In Vitro Effects of Tea Tree Oil (Melaleuca Alternifolia Essential Oil) and its Principal Component Terpinen-4-ol on Swine Spermatozoa
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
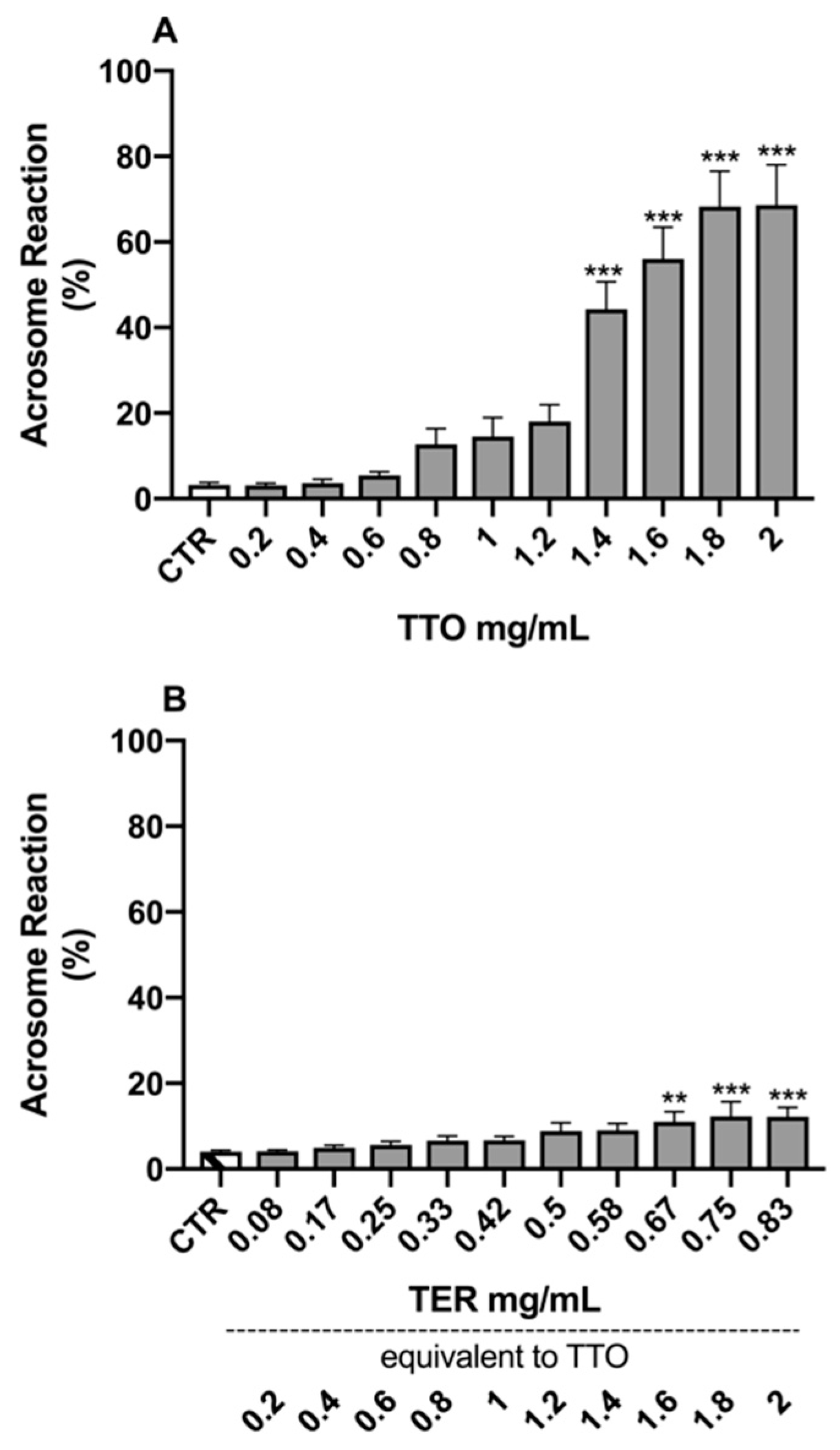
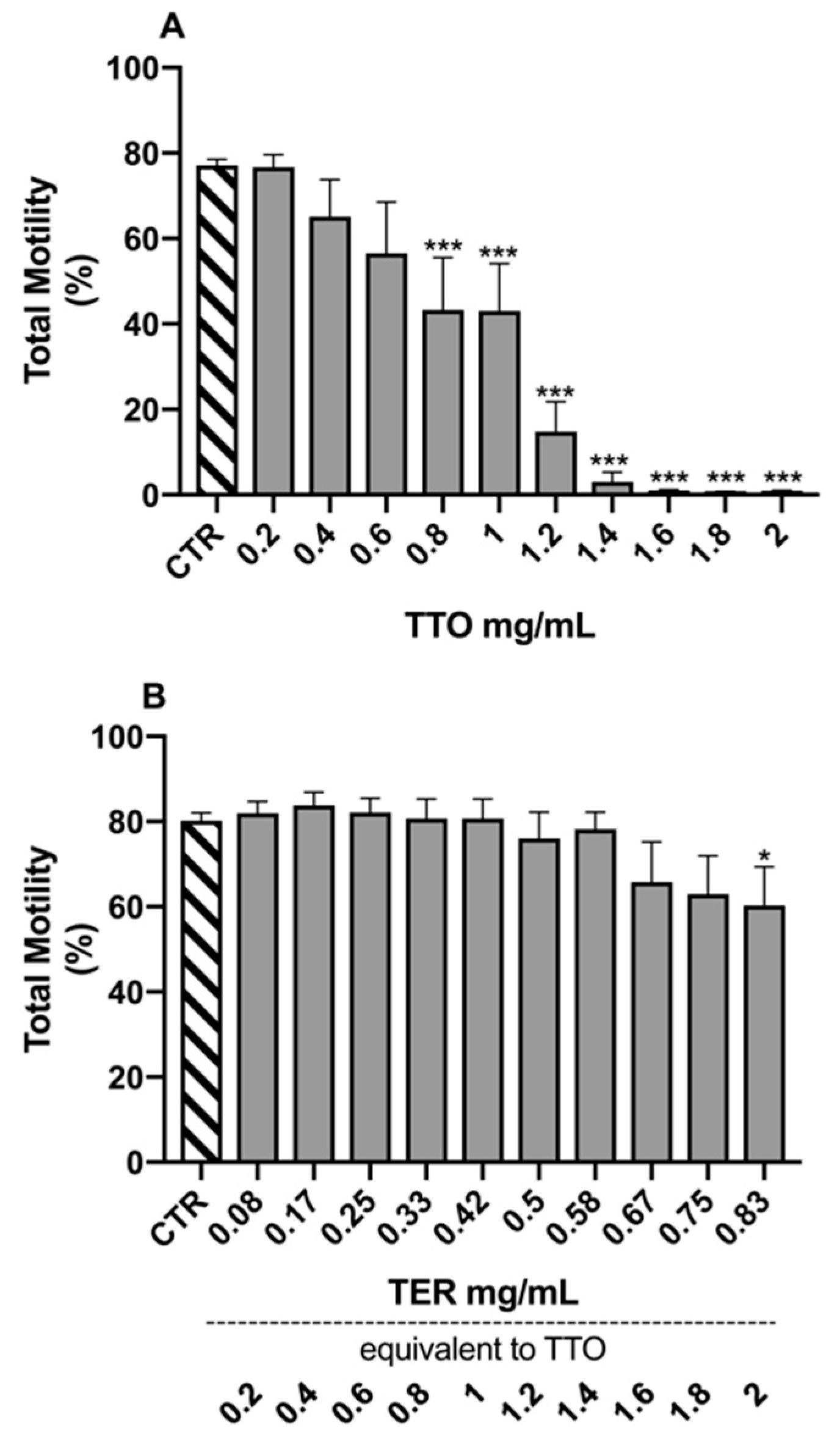
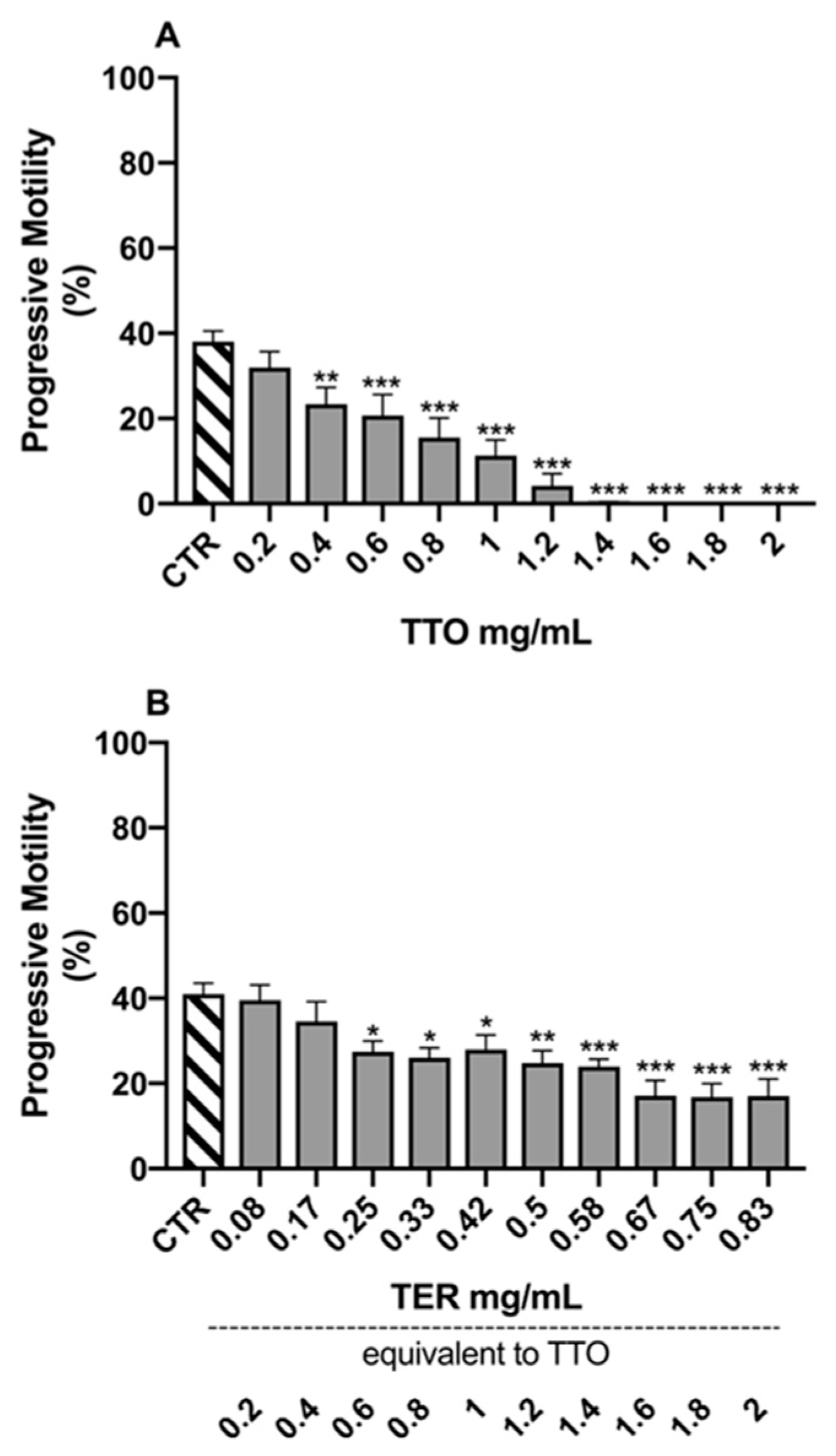
2.2. Sperm Morpho-Functional Parameters
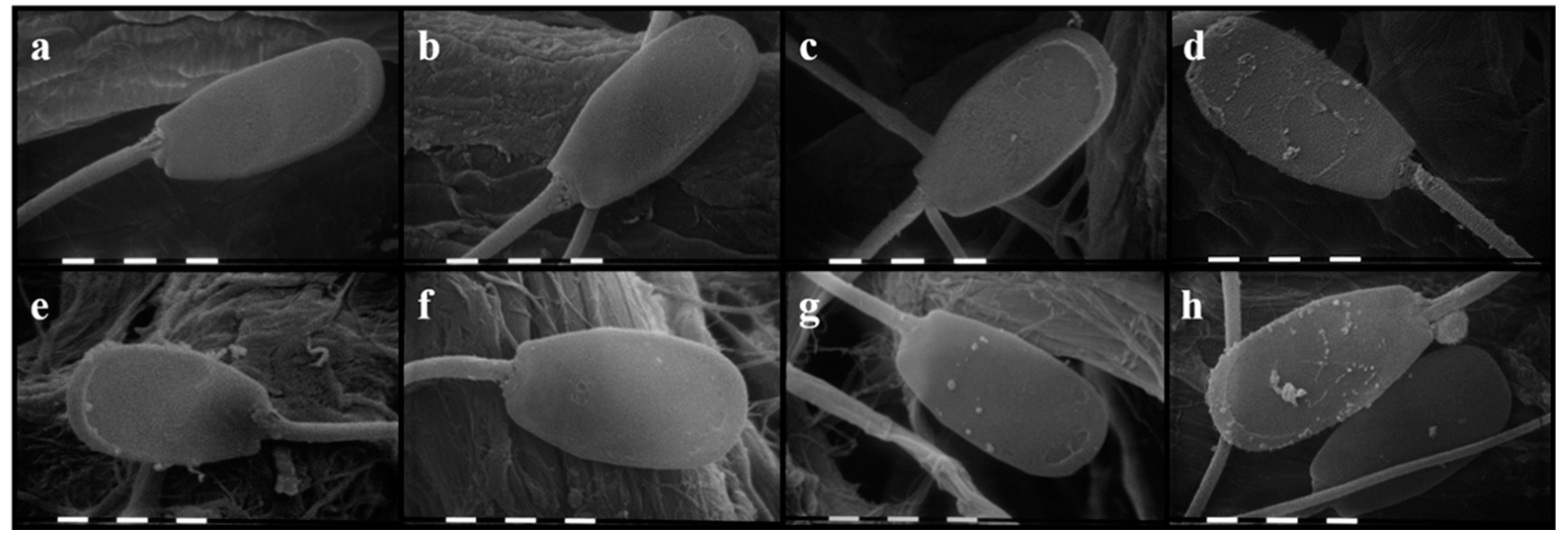
2.3. Sperm Morphological Evaluation by Scanning Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Chemo-Characterization of the M. alternifolia EO
4.1.1. Gas Chromatography-Mass Detector (GC-MS) Analysis
4.1.2. Gas Chromatography-Flame Ionization Detector (GC-FID) Analysis
4.1.3. Qualitative and Semi-Quantitative Analysis
4.2. Boars and Ejaculates
4.3. Experimental Protocol
4.4. Evaluation of Spermatozoa Morpho-Functional Parameters
4.5. Scanning Electron Microscopy
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeste, M. State-of-the-art of boar sperm preservation in liquid and frozen state. Anim. Reprod. 2017, 14, 69–81. [Google Scholar] [CrossRef]
- Yeste, M. Recent advances in boar sperm cryopreservation: State of the art and current perspectives. Reprod. Domest. Anim. 2015, 50 (Suppl. S2), 71–79. [Google Scholar] [CrossRef]
- Pezo, F.; Romero, F.; Zambrano, F.; Sánchez, R.S. Preservation of boar semen: An update. Reprod. Domest. Anim. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Monton, A.; Gil, L.; Malo, C.; Olaciregui, M.; Gonzalez, N.; de Blas, I. Sage (Salvia officinalis) and fennel (Foeniculum vulgare) improve cryopreserved boar epididymal semen quality study. Cryoletters 2015, 36, 83–90. [Google Scholar]
- Schulze, M.; Schäfer, J.; Simmet, C.; Jung, M.; Gabler, C. Detection and characterization of Lactobacillus spp. in the porcine seminal plasma and their influence on boar semen quality. PLoS ONE 2018, 13, e0202699. [Google Scholar] [CrossRef]
- Barone, F.; Ventrella, D.; Zannoni, A.; Forni, M.; Bacci, M.L. Can microfiltered seminal plasma preserve the morphofunctional characteristics of porcine spermatozoa in the absence of antibiotics? A preliminary study. Reprod. Domest. Anim. 2016, 51, 604–610. [Google Scholar] [CrossRef]
- Althouse, G.C.; Kuster, C.E.; Clark, S.G.; Weisiger, R.M. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology 2000, 53, 1167–1176. [Google Scholar] [CrossRef]
- EUR-Lex—31990L0429. Available online: http://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31990L0429 (accessed on 5 July 2017).
- Barton, M.D. Impact of antibiotic use in the swine industry. Curr. Opin. Microbiol. 2014, 19, 9–15. [Google Scholar] [CrossRef]
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Costa, M.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Davies, N.W.; Southwell, I.A.; Stiff, I.A.; Williams, L.R. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J. Agric. Food Chem. 1989, 37, 1330–1335. [Google Scholar] [CrossRef]
- Homeyer, D.C.; Sanchez, C.J.; Mende, K.; Beckius, M.L.; Murray, C.K.; Wenke, J.C.; Akers, K.S. In vitro activity of Melaleuca alternifolia (tea tree) oil on filamentous fungi and toxicity to human cells. Med. Mycol. 2015, 53, 285–294. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca genus as antimicrobial agents: From farm to pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef]
- ISO/TR 21092:2004 (en), Essential oils—Characterization. Available online: https://www.iso.org/obp/ui/#iso:std:iso:tr:21092:ed-1:en (accessed on 28 June 2018).
- Wu, C.-S.; Chen, Y.-J.; Chen, J.J.W.; Shieh, J.-J.; Huang, C.-H.; Lin, P.-S.; Chang, G.-C.; Chang, J.-T.; Lin, C.-C. Terpinen-4-ol Induces apoptosis in human nonsmall cell lung cancer in vitro and in vivo. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef]
- Paul, S.; Kang, S.C. Studies on the viability and membrane integrity of human spermatozoa treated with essential oil of Trachyspermum ammi (L.) sprague ex turrill fruit. Andrologia 2012, 44, 117–125. [Google Scholar]
- Chikhoune, A.; Stouvenel, L.; Iguer-Ouada, M.; Hazzit, M.; Schmitt, A.; Lorès, P.; Wolf, J.P.; Aissat, K.; Auger, J.; Vaiman, D.; et al. In-vitro effects of Thymus munbyanus essential oil and thymol on human sperm motility and function. Reprod. Biomed. Online 2015, 31, 411–420. [Google Scholar] [CrossRef]
- Touazi, L.; Aberkane, B.; Bellik, Y.; Moula, N.; Iguer-Ouada, M. Effect of the essential oil of Rosmarinus officinalis (L.) on rooster sperm motility during 4 °C short-term storage. Vet. World 2018, 11, 590–597. [Google Scholar]
- Elmi, A.; Ventrella, D.; Barone, F.; Filippini, G.; Benvenuti, S.; Pisi, A.; Scozzoli, M.; Bacci, M.L. Thymbra capitata (L.) cav. and Rosmarinus officinalis (L.) essential oils: In vitro effects and toxicity on swine spermatozoa. Molecules 2017, 22, 2162. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, E.; Salo, J.; Toivonen, M.S.; Marik, T.; Mikkola, R.; Kredics, L.; Vicente-Carrillo, A.; Nagy, S.; Andersson, M.T.; Andersson, M.A.; et al. An evaluation of boar spermatozoa as a biosensor for the detection of sublethal and lethal toxicity. Toxins 2018, 10, 463. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A.; Edebert, I.; Garside, H.; Cotgreave, I.; Rigler, R.; Loitto, V.; Magnusson, K.E.; Rodríguez-Martínez, H. Boar spermatozoa successfully predict mitochondrial modes of toxicity: Implications for drug toxicity testing and the 3R principles. Toxicol. In Vitro 2015, 29, 582–591. [Google Scholar] [CrossRef]
- Elmi, A.; Banchelli, F.; Barone, F.; Fantinati, P.; Ventrella, D.; Forni, M.; Bacci, M.L. Semen evaluation and in vivo fertility in a Northern Italian pig farm: Can advanced statistical approaches compensate for low sample size? An observational study. Anim. Reprod. Sci. 2018, 192, 61–68. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef]
- Gil, M.C.; García-Herreros, M.; Barón, F.J.; Aparicio, I.M.; Santos, A.J.; García-Marín, L.J. Morphometry of porcine spermatozoa and its functional significance in relation with the motility parameters in fresh semen. Theriogenology 2009, 71, 254–263. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef]
- Ziółkowska-Klinkosz, M.; Kedzia, A.; Meissner, H.O.; Kedzia, A.W. Evaluation of the tea tree oil activity to anaerobic bacteria—In vitro study. Acta Pol. Pharm. 2016, 73, 389–394. [Google Scholar]
- Papadopoulos, C.J.; Carson, C.F.; Hammer, K.A.; Riley, T.V. Susceptibility of pseudomonads to Melaleuca alternifolia (tea tree) oil and components. J. Antimicrob. Chemother. 2006, 58, 449–451. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Dang Vu, K.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2016, 53, 24–30. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Pugnaloni, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes virulence by Cannabis sativa L. essential oil. Front. Cell. Infect. Microbiol. 2018, 8, 293. [Google Scholar] [CrossRef]
- Cremades, T.; Roca, J.; Rodriguez-Martinez, H.; Abaigar, T.; Vazquez, J.M.; Martinez, E.A. Kinematic changes during the cryopreservation of boar spermatozoa. J. Androl. 2005, 26, 610–618. [Google Scholar] [CrossRef]
- Akashi, T.; Watanabe, A.; Komiya, A.; Fuse, H. Evaluation of the sperm motility analyzer system (SMAS) for the assessment of sperm quality in infertile men. Syst. Biol. Reprod. Med. 2010, 56, 473–477. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components By Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp.: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Fantinati, P.; Zannoni, A.; Bernardini, C.; Forni, M.; Tattini, A.; Seren, E.; Bacci, M.L. Evaluation of swine fertilisation medium (SFM) efficiency in preserving spermatozoa quality during long-term storage in comparison to four commercial swine extenders. Animal 2009, 3, 269–274. [Google Scholar] [CrossRef]
- Björndahl, L.; Söderlund, I.; Johansson, S.; Mohammadieh, M.; Pourian, M.R.; Kvist, U. Why the WHO recommendations for eosin-nigrosin staining techniques for human sperm vitality assessment must change. J. Androl. 2004, 25, 671–678. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds used in the present study are available from the authors. |

| Compound | LRI 1 | Area% |
|---|---|---|
| terpinen-4-ol | 1185 | 41.49 |
| γ-terpinene | 1061 | 20.55 |
| α-terpinene | 1018 | 9.59 |
| α-terpineol | 1194 | 4.42 |
| α-pinene | 933 | 4.4 |
| p-cymene | 1025 | 3.66 |
| terpinolene | 1089 | 3.18 |
| 1,8-cineole | 1031 | 2.15 |
| limonene | 1029 | 1.78 |
| aromadendrene | 1446 | 1.38 |
| caryophyllene oxide | 1594 | 0.76 |
| myrcene | 992 | 0.72 |
| allo-aromadendrene | 1468 | 0.18 |
| α-felladrene | 1005 | 0.17 |
| sabinene | 973 | 0.03 |
| β-pinene | 975 | 0.03 |
| α-humulene | 1460 | 0.02 |
| β-caryophyllene | 1425 | 0.02 |
| Total | 94.53 |
| TTO (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| CTR | 0.2 | 0.4 | 0.6 | 0.8 | 1 | |
| VAP (μm/s) | 80.96 (2.63) | 77.70 (3.89) | 81.02 (5.63) | 79.04 (4.37) | 72.47 (3.15) | 54.95 (7.87) *** |
| VCL (μm/s) | 180.75 (5.62) | 173.34 (8.61) | 186.84 (12.73) | 180.77 (6.22) | 165.14 (6.14) | 132.98 (16.83) ** |
| VSL (μm/s) | 41.10 (1.73) | 37.30 (2.00) | 34.35 (2.29) | 33.33 (2.19) | 32.02 (1.27) | 25.36 (2.16) *** |
| DAP (μm) | 48.19 (1.39) | 47.37 (1.89) | 49.14 (2.77) | 48.08 (2.41) | 44.78 (2.11) | 36.71 (4.19) ** |
| DCL (μm) | 110.20 (3.49) | 106.94 (4.83) | 115.65 (6.59) | 112.55 (3.62) | 104.97 (5.06) | 86.34 (9.86) * |
| DSL (μm) | 22.71 (0.79) | 20.88 (0.96) | 18.45 (0.94) * | 18.27 (1.12) * | 18.11 (0.32) * | 15.24 (0.83) *** |
| LIN (%) | 22.74 (0.60) | 22.58 (0.55) | 19.54 (0.86) | 20.07 (1.32) | 21.03 (1.25) | 21.41 (1.60) |
| STR (%) | 50.90 (1.09) | 48.67 (0.97) | 43.19 (1.28) ** | 43.37 (2.16) * | 46.48 (2.42) | 47.43 (3.03) |
| WOB (%) | 45.16 (0.32) | 45.00 (0.35) | 43.64 (0.89) | 44.48 (1.02) | 44.06 (0.83) | 43.96 (0.97) |
| ALH (μm) | 9.50 (0.26) | 8.88 (0.38) | 9.55 (0.69) | 10.01 (0.44) | 9.54 (0.66) | 8.65 (1.01) |
| BCF(Hz) | 37.75 (1.15) | 37.34 (0.77) | 38.15 (0.99) | 36.60 (2.89) | 34.94 (1.38) | 35.11 (2.75) |
| TER (mg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | 0.08 | 0.17 | 0.25 | 0.33 | 0.42 | 0.50 | 0.58 | 0.67 | 0.75 | 0.83 | |
| Equivalent to TTO (mg/mL) | |||||||||||
| 0.2 | 0.4 | 0.6 | 0.8 | 1 | 1.2 | 1.4 | 1.6 | 1.8 | 2 | ||
| VAP (µm/s) | 88.12 (2.26) | 92.15 (4.46) | 92.29 (4.22) | 95.19 (5.92) | 90.17 (5.92) | 86.83 (6.27) | 87.91 (5.09) | 87.89 (5.61) | 78.58 (5.71) | 75.39 (6.40) | 75.41 (4.92) |
| VCL (µm/s) | 200.47 (5.82) | 212.65 (12.22) | 219.15 (7.81) | 223.22 (8.89) | 212.76 (9.48) | 200.71 (12.55) | 202.52 (11.31) | 203.59 (11.60) | 188.61 (13.59) | 172.23 (12.16) | 170.46 (10.83) |
| VSL (µm/s) | 44.32 (1.63) | 44.77 (1.56) | 36.71 (3.73) | 37.25 (1.88) | 36.68 (3.25) | 34.88 (2.86) * | 35.54 (2.87) ** | 33.78 (1.90) ** | 27.72 (2.33) *** | 28.15 (1.97) *** | 29.19 (2.61) *** |
| DAP (µm) | 51.32 (1.53) | 54.01 (2.78) | 54.23 (2.78) | 55.39 (3.32) | 53.45 (3.73) | 51.58 (3.34) | 51.15 (3.42) | 50.77 (4.66) | 48.48 (3.01) | 44.37 (4.45) | 45.79 (2.48) |
| DCL (µm) | 119.94 (3.40) | 127.73 (8.35) | 132.63 (3.80) | 134.08 (5.57) | 129.96 (7.87) | 123.33 (6.62) | 122.08 (7.39) | 124.67 (7.34) | 121.17 (7.45) | 108.95 (6.67) | 107.95 (6.26) |
| DSL (µm) | 23.62 (1.31) | 24.27 (0.98) | 19.08 (2.18) | 19.36 (1.33) | 19.28 (0.99) | 18.06 (0.82) * | 18.25 (0.94) * | 17.88 (1.10) * | 15.32 (1.25) *** | 15.24 (1.10) *** | 16.13 (1.41) *** |
| LIN (%) | 22.93 (1.28) | 22.67 (1.68) | 18.15 (1.81) | 18.16 (1.25) | 18.74 (1.42) | 20.09 (1.35) | 17.03 (1.15) * | 17.47 (0.50) * | 15.95 (0.66) * | 16.66 (0.69) * | 16.23 (1.81) ** |
| STR (%) | 49.45 (2.11) | 48.83 (2.65) | 41.79 (3.25) | 41.04 (2.60) | 41.66 (2.51) | 41.52 (1.71) | 41.32 (0.91) | 39.64 (1.20) * | 36.99 (1.48) ** | 38.67 (2.19) ** | 39.01 (1.62) ** |
| WOB (%) | 10.31 (0.30) | 10.57 (0.59) | 174.74 (0.36) | 11.38 (0.34) | 11.08 (0.52) | 10.95 (0.37) | 43.55 (0.37) | 42.98 (0.68) | 42.28 (0.78) | 43.54 (0.81) | 44.29 (1.16) |
| ALH (µm) | 10.31 (0.30) | 10.57 (0.59) | 11.25 (0.39) | 11.38 (0.36) | 33.08 (0.34) | 10.95 (0.52) | 11.44 (0.42) | 11.30 (0.51) | 12.34 (1.59) | 10.67 (0.58) | 10.67 (0.39) |
| BCF (Hz) | 45.05 (3.30) | 43.52 (3.95) | 48.68 (7.60) | 42.48 (4.63) | 41.13 (5.56) | 36.21 (1.82) | 32.47 (1.01) | 36.27 (1.82) | 40.78 (5.74) | 34.56 (2.00) | 33.38 (2.29) |
| Tea Tree Oil | Terpinen-4-ol | |
|---|---|---|
| β (C.I. 95%) | β (C.I. 95%) | |
| V % | −0.091 (−0.104; −0.079) p < 0.0001 | 0.094 (0.010; 0.178) p = 0.0279 |
| TotM % | −0.080 (−0.090: −0.070) p < 0.0001 | 0.049 (0.008; 0.091) p = 0.020 |
| ProgM % | −0.163 (−0.185; −0.141) p < 0.0001 | 0.017 (−0.043; 0.077) p =0.564 |
| AR % | 0.100 (0.086; 0.114) p < 0.0001 | −0.089 (−0.237; 0.060) p = 0.239 |
| pH value | −2.071 (−10.308; 6.166) p = 0.619 | 4.348 (−8.203; 16.899) p = 0.491 |
| TTO (mg/mL) | TER (41.5 %) (mg/mL) |
|---|---|
| 2 | 0.83 |
| 1.8 | 0.75 |
| 1.6 | 0.67 |
| 1.4 | 0.58 |
| 1.2 | 0.5 |
| 1 | 0.42 |
| 0.8 | 0.33 |
| 0.6 | 0.25 |
| 0.4 | 0.17 |
| 0.2 | 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmi, A.; Ventrella, D.; Barone, F.; Carnevali, G.; Filippini, G.; Pisi, A.; Benvenuti, S.; Scozzoli, M.; Bacci, M.L. In Vitro Effects of Tea Tree Oil (Melaleuca Alternifolia Essential Oil) and its Principal Component Terpinen-4-ol on Swine Spermatozoa. Molecules 2019, 24, 1071. https://doi.org/10.3390/molecules24061071
Elmi A, Ventrella D, Barone F, Carnevali G, Filippini G, Pisi A, Benvenuti S, Scozzoli M, Bacci ML. In Vitro Effects of Tea Tree Oil (Melaleuca Alternifolia Essential Oil) and its Principal Component Terpinen-4-ol on Swine Spermatozoa. Molecules. 2019; 24(6):1071. https://doi.org/10.3390/molecules24061071
Chicago/Turabian StyleElmi, Alberto, Domenico Ventrella, Francesca Barone, Giacomo Carnevali, Gianfranco Filippini, Annamaria Pisi, Stefania Benvenuti, Maurizio Scozzoli, and Maria Laura Bacci. 2019. "In Vitro Effects of Tea Tree Oil (Melaleuca Alternifolia Essential Oil) and its Principal Component Terpinen-4-ol on Swine Spermatozoa" Molecules 24, no. 6: 1071. https://doi.org/10.3390/molecules24061071
APA StyleElmi, A., Ventrella, D., Barone, F., Carnevali, G., Filippini, G., Pisi, A., Benvenuti, S., Scozzoli, M., & Bacci, M. L. (2019). In Vitro Effects of Tea Tree Oil (Melaleuca Alternifolia Essential Oil) and its Principal Component Terpinen-4-ol on Swine Spermatozoa. Molecules, 24(6), 1071. https://doi.org/10.3390/molecules24061071