Halogen-Substituted Triazolethioacetamides as a Potent Skeleton for the Development of Metallo-β-Lactamase Inhibitors
Abstract
1. Introduction
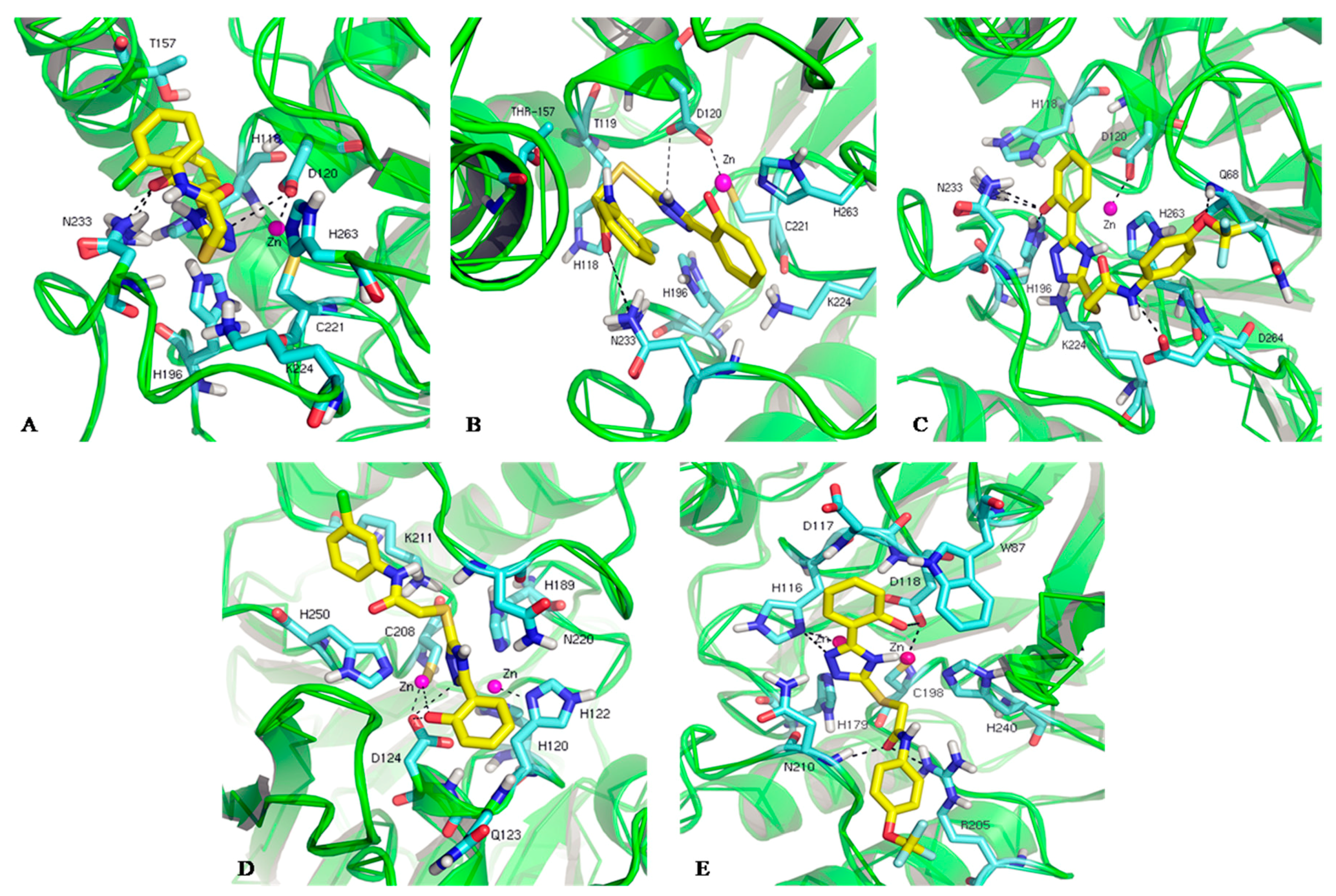
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.3 Determination of IC50 and Ki values
3.4. Docking Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Docquier, J.D.; Mangani, S. An update on β-lactamase inhibitor discovery and development. Drug Resist. Updat. 2018, 36, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, K.; Ren, J.; Qu, X. A β-lactamase-imprinted responsive hydrogel for the treatment of antibiotic-resistant bacteria. Angew. Chem. Int. Ed. Engl. 2016, 55, 8049–8053. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.A.; Kanafani, Z.A.; Tabaja, H.Z.; Kanj, S.S. Extended infusion of β-lactam antibiotics: Optimizing therapy in critically-ill patients in the era of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2017, 15, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 2018, 62, 01076–01118. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. Interplay between β-lactamases and new β-lactamase inhibitors. Nat. Rev. Microbiol. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Brem, J.; Cain, R.; Cahill, S.; McDonough, M.A.; Clifton, I.J.; Jimenez-Castellanos, J.C.; Avison, M.B.; Spencer, J.; Fishwick, C.W.; Schofield, C.J. Structural basis of metallo-β -lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crowder, M.W.; Spencer, J.; Vila, A.J. Metallo-β-lactamases: Novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 2006, 39, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.C.; Cheng, Z.; Fast, W.; Bonomo, R.A.; Crowder, M.W. The continuing challenge of metallo-β-lactamase inhibition: Mechanism matters. Trends Pharmacol. Sci. 2018, 39, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Ali, A.; Gupta, D.; Srivastava, G.; Sharma, A.; Khan, A.U. Molecular and computational approaches to understand resistance of New Delhi metallo β-lactamase variants (NDM-1, NDM-4, NDM-5, NDM-6, NDM-7)-producing strains against carbapenems. J. Biomol. Struct Dyn. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, W.; Liang, H.; Zhao, S.; Zhang, W.; Liu, J.; Jin, C.; Hu, H. NDM-1 encoded by a pNDM-HN380-like plasmid pNDM-BJ03 in clinical Enterobacter cloacae. Diagn Microbiol. Infect Dis. 2018, 90, 153–155. [Google Scholar]
- Chen, J.; Wang, J.; Zhu, W. Zinc ion-induced conformational changes in new Delphi metallo-beta-lactamase 1 probed by molecular dynamics simulations and umbrella sampling. Phys. Chem. Chem. Phys. 2017, 19, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; Bonomo, R.A.; Fast, W. B1-metallo-β-lactamases: Where do we stand? Curr. Drug Targets 2016, 17, 1029–1050. [Google Scholar] [CrossRef]
- Mollard, C.; Moali, C.; Papamicael, C.; Damblon, C.; Vessilier, S.; Amicosante, G.; Schofield, C.J.; Galleni, M.; Frere, J.M.; Roberts, G.C. Thiomandelic acid, a broad spectrum inhibitor of zinc β-lactamases: Kinetic and spectroscopic studies. J. Biol. Chem. 2001, 276, 45015–45023. [Google Scholar]
- Weide, T.; Saldanha, S.A.; Minond, D.; Spicer, T.P.; Fotsing, J.R.; Spaargaren, M.; Frere, J.M.; Bebrone, C.; Sharpless, K.B.; Hodder, P.S.; et al. NH-1,2,3-Triazole-based inhibitors of the VIM-2 metallo-β-lactamase: Synthesis and structure-activity studies. ACS Med. Chem. Lett. 2010, 1, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Vella, P.; Hussein, W.M.; Leung, E.W.; Clayton, D.; Ollis, D.L.; Mitic, N.; Schenk, G.; McGeary, R.P. The identification of new metallo-β-lactamase inhibitor leads from fragment-based screening. Bioorg. Med. Chem. Lett. 2011, 21, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Yang, K.W.; Zhou, Y.J.; LaCuran, A.E.; Oelschlaeger, P.; Crowder, M.W. Diaryl-substituted azolylthioacetamides: Inhibitor discovery of New Delhi metallo-β -lactamase-1 (NDM-1). Chem. Med. Chem. 2014, 9, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, Y.L.; Kang, J.S.; Oelschlaeger, P.; Xiao, L.; Nie, S.S.; Yang, K.W. Triazolylthioacetamide: A valid scaffold for the development of New Delhi metallo-β-lactmase-1 (NDM-1) inhibitors. ACS Med. Chem. Lett. 2016, 7, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Chang, Y.N.; Ge, Y.; Kang, J.S.; Zhang, Y.L.; Liu, X.L.; Oelschlaeger, P.; Yang, K.W. Azolylthioacetamides as a potent scaffold for the development of metallo-β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 5225–5229. [Google Scholar]
- Shao-Kang, Y.; Kang, J.S.; Peter, O.; Ke-Wu, Y. Azolylthioacetamide: A Highly Promising Scaffold for the Development of Metallo-β-lactamase Inhibitors. ACS Med. Chem. Lett. 2015, 6, 455–460. [Google Scholar]
- Yang, H.; Aitha, M.; Hetrick, A.M.; Richmond, T.K.; Tierney, D.L.; Crowder, M.W. Mechanistic and spectroscopic studies of metallo-β-lactamase NDM-1. Biochemistry 2012, 51, 3839–3847. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.A.; Sharma, N.; Chandrasekar, S.; Sigdel, T.; Walsh, T.R.; Spencer, J.; Crowder, M.W. Over-expression, purification, and characterization of metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Protein Expr. Purif. 2004, 36, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Crowder, M.W.; Walsh, T.R.; Banovic, L.; Pettit, M.; Spencer, J. Overexpression, purification, and characterization of the cloned metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1998, 42, 921–926. [Google Scholar] [CrossRef]
- Moya, B.; Barcelo, I.M.; Bhagwat, S.; Patel, M.; Bou, G.; Papp-Wallace, K.M.; Bonomo, R.A.; Oliver, A. WCK 5107 (Zidebactam) and WCK 5153 Are Novel Inhibitors of PBP2 Showing Potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-Lactamase-producing high-risk clones. Antimicrob. Agents Chemother. 2017, 61, 02529–02616. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: All the target compounds halogen-substituted triazolethioacetamides are available from the authors, the synthetic raw materials are purchased from the reagent company and the metallo-beta-lactamase used in the manuscript were provided from Professor Ke-wu Yang. |
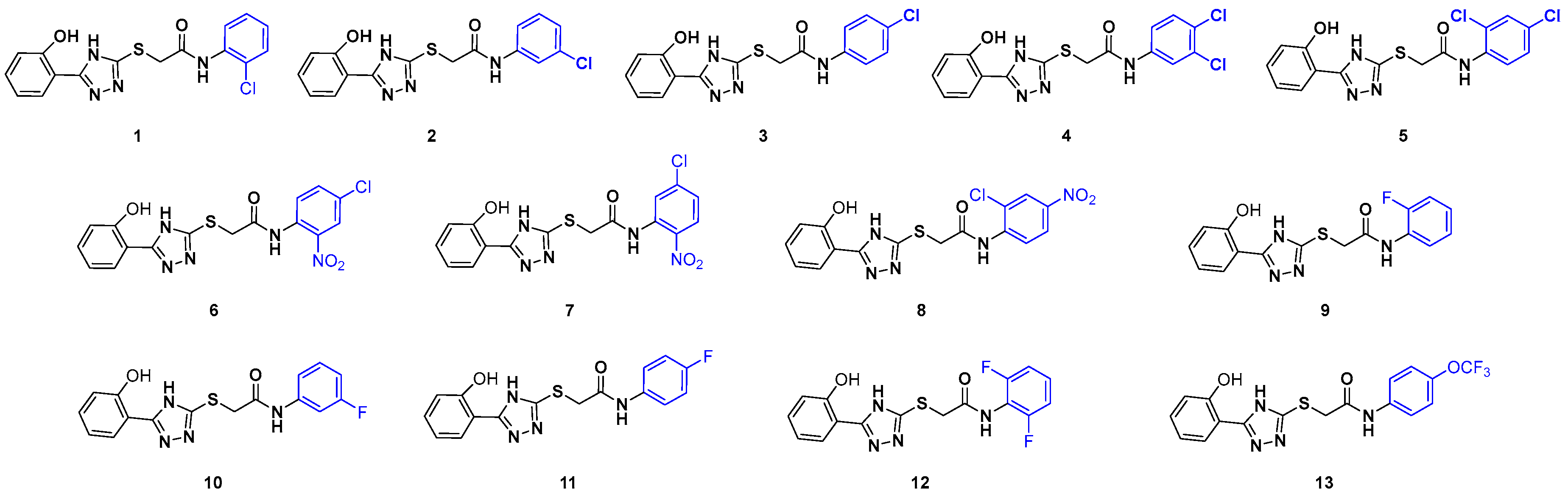
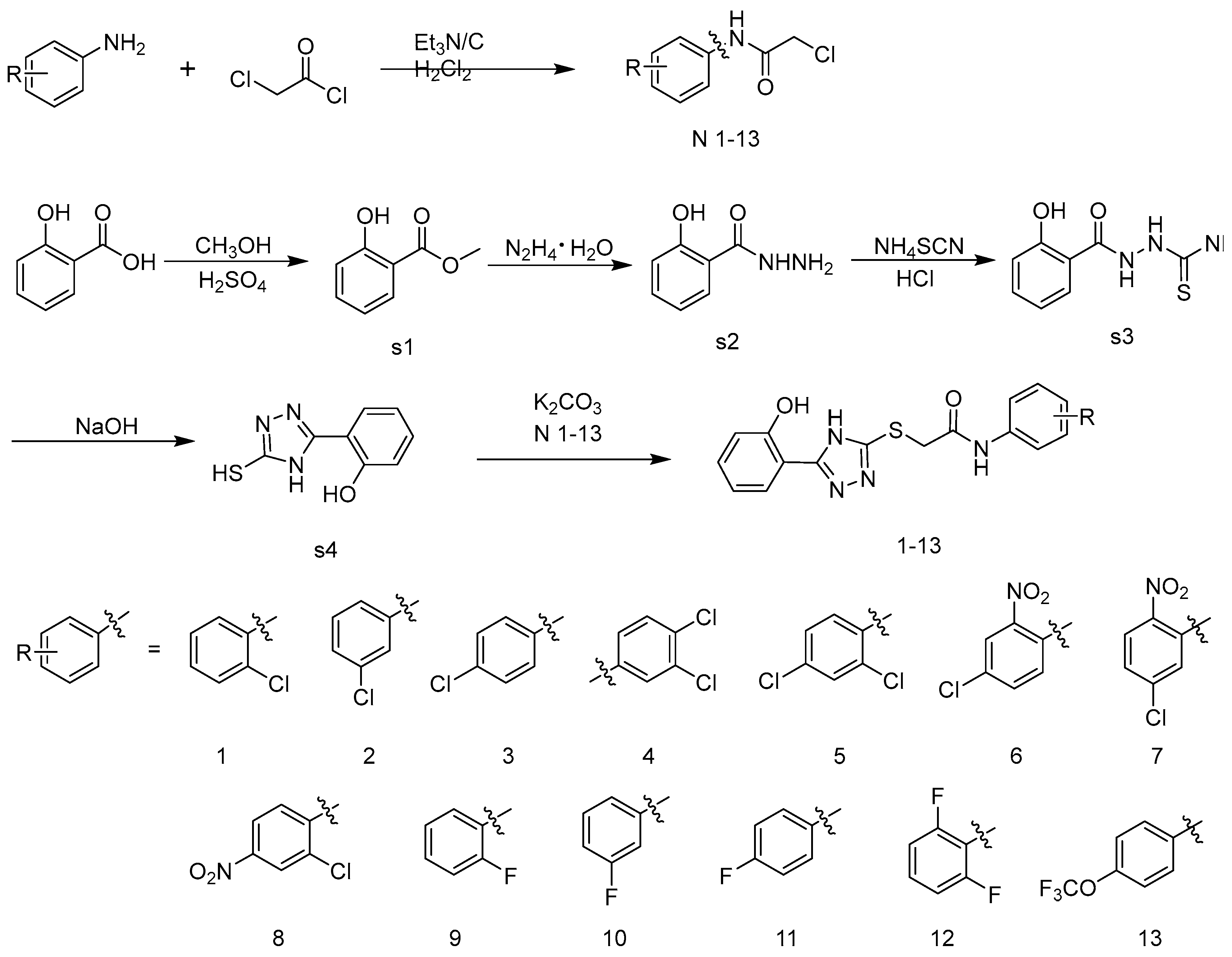

| Compds | ImiS | NDM-1 | VIM-2 |
|---|---|---|---|
| 1 | 0.032 ± 0.004 | 0.96 ± 0.07 | - 1 |
| 2 | 0.045 ± 0.002 | 0.17 ± 0.02 | - |
| 3 | 0.051 ± 0.006 | 0.37 ± 0.05 | - |
| 4 | 0.27 ± 0.02 | - | - |
| 5 | 0.16 ± 0.03 | - | - |
| 6 | 0.78 ± 0.08 | - | - |
| 7 | 0.20 ± 0.04 | - | - |
| 8 | 0.26 ± 0.03 | - | - |
| 9 | 0.072 ± 0.005 | - | - |
| 10 | 0.98 ± 0.04 | - | - |
| 11 | >50 | - | - |
| 12 | 15.64 ± 3 | - | 38.9 ± 5 |
| 13 | 0.23 ± 0.09 | - | 2.8 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yan, Y.; Liang, L.; Feng, J.; Wang, X.; Li, L.; Yang, K. Halogen-Substituted Triazolethioacetamides as a Potent Skeleton for the Development of Metallo-β-Lactamase Inhibitors. Molecules 2019, 24, 1174. https://doi.org/10.3390/molecules24061174
Zhang Y, Yan Y, Liang L, Feng J, Wang X, Li L, Yang K. Halogen-Substituted Triazolethioacetamides as a Potent Skeleton for the Development of Metallo-β-Lactamase Inhibitors. Molecules. 2019; 24(6):1174. https://doi.org/10.3390/molecules24061174
Chicago/Turabian StyleZhang, Yilin, Yong Yan, Lufan Liang, Jie Feng, Xuejun Wang, Li Li, and Kewu Yang. 2019. "Halogen-Substituted Triazolethioacetamides as a Potent Skeleton for the Development of Metallo-β-Lactamase Inhibitors" Molecules 24, no. 6: 1174. https://doi.org/10.3390/molecules24061174
APA StyleZhang, Y., Yan, Y., Liang, L., Feng, J., Wang, X., Li, L., & Yang, K. (2019). Halogen-Substituted Triazolethioacetamides as a Potent Skeleton for the Development of Metallo-β-Lactamase Inhibitors. Molecules, 24(6), 1174. https://doi.org/10.3390/molecules24061174




