Differential Proteomics Reveals miR-155 as a Novel Indicator of Liver and Spleen Pathology in the Symptomatic Niemann-Pick Disease, Type C1 Mouse Model
Abstract
1. Introduction
2. Results
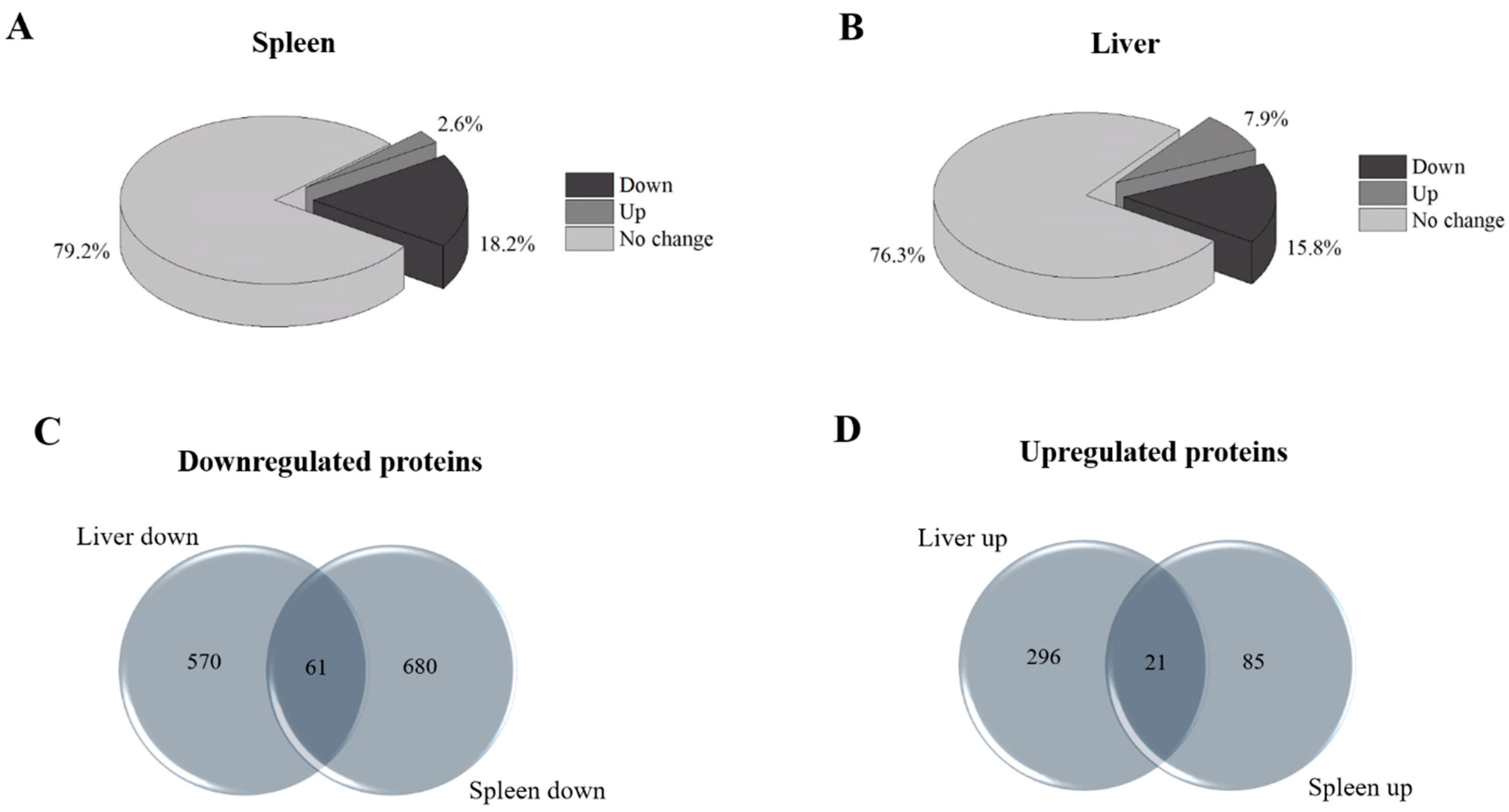
2.1. Evaluation of the Differential Proteomes
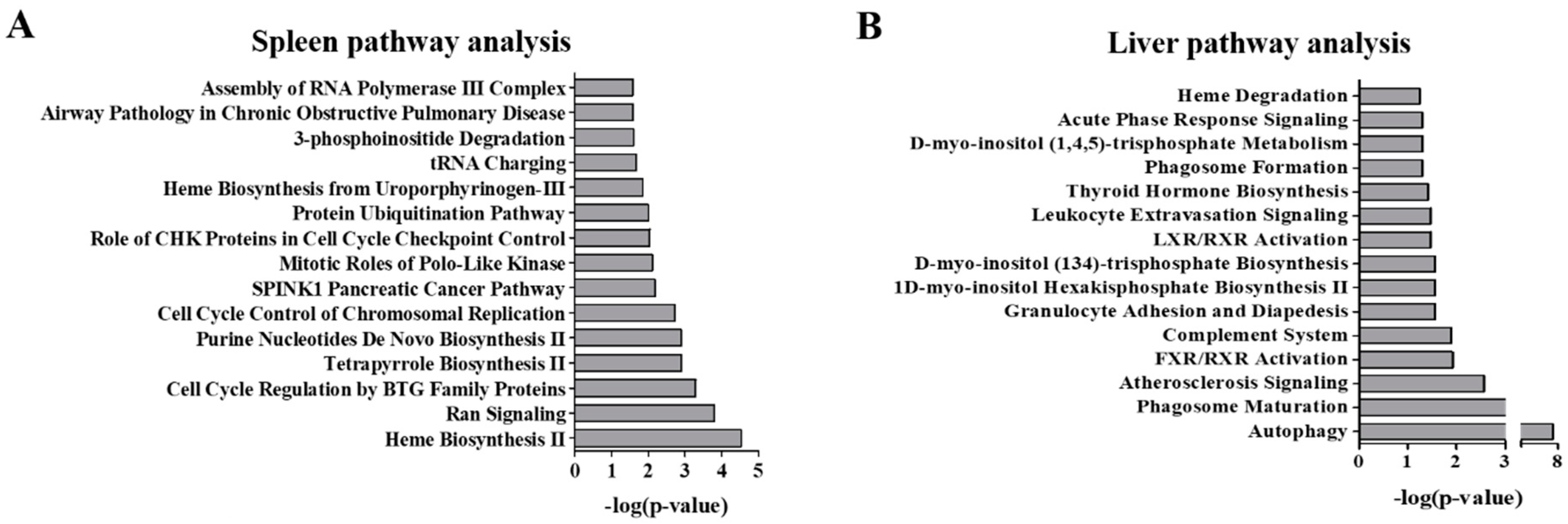
2.2. Pathway Analysis
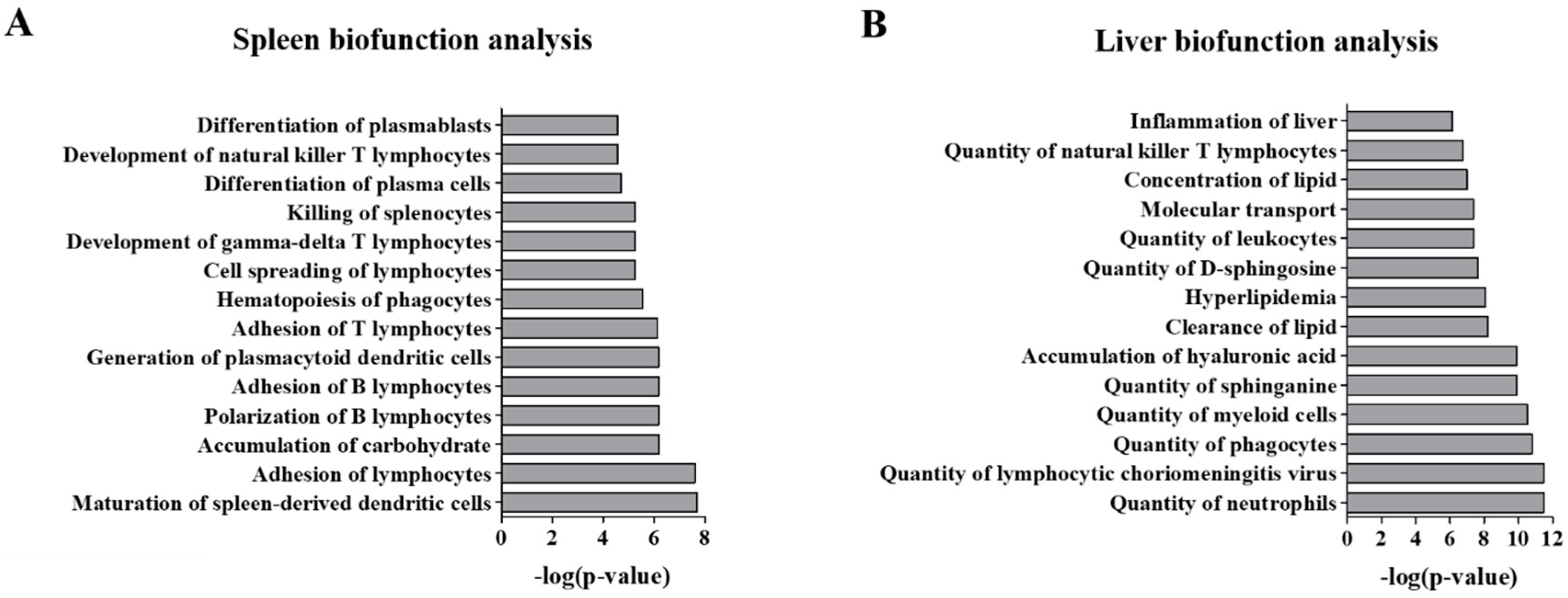
2.3. Functional Analysis
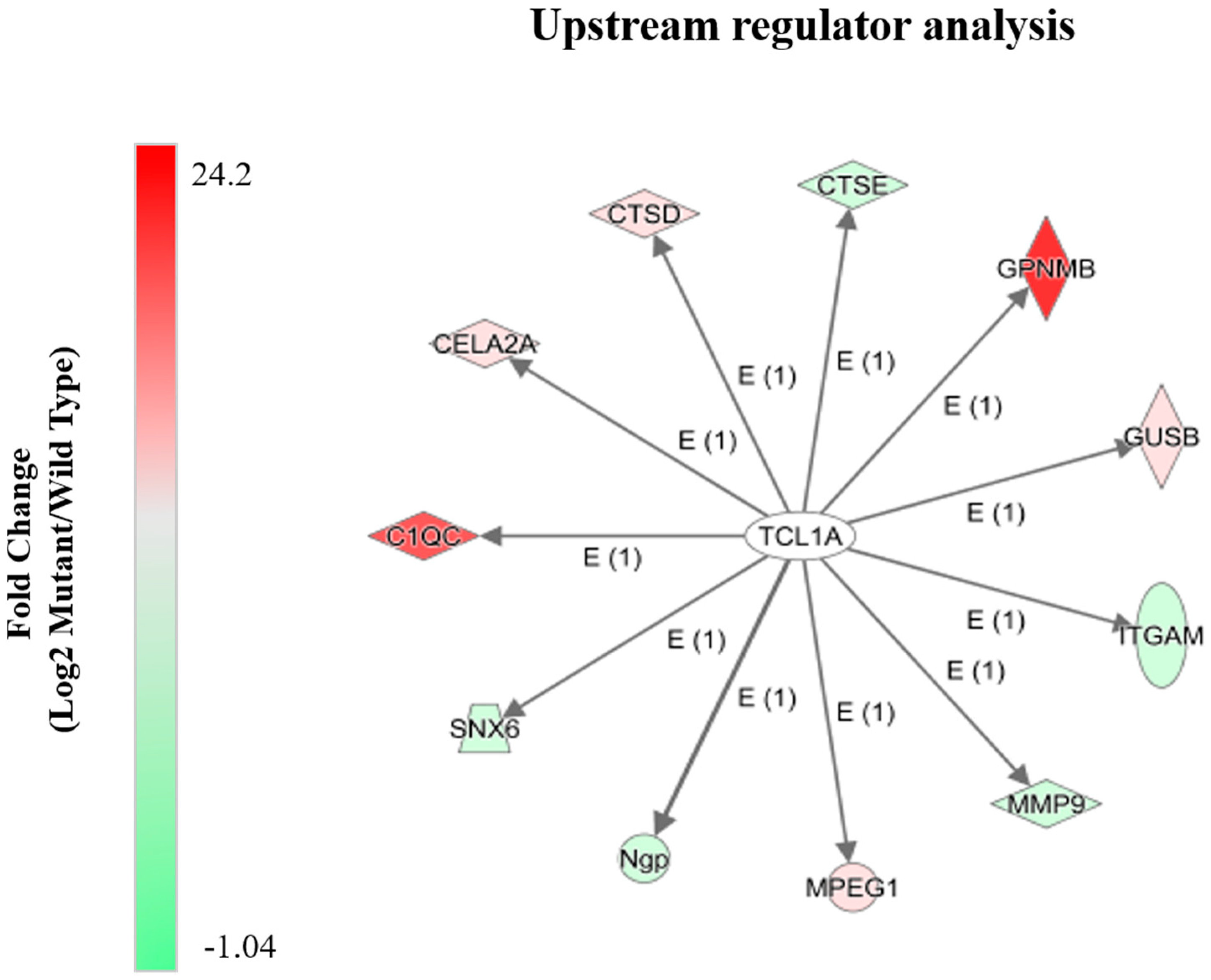
2.4. Alterations in Proteins Known to Be Transcriptionally Regulated by Tcl1a
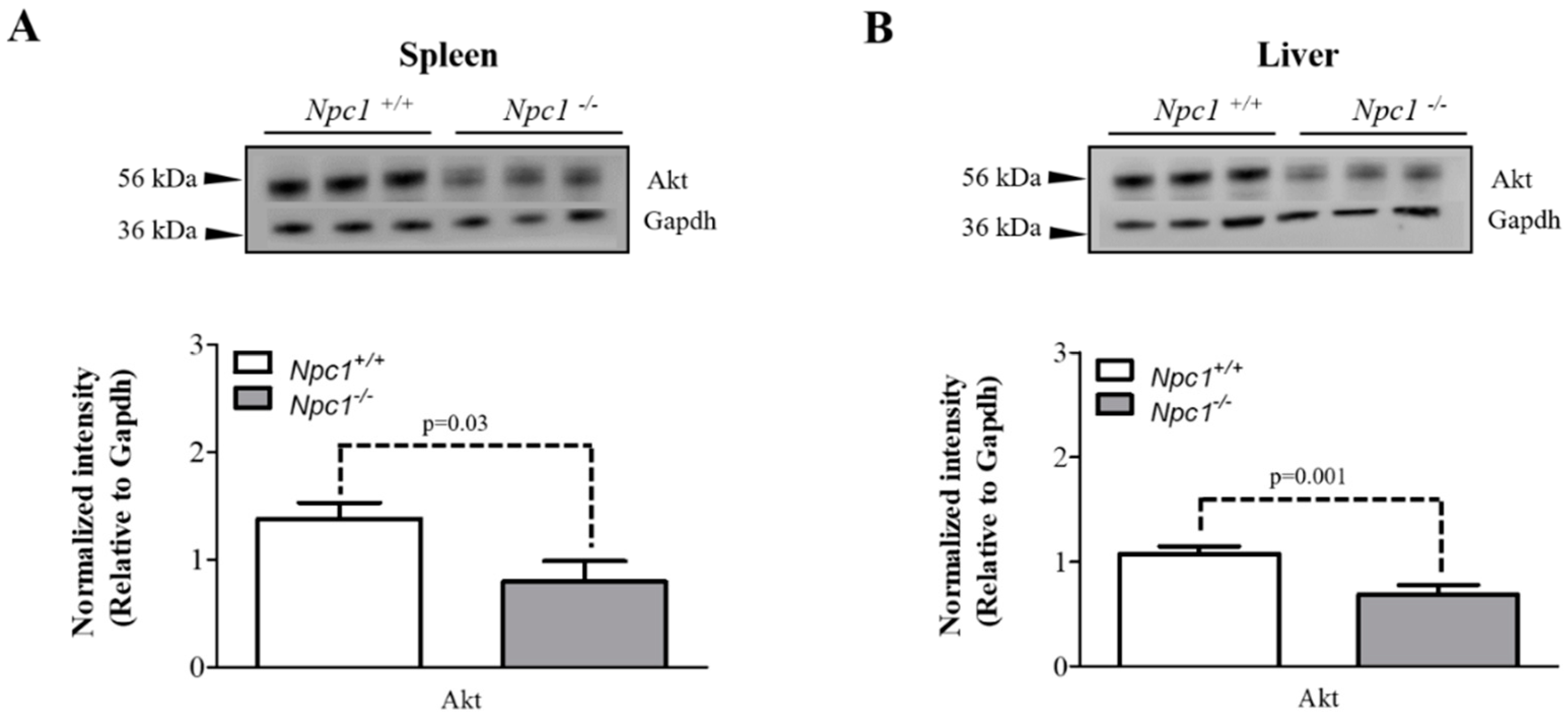
2.5. Altered Expression of Akt in the Spleen and Liver
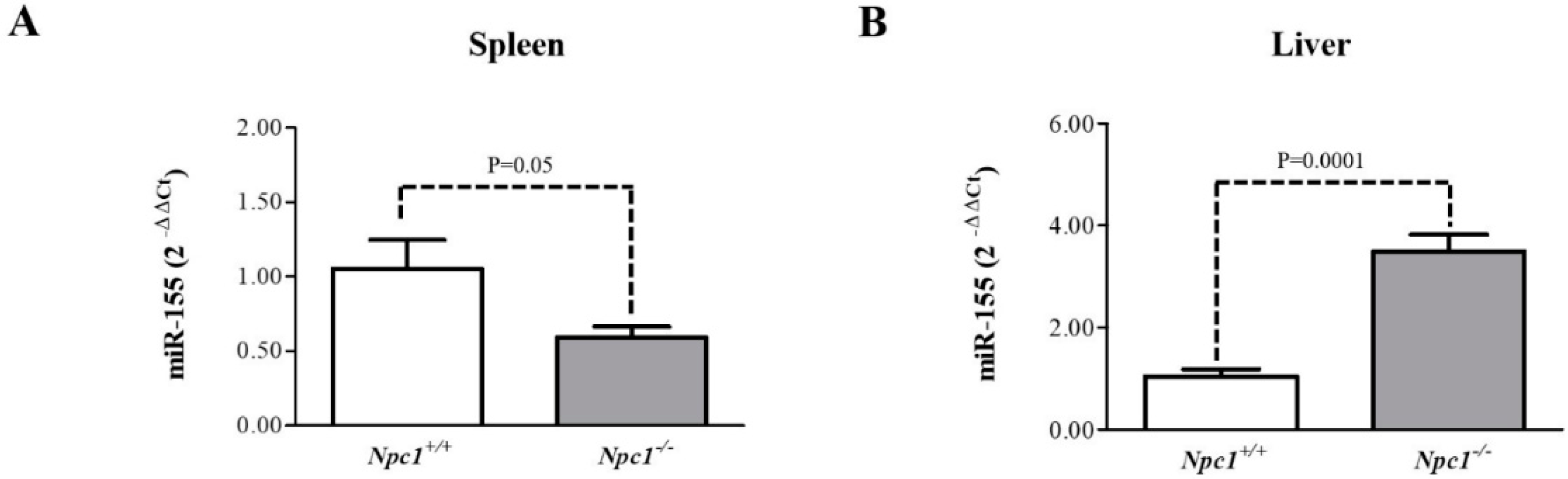
2.6. miR-155 May Be a Marker of NPC1 Spleen and Liver Pathology
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Animal Breeding and Tissue Collection
4.3. Sample Preparation for Proteomic Analysis
4.4. LC-MS/MS Analysis
4.5. Protein Identification and Relative Quantification
4.6. Differential Proteome Analysis
4.7. Western Blot Analysis
4.8. RNA Isolation and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanier, M.T.; Millat, G. Niemann-Pick disease type C. Clin. Genet. 2003, 64, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Sturley, S.L.; Patterson, M.C.; Balch, W.; Liscum, L. The pathophysiology and mechanisms of NP-C disease. Biochim. Biophys. Acta 2004, 1685, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.S. Niemann-Pick type C: A disorder of cellular cholesterol trafficking. Biochim. Biophys. Acta 2000, 1529, 331–339. [Google Scholar] [CrossRef]
- Kelly, D.A.; Portmann, B.; Mowat, A.P.; Sherlock, S.; Lake, B.D. Niemann-Pick disease type C: Diagnosis and outcome in children, with particular reference to liver disease. J. Pediatr. 1993, 123, 242–247. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. J. Lipid Res. 2008, 49, 663–669. [Google Scholar] [CrossRef]
- Morris, M.D.; Bhuvaneswaran, C.; Shio, H.; Fowler, S. Lysosome lipid storage disorder in NCTR-BALB/c mice. I. Description of the disease and genetics. Am. J. Pathol. 1982, 108, 140–149. [Google Scholar]
- Harzer, K.; Massenkeil, G.; Frohlich, E. Concurrent increase of cholesterol, sphingomyelin and glucosylceramide in the spleen from non-neurologic Niemann-Pick type C patients but also patients possibly affected with other lipid trafficking disorders. FEBS Lett. 2003, 537, 177–181. [Google Scholar] [CrossRef]
- Loftus, S.K.; Morris, J.A.; Carstea, E.D.; Gu, J.Z.; Cummings, C.; Brown, A.; Ellison, J.; Ohno, K.; Rosenfeld, M.A.; Tagle, D.A.; et al. Murine model of Niemann-Pick C disease: Mutation in a cholesterol homeostasis gene. Sciences (New York N. Y.) 1997, 277, 232–235. [Google Scholar] [CrossRef]
- Parra, J.; Klein, A.D.; Castro, J.; Morales, M.G.; Mosqueira, M.; Valencia, I.; Cortes, V.; Rigotti, A.; Zanlungo, S. Npc1 deficiency in the C57BL/6J genetic background enhances Niemann-Pick disease type C spleen pathology. Biochem. Biophys. Res. Commun. 2011, 413, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Beltroy, E.P.; Richardson, J.A.; Horton, J.D.; Turley, S.D.; Dietschy, J.M. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology 2005, 42, 886–893. [Google Scholar] [CrossRef] [PubMed]
- vom Dahl, S.; Mengel, E. Lysosomal storage diseases as differential diagnosis of hepatosplenomegaly. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Volk, B.W.; Schneck, L. Animal model of human disease: Niemann-Pick Disease type C. Am. J. Pathol. 1976, 85, 229–232. [Google Scholar] [PubMed]
- Zhang, B.; Yang, C.; Qiao, L.; Li, Q.; Wang, C.; Yan, X.; Lin, J. Telocytes: A potential defender in the spleen of Npc1 mutant mice. J. Cell. Mol. Med. 2017, 21, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Pergande, M.R.; Zarate, E.; Haney-Ball, C.; Scesa, G.; Davidson, C.D.; Givogri, M.I.; Bongarzone, E.R.; Cologna, S.M. Proteomic analysis via standard-flow liquid chromatography and thermal focusing electrospray ionization elucidates altered liver proteins in late stage Niemann-Pick, type C1 disease. Bioanalysis. (under review).
- Pacheco, C.D.; Kunkel, R.; Lieberman, A.P. Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum. Mol. Genet. 2007, 16, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, L.; Ying, H.; Yu, Y.; Zhang, D.; Deng, H.; Zhang, H.; Chai, J. Acute downregulation of miR-155 leads to a reduced collagen synthesis through attenuating macrophages inflammatory factor secretion by targeting SHIP1. J. Mol. Histol. 2018, 49, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Wu, J.J.; Jiang, W.L.; Xiao, J.H.; Tao, K.Z.; Ma, L.; Zheng, P.; Wan, R.; Wang, X.P. MicroRNA-155 promotes the pathogenesis of experimental colitis by repressing SHIP-1 expression. World J. Gastroenterol. 2017, 23, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Getz, M.; Safeukui, I.; Yi, S.; Tamez, P.; Shin, J.; Velazquez, P.; Haldar, K. Genomic expression analyses reveal lysosomal, innate immunity proteins, as disease correlates in murine models of a lysosomal storage disorder. PLoS ONE 2012, 7, e48273. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, L.; Lazzeri, C.; Bichi, R.; Nibu, K.; Narducci, M.G.; Russo, G.; Rothstein, J.L.; Croce, C.M. Deregulated expression of TCL1 causes T cell leukemia in mice. Proc. Natl. Acad. Sci. USA 1998, 95, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Gaudio, E.; Mensah, A.A.; Pinton, S.; Bertoni, F.; Trapasso, F. T-Cell Leukemia/Lymphoma 1 (TCL1): An Oncogene Regulating Multiple Signaling Pathways. Front. Oncol. 2018, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, V.M.; Irvine, K.M.; Ravasi, T.; Sweet, M.J.; Hume, D.A. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J. Immunol. (Baltimore, MD 1950) 2007, 178, 6557–6566. [Google Scholar] [CrossRef]
- Bertoni, A.; Alabiso, O.; Galetto, A.S.; Baldanzi, G. Integrins in T Cell Physiology. Int. J. Mol. Sci. 2018, 19, 485. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, C.; Korpos, E.; Zhang, X.; Agrawal, S.M.; Wang, Y.; Faber, C.; Schafers, M.; Korner, H.; Opdenakker, G.; et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015, 10, 1040–1054. [Google Scholar] [CrossRef] [PubMed]
- Spilsbury, K.; O’Mara, M.A.; Wu, W.M.; Rowe, P.B.; Symonds, G.; Takayama, Y. Isolation of a novel macrophage-specific gene by differential cDNA analysis. Blood 1995, 85, 1620–1629. [Google Scholar] [PubMed]
- Roumenina, L.T.; Sene, D.; Radanova, M.; Blouin, J.; Halbwachs-Mecarelli, L.; Dragon-Durey, M.A.; Fridman, W.H.; Fremeaux-Bacchi, V. Functional complement C1q abnormality leads to impaired immune complexes and apoptotic cell clearance. J. Immunol. (Baltimore MD 1950) 2011, 187, 4369–4373. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.E.; Klein, A.D.; Scott, M.P. Complement is dispensable for neurodegeneration in Niemann-Pick disease type C. J. Neuroinflamm. 2012, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, C.V.; Watkins-Chow, D.E.; Fu, R.; Borate, B.; Yanjanin, N.; Dail, M.K.; Davidson, C.D.; Walkley, S.U.; Ory, D.S.; Wassif, C.A.; et al. Microarray expression analysis and identification of serum biomarkers for Niemann-Pick disease, type C1. Hum. Mol. Genet. 2012, 21, 3632–3646. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.H.; Calado, D.P.; Casola, S.; Ansel, K.M.; Xiao, C.; Xue, Y.; Murphy, A.; Frendewey, D.; Valenzuela, D.; Kutok, J.L.; et al. Regulation of the germinal center response by microRNA-155. Sciences (New York N. Y.) 2007, 316, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Mycko, M.P.; Cichalewska, M.; Cwiklinska, H.; Selmaj, K.W. miR-155-3p Drives the Development of Autoimmune Demyelination by Regulation of Heat Shock Protein 40. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 16504–16515. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jia, J.; Du, T.; Li, W.; Wang, X.; Wei, J.; Lin, X.; Zeng, H.; Yao, L.; Chen, X.; et al. Overexpression of miR-155 in the liver of transgenic mice alters the expression profiling of hepatic genes associated with lipid metabolism. PLoS ONE 2015, 10, e0118417. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Ibrahim, N.; Murnane, M.J.; Diamond, A.; Forget, B.G.; Levere, R.D. Hemin control of heme biosynthesis and catabolism in a human leukemia cell line. Blood 1980, 56, 567–570. [Google Scholar] [PubMed]
- Bottomley, S.S.; Muller-Eberhard, U. Pathophysiology of heme synthesis. Semin. Hematol. 1988, 25, 282–302. [Google Scholar] [PubMed]
- Braidotti, G.; Borthwick, I.A.; May, B.K. Identification of regulatory sequences in the gene for 5-aminolevulinate synthase from rat. J. Biol. Chem. 1993, 268, 1109–1117. [Google Scholar] [PubMed]
- Nicoli, E.R.; Al Eisa, N.; Cluzeau, C.V.; Wassif, C.A.; Gray, J.; Burkert, K.R.; Smith, D.A.; Morris, L.; Cologna, S.M.; Peer, C.J.; et al. Defective Cytochrome P450-Catalysed Drug Metabolism in Niemann-Pick Type C Disease. PLoS ONE 2016, 11, e0152007. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, N.; Epstein, O.; Lahav, M.; Mamet, R.; Shaklai, M.; Atsmon, A. The heme biosynthetic pathway in lymphocytes of patients with malignant lymphoproliferative disorders. Cancer Lett. 1988, 43, 43–48. [Google Scholar] [CrossRef]
- Nieland, J.D.; Haks, M.C.; Kremers, B.L.; Leupers, T.J.; Bakker, A.Q.; Offringa, R.; Kruisbeek, A.M. Functional analysis of Ran/TC4 as a protein regulating T-cell costimulation. Cancer Gene Ther. 1998, 5, 259–273. [Google Scholar] [PubMed]
- Sekimoto, T.; Imamoto, N.; Nakajima, K.; Hirano, T.; Yoneda, Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 1997, 16, 7067–7077. [Google Scholar] [CrossRef] [PubMed]
- Reich, N.C. STATs get their move on. JAK-STAT 2013, 2, e27080. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sugimoto, Y.; Ohsaki, Y.; Ueno, M.; Kato, S.; Kitamura, Y.; Hosokawa, H.; Davies, J.P.; Ioannou, Y.A.; et al. Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: A potential basis for glial cell activation in the NPC brain. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 1879–1891. [Google Scholar]
- Wos, M.; Komiazyk, M.; Pikula, S.; Tylki-Szymanska, A.; Bandorowicz-Pikula, J. Activation of mammalian terget of rapamycin kinase and glycogen synthase kinase-3beta accompanies abnormal accumulation of cholesterol in fibroblasts from Niemann-Pick type C patients. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Laine, J.; Kunstle, G.; Obata, T.; Sha, M.; Noguchi, M. The protooncogene TCL1 is an Akt kinase coactivator. Mol. Cell 2000, 6, 395–407. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Jo, S.R.; Liu, X.; Qi, Y.; Osborne, B.; Byrne, F.L.; Smith, G.C.; Turner, N.; Hoehn, K.L.; et al. Akt activation increases cellular cholesterol by promoting the proteasomal degradation of Niemann-Pick C1. Biochem. J. 2015, 471, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Croce, C.M.; Michaille, J.J. miR-155: On the crosstalk between inflammation and cancer. Int. Rev. Immunol. 2009, 28, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of bic/microRNA-155 for normal immune function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, A.; Kroesen, B.J.; Kooistra, K.; de Jong, D.; Briggs, J.; Blokzijl, T.; Jacobs, S.; Kluiver, J.; Diepstra, A.; Maggio, E.; et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Geneschromosomes Cancer 2003, 37, 20–28. [Google Scholar] [CrossRef]
- Singh, A.; Sen, D. MicroRNAs in Parkinson’s disease. Exp. Brain Res. 2017, 235, 2359–2374. [Google Scholar] [CrossRef] [PubMed]
- Ozsait, B.; Komurcu-Bayrak, E.; Levula, M.; Erginel-Unaltuna, N.; Kahonen, M.; Rai, M.; Lehtimaki, T.; Laaksonen, R. Niemann-Pick type C fibroblasts have a distinct microRNA profile related to lipid metabolism and certain cellular components. Biochem. Biophys. Res. Commun. 2010, 403, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.R. Quantitative Evaluation of Filter Aided Sample Preparation (FASP) and Multienzyme Digestion FASP Protocols. Anal. Chem. 2016, 88, 5438–5443. [Google Scholar] [CrossRef] [PubMed]
- Pergande, M.R.; Nguyen, T.T.A.; Haney-Ball, C.; Davidson, C.D.; Cologna, S.M. Quantitative, Label-Free Proteomics in the Symptomatic Niemann-Pick, Type C1 Mouse Model Using Standard Flow Liquid Chromatography and Thermal Focusing Electrospray Ionization. Proteomics 2019. (accepted). [Google Scholar]
- Batth, T.S.; Francavilla, C.; Olsen, J.V. Off-line high-pH reversed-phase fractionation for in-depth phosphoproteomics. J. Proteome Res. 2014, 13, 6176–6186. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergande, M.R.; Cougnoux, A.; Rathnayake, R.A.C.; Porter, F.D.; Cologna, S.M. Differential Proteomics Reveals miR-155 as a Novel Indicator of Liver and Spleen Pathology in the Symptomatic Niemann-Pick Disease, Type C1 Mouse Model. Molecules 2019, 24, 994. https://doi.org/10.3390/molecules24050994
Pergande MR, Cougnoux A, Rathnayake RAC, Porter FD, Cologna SM. Differential Proteomics Reveals miR-155 as a Novel Indicator of Liver and Spleen Pathology in the Symptomatic Niemann-Pick Disease, Type C1 Mouse Model. Molecules. 2019; 24(5):994. https://doi.org/10.3390/molecules24050994
Chicago/Turabian StylePergande, Melissa R., Antony Cougnoux, Rathnayake A. C. Rathnayake, Forbes D. Porter, and Stephanie M. Cologna. 2019. "Differential Proteomics Reveals miR-155 as a Novel Indicator of Liver and Spleen Pathology in the Symptomatic Niemann-Pick Disease, Type C1 Mouse Model" Molecules 24, no. 5: 994. https://doi.org/10.3390/molecules24050994
APA StylePergande, M. R., Cougnoux, A., Rathnayake, R. A. C., Porter, F. D., & Cologna, S. M. (2019). Differential Proteomics Reveals miR-155 as a Novel Indicator of Liver and Spleen Pathology in the Symptomatic Niemann-Pick Disease, Type C1 Mouse Model. Molecules, 24(5), 994. https://doi.org/10.3390/molecules24050994





