaoka, T. (2019). Concerted Catalysis by Nanocellulose and Proline in Organocatalytic Michael Additions. Molecules, 24(7), 1231. https://doi.org/10.3390/molecules24071231







