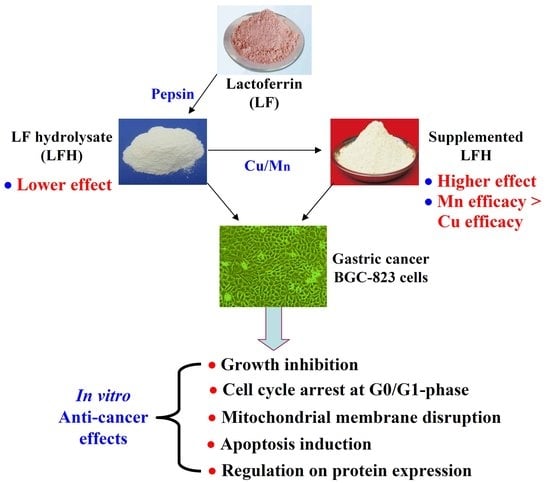
Effect of Cu/Mn-Fortification on In Vitro Activities of the Peptic Hydrolysate of Bovine Lactoferrin against Human Gastric Cancer BGC-823 Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Features of LF, BLH, and Mixtures I–IV
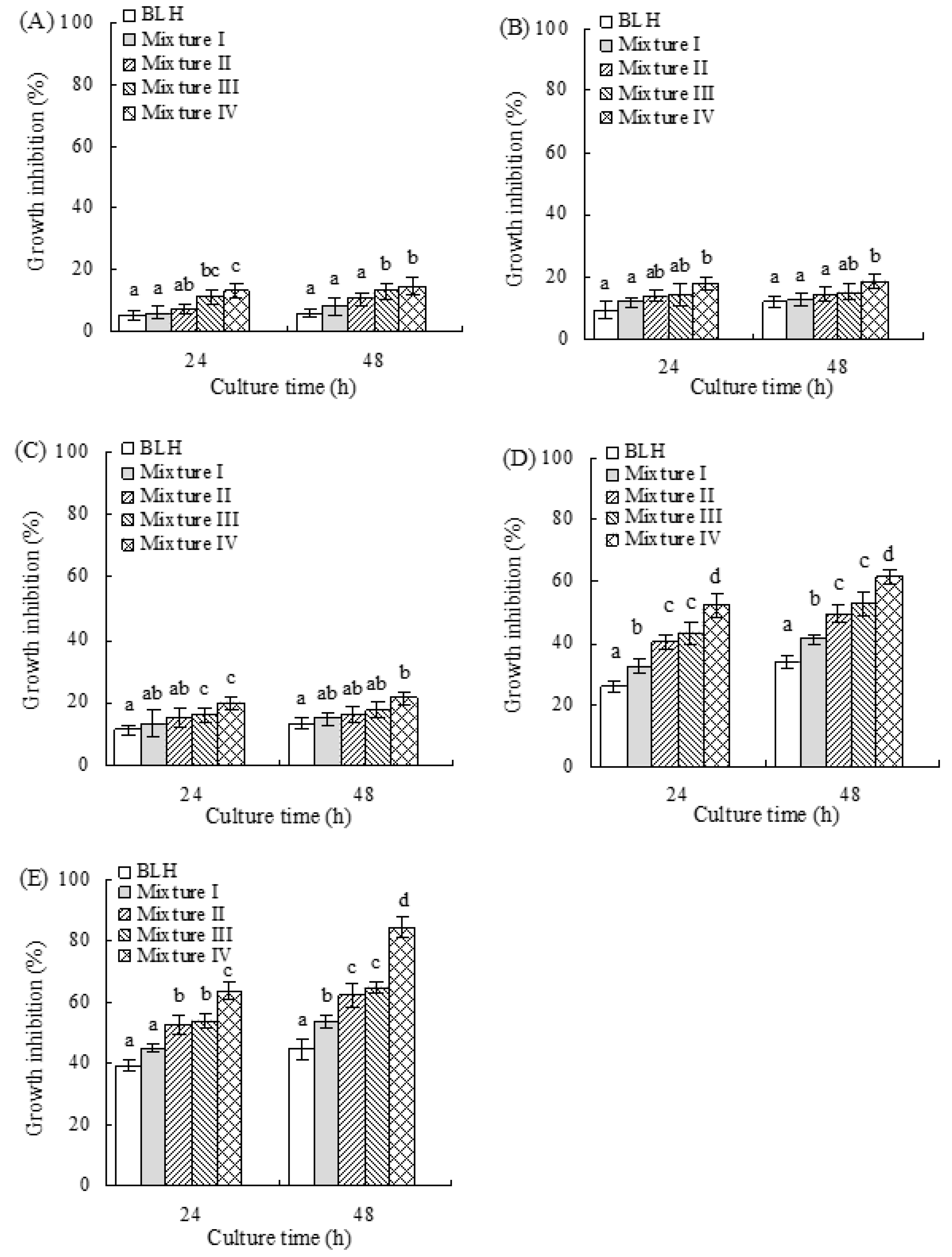
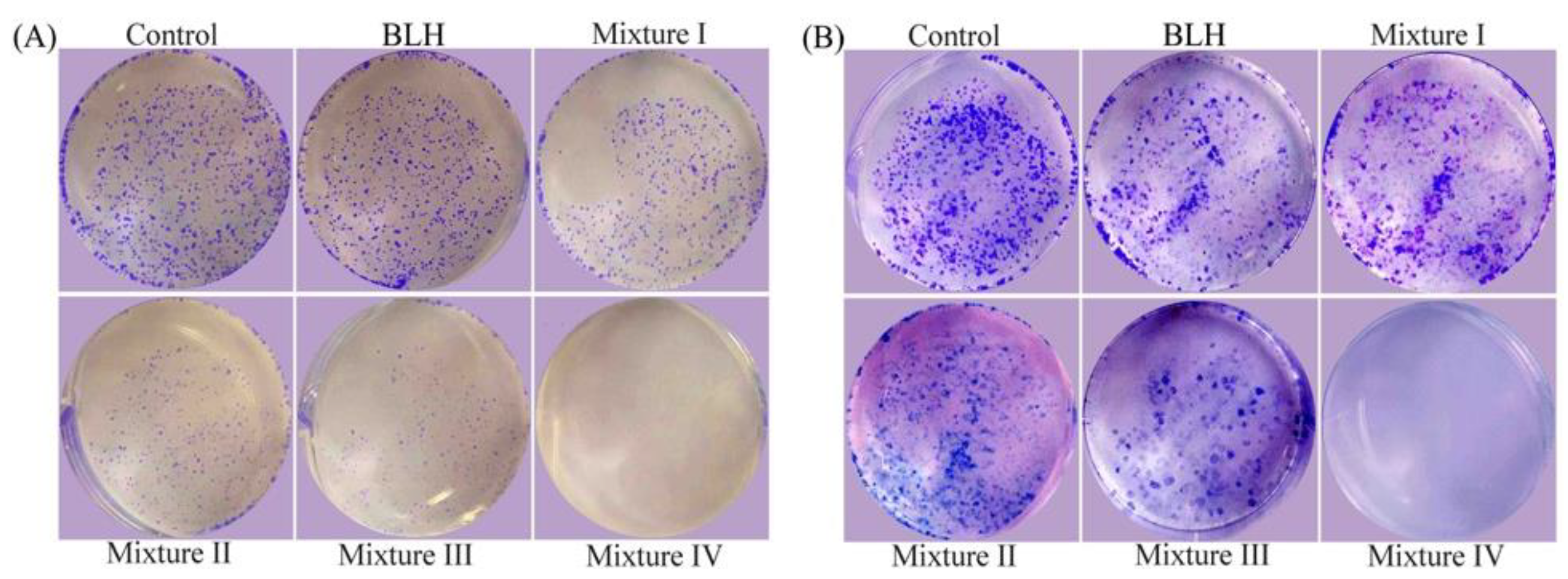
2.2. Growth Inhibition of BLH and Mixtures I–IV on the BGC-823 Cells
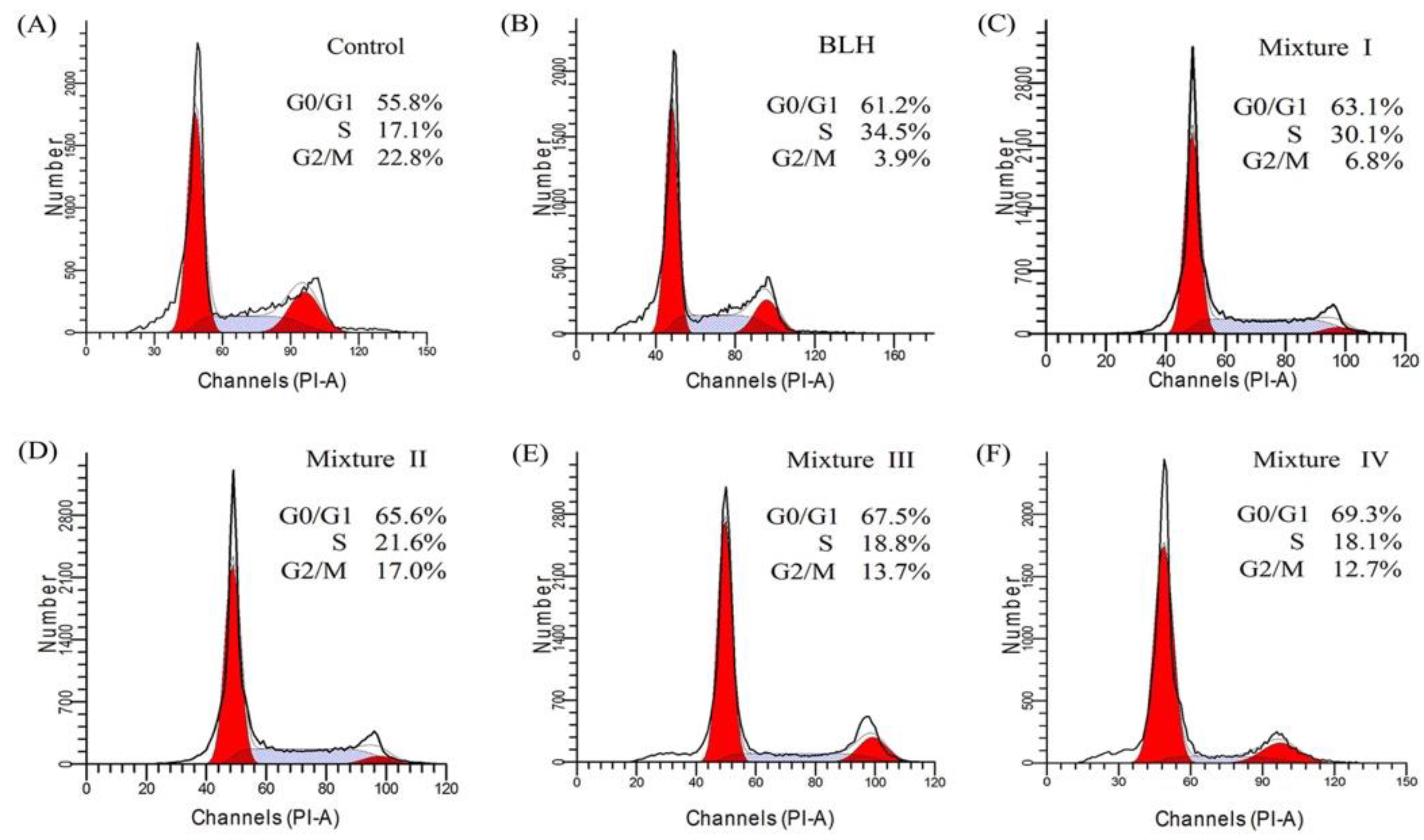
2.3. Effects of BLH and Mixtures I–IV on Cell-Cycle Progression of the BGC-823 Cells
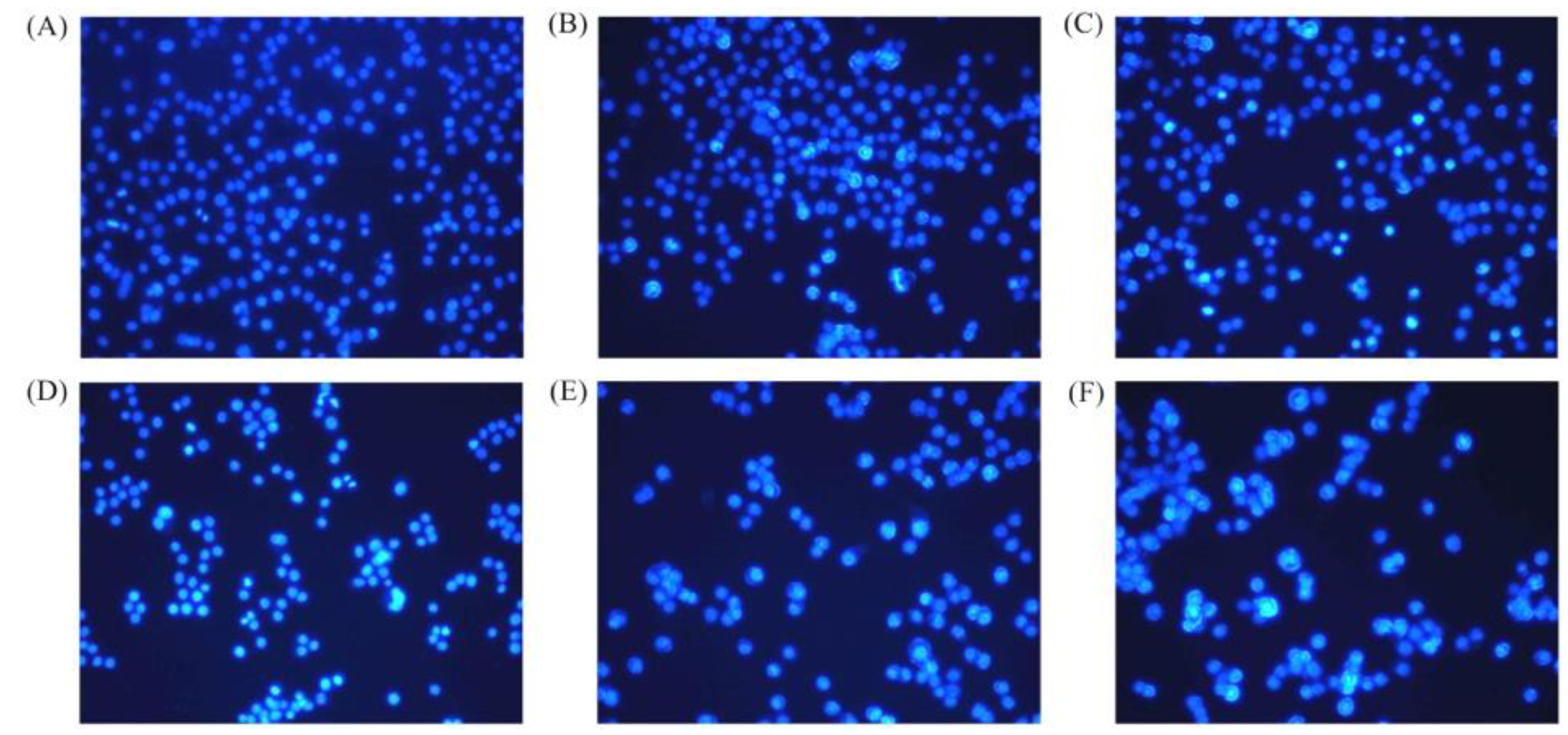
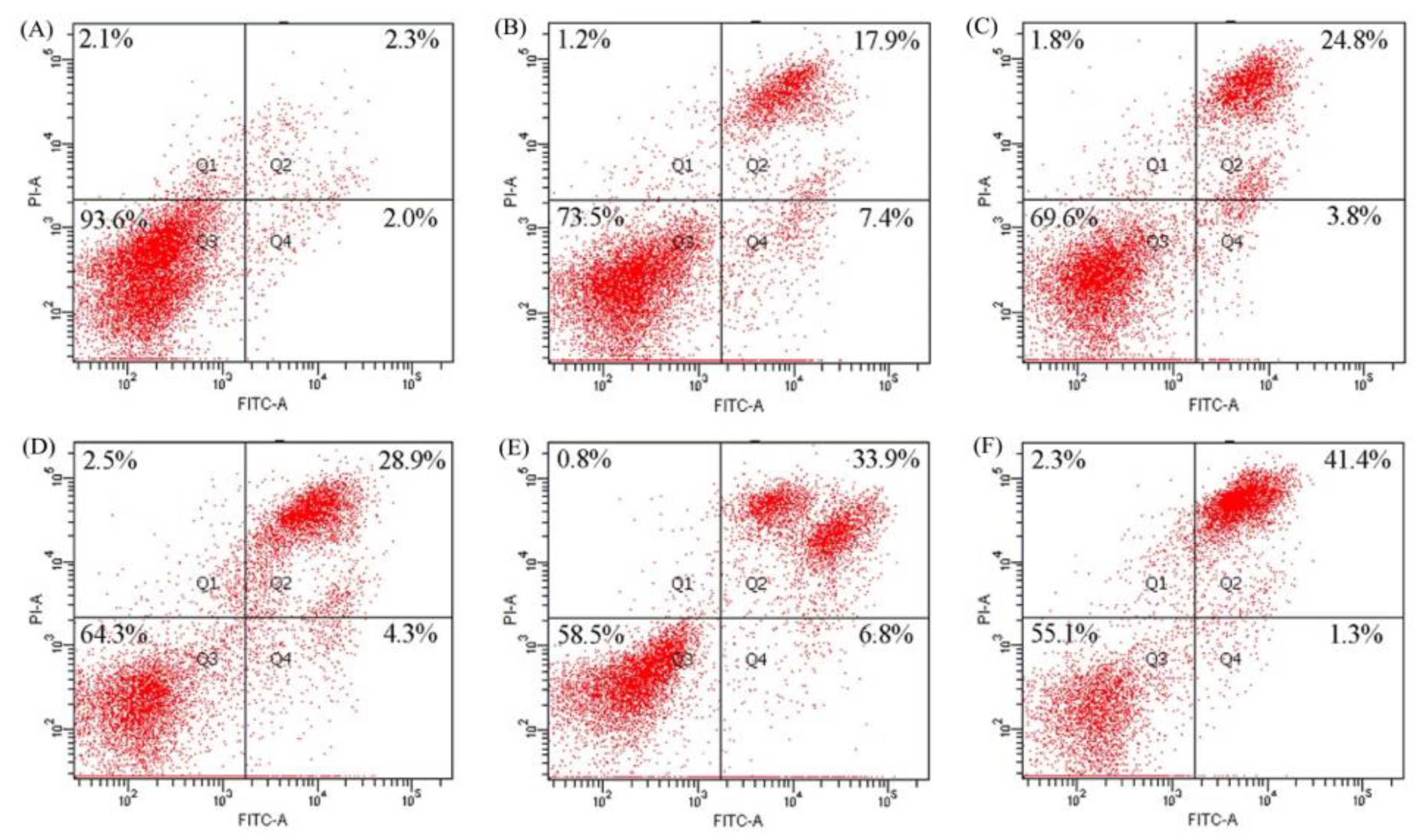
2.4. Apoptosis Induction of BLH and Mixtures I–IV to the BGC-823 Cells
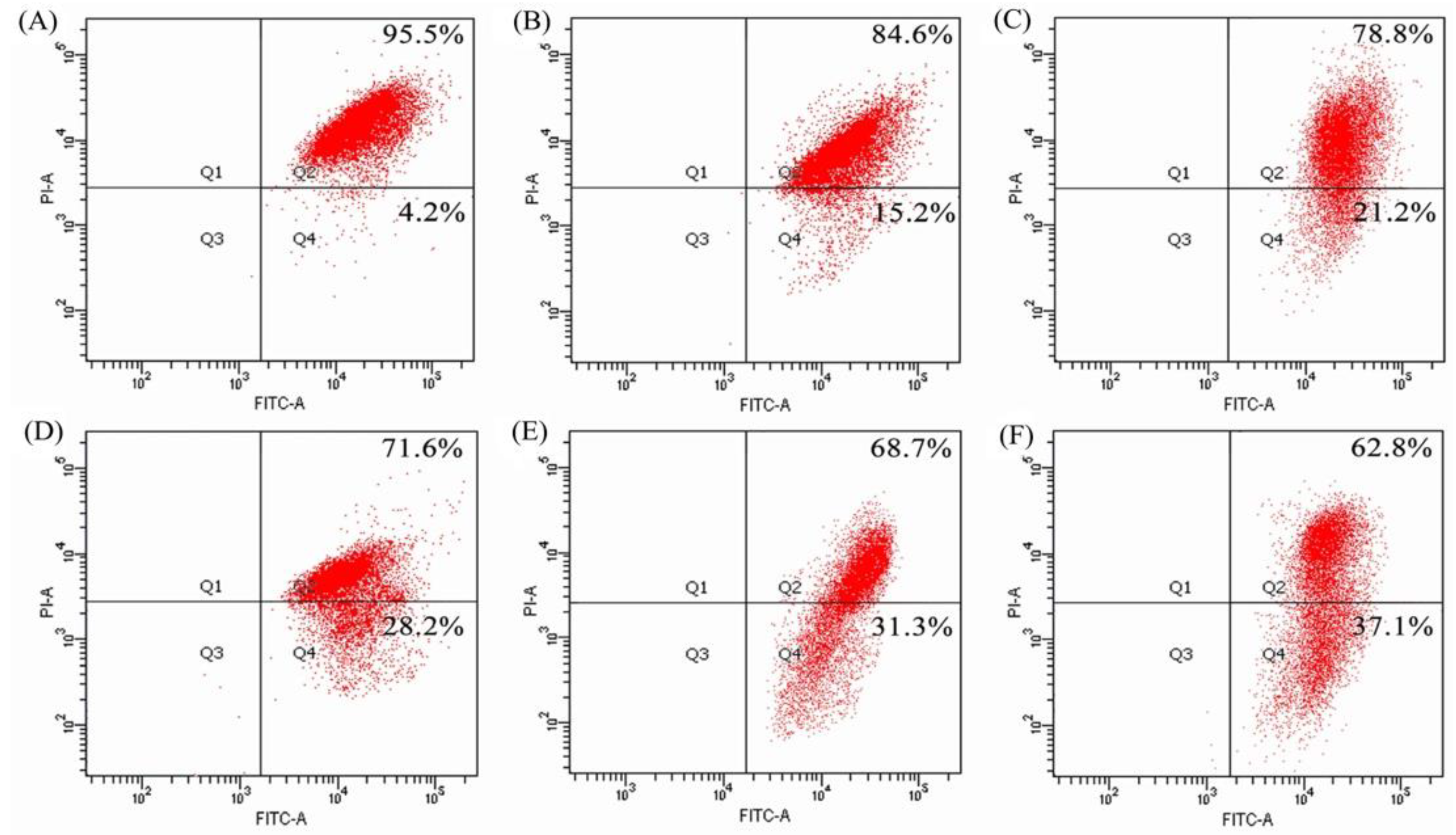
2.5. Mitochondrial Membrane Disruption of the BGC-823 Cells by BLH and Mixtures I–IV
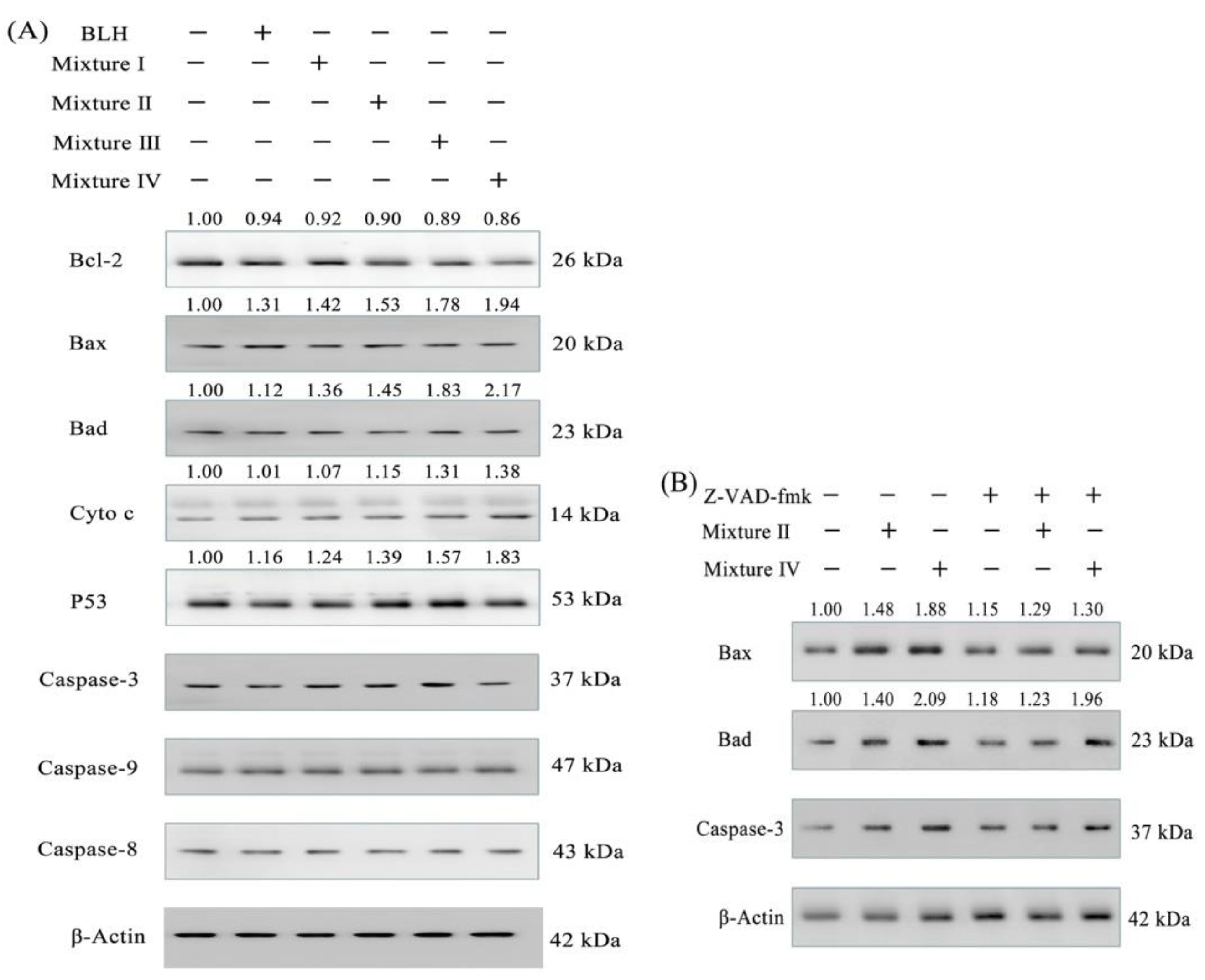
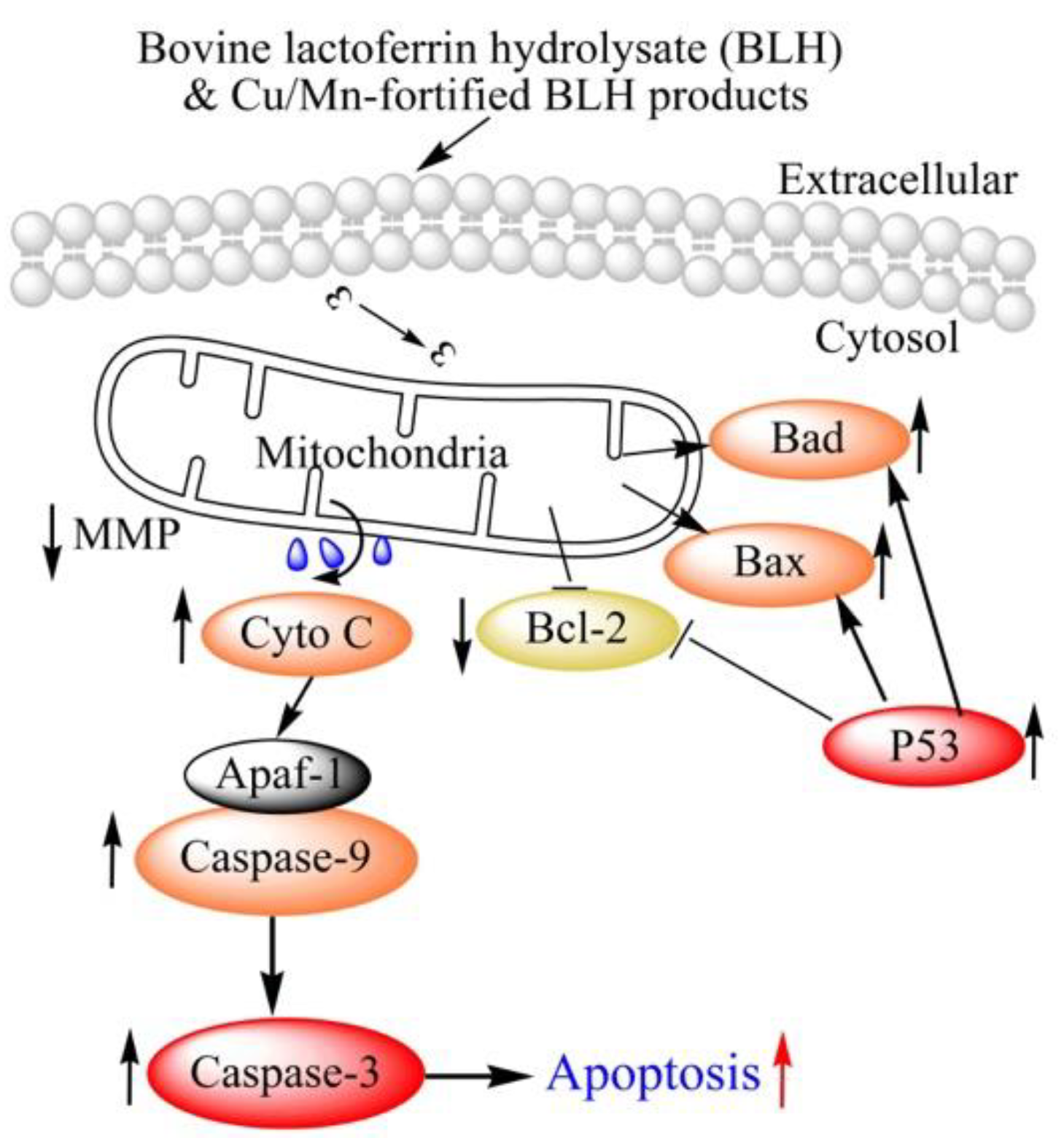
2.6. Expression Changes of Apoptosis-related Proteins in the BGC-823 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Sample Analyses
4.4. Assay of Cytotoxic Effect
4.5. Colony Formation Assay
4.6. Assay of Cell-Cycle Progression
4.7. Hoechst 33258 Staining
4.8. Assay of Mitochondrial Membrane Potential
4.9. Assay of Apoptosis Induction
4.10. Western-Blot Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BLH | Bovine lactoferrin hydrolysate |
| CCK-8 | Cell counting kit-8 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EDTA | Ethylenediamine tetra-acetic acid |
| FBS | Fetal bovine serum |
| 5-FU | 5-Fluorouracil |
| LF | Lactoferrin |
| MMP | Mitochondrial membrane potential |
| PBS | Sodium phosphate buffered solution |
| PI | Propidium iodide |
| PMSF | Phenylmethanesulfonyl fluoride |
References
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: a review. J. Food Sci. Tech. 2015, 52, 5377–5392. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Kumar, B.D.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Puchalska, P.; Esteve, C.; Marine, M.L. Vegetable foods: a cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef]
- Reddy, G.V.; Friend, B.A.; Shahani, K.M.; Farmer, R.E. Antitumor activity of yogurt components. J. Food Protect. 1983, 46, 8–11. [Google Scholar] [CrossRef]
- Sheih, I.C.; Fang, T.J.; Wu, T.K.; Lin, P.H. Anticancer and antioxidant activities of the peptides fraction from algae protein waste. J. Agric. Food Chem. 2010, 58, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Jiang, H.R.; Li, H.B.; Zhang, T.N.; Zhou, Q.; Liu, N. Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin. J. Dairy Sci. 2010, 93, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.R.; Chen, P.W.; Chen, Y.L.; Hsu, H.C.; Lin, C.C.; Chen, W.J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013, 96, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Ayuningtyas, N.F.; Subarnbhesaj, A.; Nguyen, P.T.; Shrestha, M.; Haing, S.; Ohta, K.; Takata, T. Molecular mechanism of inhibitory effects of bovine lactoferrin on the growth of oral squamous cell carcinoma. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Bellamy, W.; Takase, M.; Tomita, M. Inactivation of Listeria monocytogenes by lactoferricin, a potent antimicrobial peptide derived from cow’s milk. J. Food Protect. 1992, 55, 238–240. [Google Scholar] [CrossRef]
- Shin, K.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H.; Tomita, M.; Otsuka, Y.; Yamazak, S. Antibacterial activity of bovine lactoferrin and its peptides against Enterohaemorrhagic Escherichia coli O157: H7. Lett Appl. Microbiol. 1998, 26, 407–411. [Google Scholar] [CrossRef]
- Zhao, H.J.; Zhao, X.H. Modulatory effect of the supplemented copper ion on in vitro activity of bovine lactoferrin to murine splenocytes and RAW264.7 macrophages. Biol. Trace Res. 2018. [Google Scholar] [CrossRef]
- Li, S.T.; Zhou, H.B.; Huang, G.R.; Liu, N. Inhibition of HBV infection by bovine lactoferrin and iron-, zinc-saturated lactoferrin. Med. Microbiol. Immun. 2009, 198, 19–25. [Google Scholar] [CrossRef]
- Bogden, J.D. The Essential Trace Elements and Minerals; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]
- Mertz, W. The essential trace elements. Science 1981, 1213, 1332–1338. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Trace element research-historical and future aspects. J. Trace Elem. Med. Biol. 2016, 38, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Shang, Z.M.; Quan, Y. High energy transition state complex increased anticancer activity: a case study on CuII-complexes. Inorg. Chem. Commun. 2018, 91, 119–123. [Google Scholar] [CrossRef]
- Zhou, D.F.; Chen, Q.Y.; Qi, Y.; Fu, H.J.; Li, Z.; Zhao, K.D.; Gao, J. Anticancer activity, attenuation on the absorption of calcium in mitochondria, and catalase activity for manganese complexes of N-substituted di(picolyl)amine. Inorg. Chem. 2011, 50, 6929–6937. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.Y.; Li, T.J.; Zhao, X.H. Copper or magnesium supplementation endows the peptic hydrolysate from bovine lactoferrin with enhanced activity to human gastric cancer AGS cells. Bio. Trace Elem. Res. 2018, 1–11. [Google Scholar]
- Duarte, D.C.; Nicolau, A.; Teixeira, J.A.; Rodrigues, L.R. The effect of bovine milk lactoferrin on human breast cancer cell lines. J. Dairy Sci. 2011, 94, 66–76. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Chi, C.F.; Wang, B. Anticancer activity of hexapeptide from skate (Raja porosa) cartilage protein hydrolysate in Hela cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Hilchie, A.L.; Haney, E.F.; Bolscher, J.G.; Hyndman, M.E.; Hancock, R.E.; Vogel, H.J. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem. Cell Biol. 2017, 95, 91–98. [Google Scholar]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar]
- Guedes, J.P.; Pereira, C.S.; Rodrigues, L.R.; Cortereal, M. Bovine milk lactoferrin selectively kills highly metastatic prostate cancer PC-3 and osteosarcoma MG-63 cells in vitro. Front Oncol. 2018, 8, 1–12. [Google Scholar]
- Tomita, M.; Wakabayashi, H.; Shin, K.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Twenty-five years of research on bovine lactoferrin applications. Biochimie 2009, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Freiburghaus, C.; Janicke, B.; Lindmark-Mansson, H.; Oredsson, S.M.; Paulsson, M.A. Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J. Dairy Sci. 2009, 92, 2477–2484. [Google Scholar] [CrossRef]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Oves, M.; Chibber, S.; Hadi, S.M.; Ahmad, A. Mobilization of nuclear copper by green tea polyphenol epicatechin-3-gallate and subsequent prooxidant breakage of cellular DNA: implications for cancer chemotherapy. Int. J. Mol. Sci. 2017, 18, 34. [Google Scholar] [CrossRef]
- Amin, A.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Gu, J.N.; Sun, Y.; Xu, S.P.; Zhang, X.Y.; Yang, H.Y.; Ren, F.Z. Anti-proliferative and anti-tumour effect of active components in donkey milk on A549 human lung cancer cells. Int. Dairy J. 2009, 19, 703–708. [Google Scholar] [CrossRef]
- Yang, J.I.; Tang, J.Y.; Liu, Y.S.; Wang, H.R.; Lee, S.Y.; Yen, C.Y.; Chang, H.W. Roe protein hydrolysates of giant grouper (Epinephelus Lanceolatus) inhibit cell proliferation of oral cancer cells involving apoptosis and oxidative stress. Biomed. Res. Int. 2016, 23, 1–12. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Proliferat. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Hung, C.C.; Yang, Y.H.; Kuo, P.F.; Hsu, K.C. Protein hydrolysates from tuna cooking juice inhibit cell growth and induce apoptosis of human breast cancer cell line MCF-7. J. Funct. Foods 2014, 11, 563–570. [Google Scholar]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. P53 mutations in human cancers. Science 1991, 253, 9–53. [Google Scholar]
- Donovan, M.; Cotter, T.G. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Bioch. Bioph. Acta 2004, 1644, 133–147. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhou, H.; Lin, Q.L.; Li, W.J.; Liu, J.W. Rice protein hydrolysate attenuates hydrogen peroxide induced apoptosis of myocardiocytes H9c2 through the Bcl-2/Bax pathway. Food Res. Int. 2012, 48, 736–741. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Yuan, Q.; Xie, H.; Shi, J.; Ju, X. Separation and purification of an anti-tumor peptide from rapeseed (Brassica campestris L.) and the effect on cell apoptosis. Food Funct. 2016, 7, 2239–2248. [Google Scholar] [CrossRef]
- Dai, F.; Wang, Q.; Fan, G.J.; Du, Y.T.; Zhou, B. Ros-deriven and preferential killing of HepG2 over L-02 cells by a short-term cooperation of Cu(Ⅱ) and a catechol-type reveratrol analog. Food Chem. 2018, 250, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.M.; He, L.L.; Zhong, H.Y.; Distelhors, C.W. Baculovirus p35 and z-VAD-fmk inhibit thapsigargin-induced apoptosis of breast cancer cells. Oncogene 1997, 15, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacterial 1992, 73, 472–479. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nagy, A.; Marciniak-Darmochwal, K.; Krawczuk, S.; Gelencser, E. Influence of glycation and pepsin hydrolysis on immunoreactivity of albumin/globulin fraction of herbicide resistant wheat line. Czech J. Food Sci. 2009, 27, 320–329. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds bovine lactoferrin and bovine lactoferrin hydrolysate are available from the authors. |
| Samples | Protein (g/kg) | −NH2 (mmol/g Protein) | Degree of Hydrolysis (%) | Fe (mg/kg) |
|---|---|---|---|---|
| LF | 957.3 ± 3.1 | 0.49 ± 0.01 | 0 | 140.6 ± 2.8 |
| BLH | 923.4 ± 2.7 | 0.93 ± 0.03 | 5.1 ± 0.1 | 130.3 ± 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, L.-Y.; Li, T.-J.; Zhao, X.-H. Effect of Cu/Mn-Fortification on In Vitro Activities of the Peptic Hydrolysate of Bovine Lactoferrin against Human Gastric Cancer BGC-823 Cells. Molecules 2019, 24, 1195. https://doi.org/10.3390/molecules24071195
Bo L-Y, Li T-J, Zhao X-H. Effect of Cu/Mn-Fortification on In Vitro Activities of the Peptic Hydrolysate of Bovine Lactoferrin against Human Gastric Cancer BGC-823 Cells. Molecules. 2019; 24(7):1195. https://doi.org/10.3390/molecules24071195
Chicago/Turabian StyleBo, Li-Ying, Tie-Jing Li, and Xin-Huai Zhao. 2019. "Effect of Cu/Mn-Fortification on In Vitro Activities of the Peptic Hydrolysate of Bovine Lactoferrin against Human Gastric Cancer BGC-823 Cells" Molecules 24, no. 7: 1195. https://doi.org/10.3390/molecules24071195
APA StyleBo, L.-Y., Li, T.-J., & Zhao, X.-H. (2019). Effect of Cu/Mn-Fortification on In Vitro Activities of the Peptic Hydrolysate of Bovine Lactoferrin against Human Gastric Cancer BGC-823 Cells. Molecules, 24(7), 1195. https://doi.org/10.3390/molecules24071195