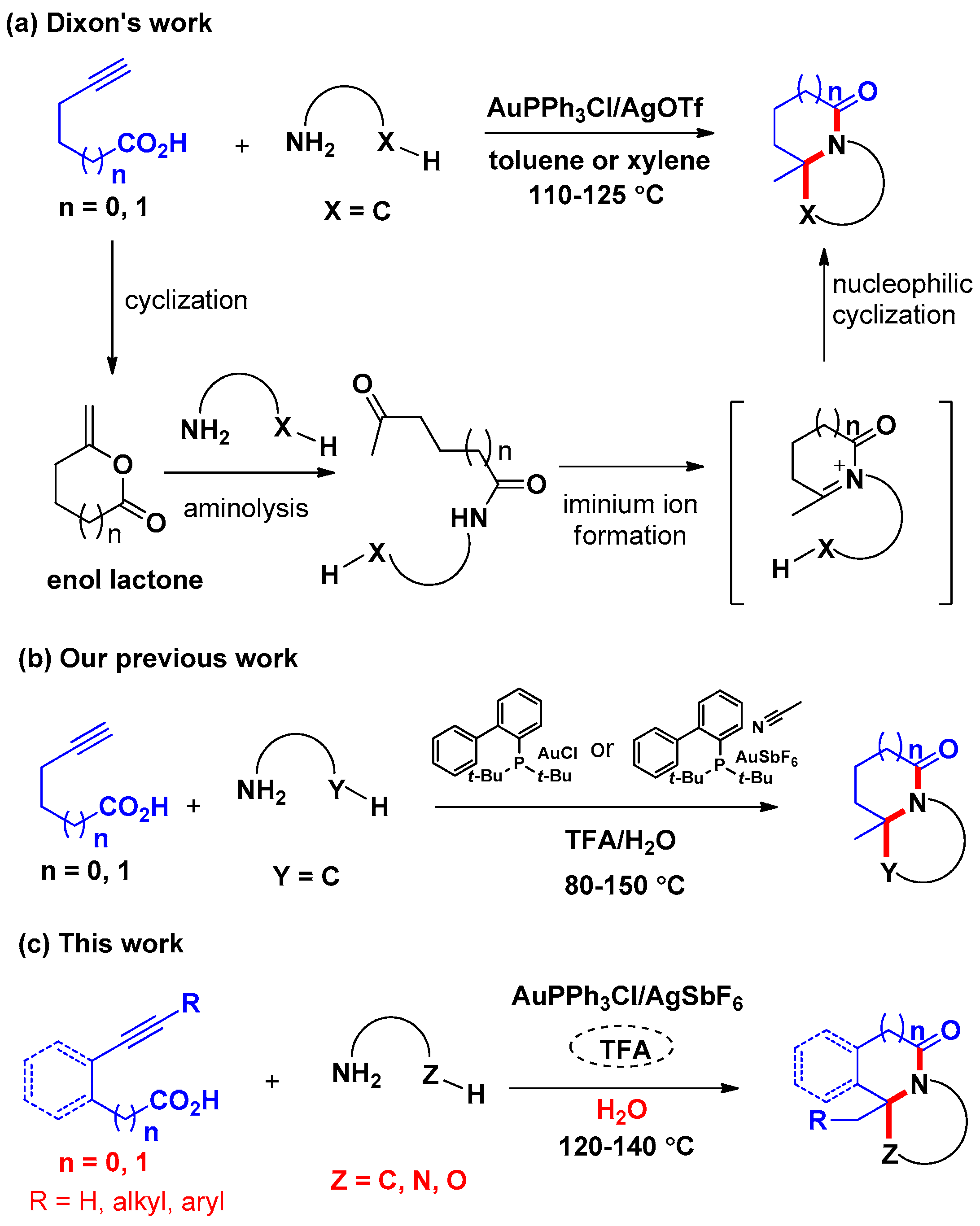
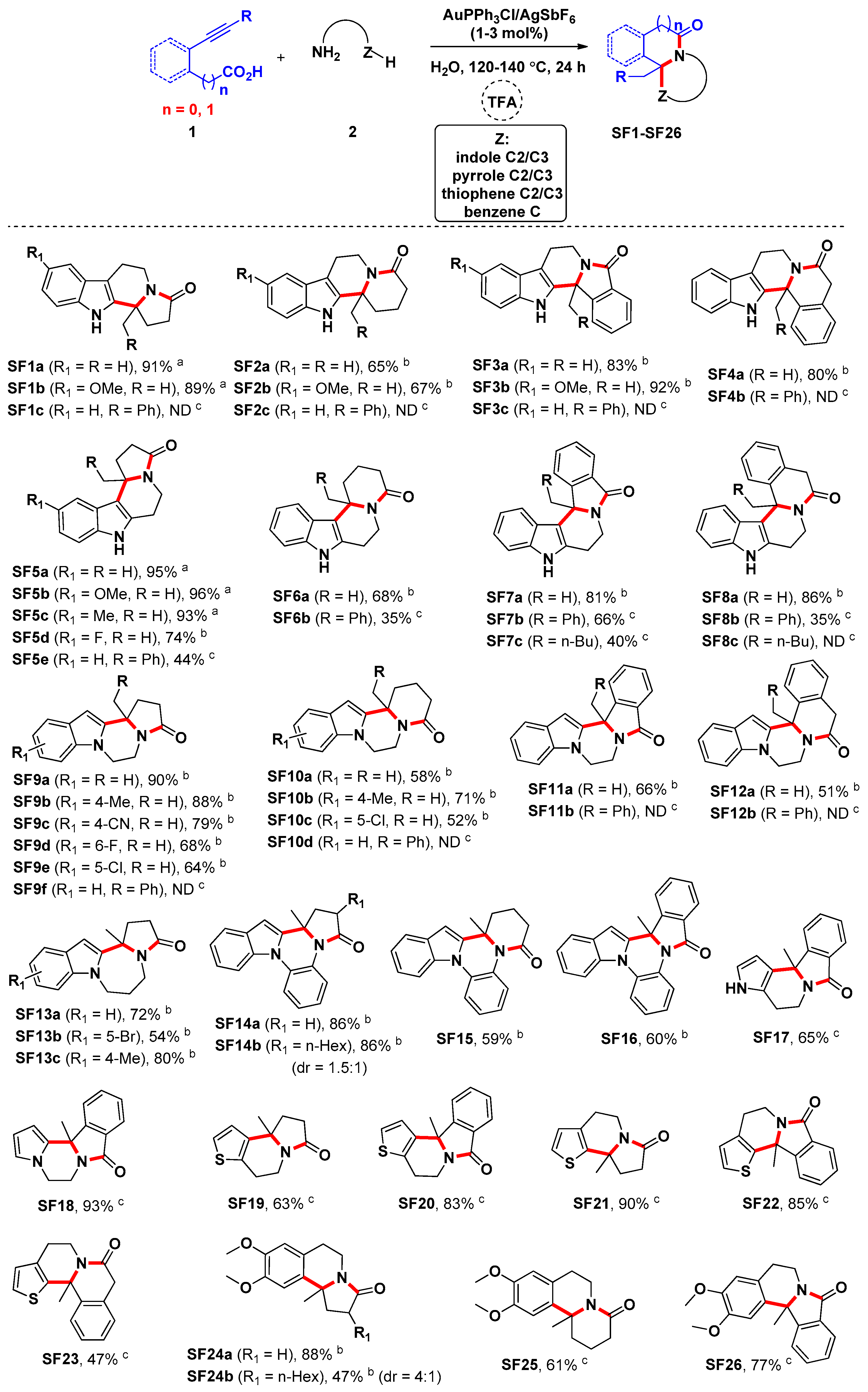
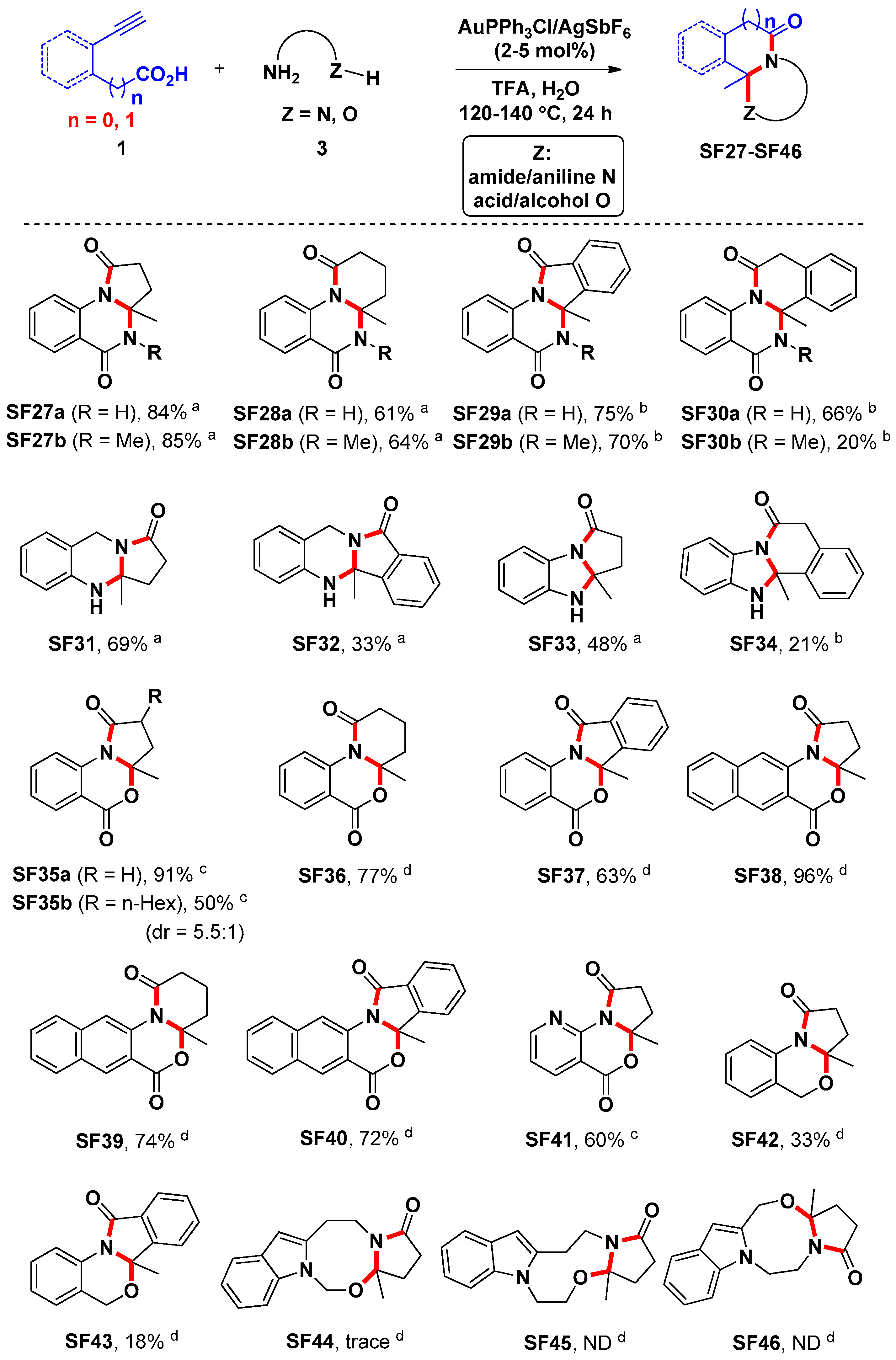
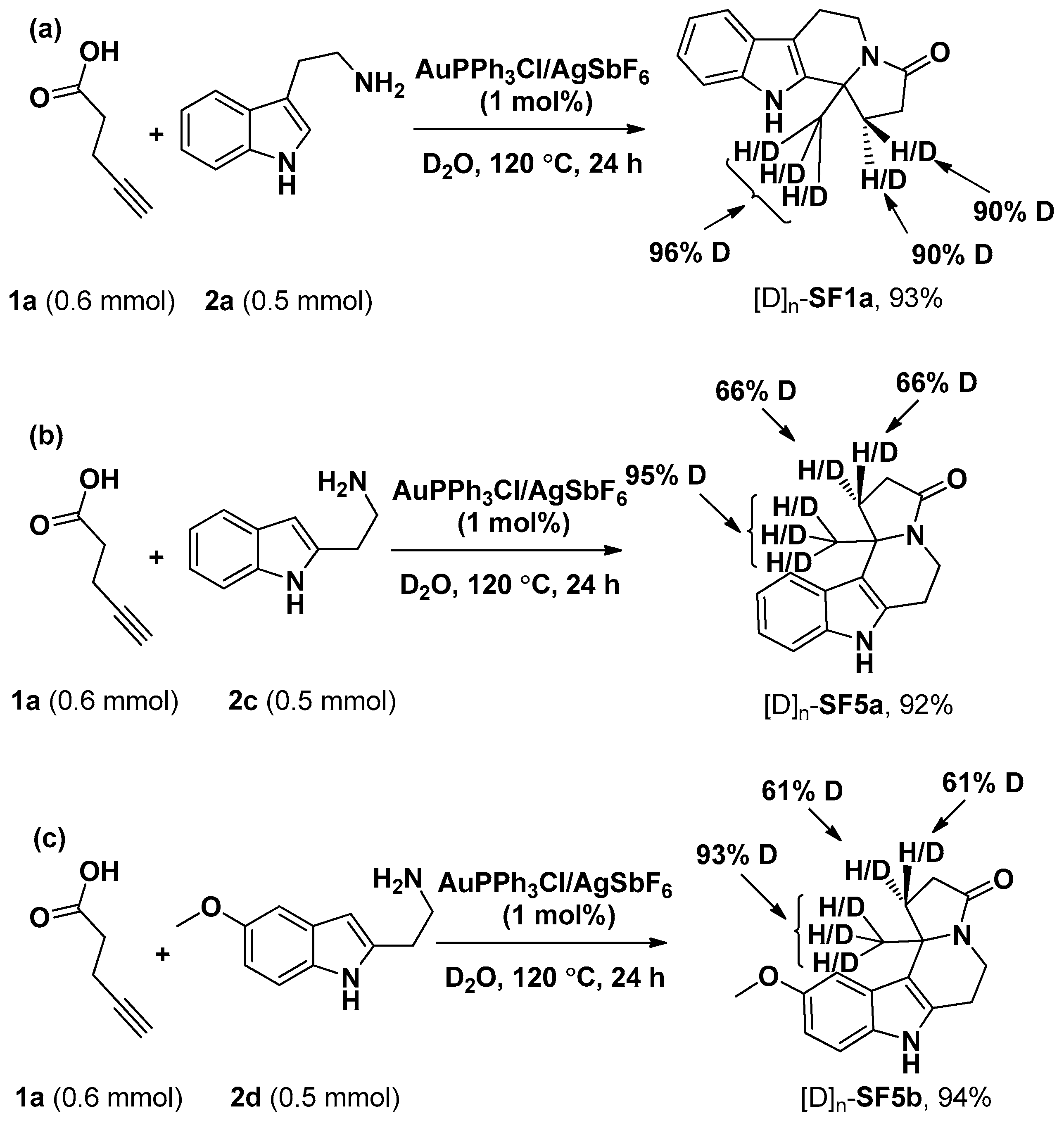
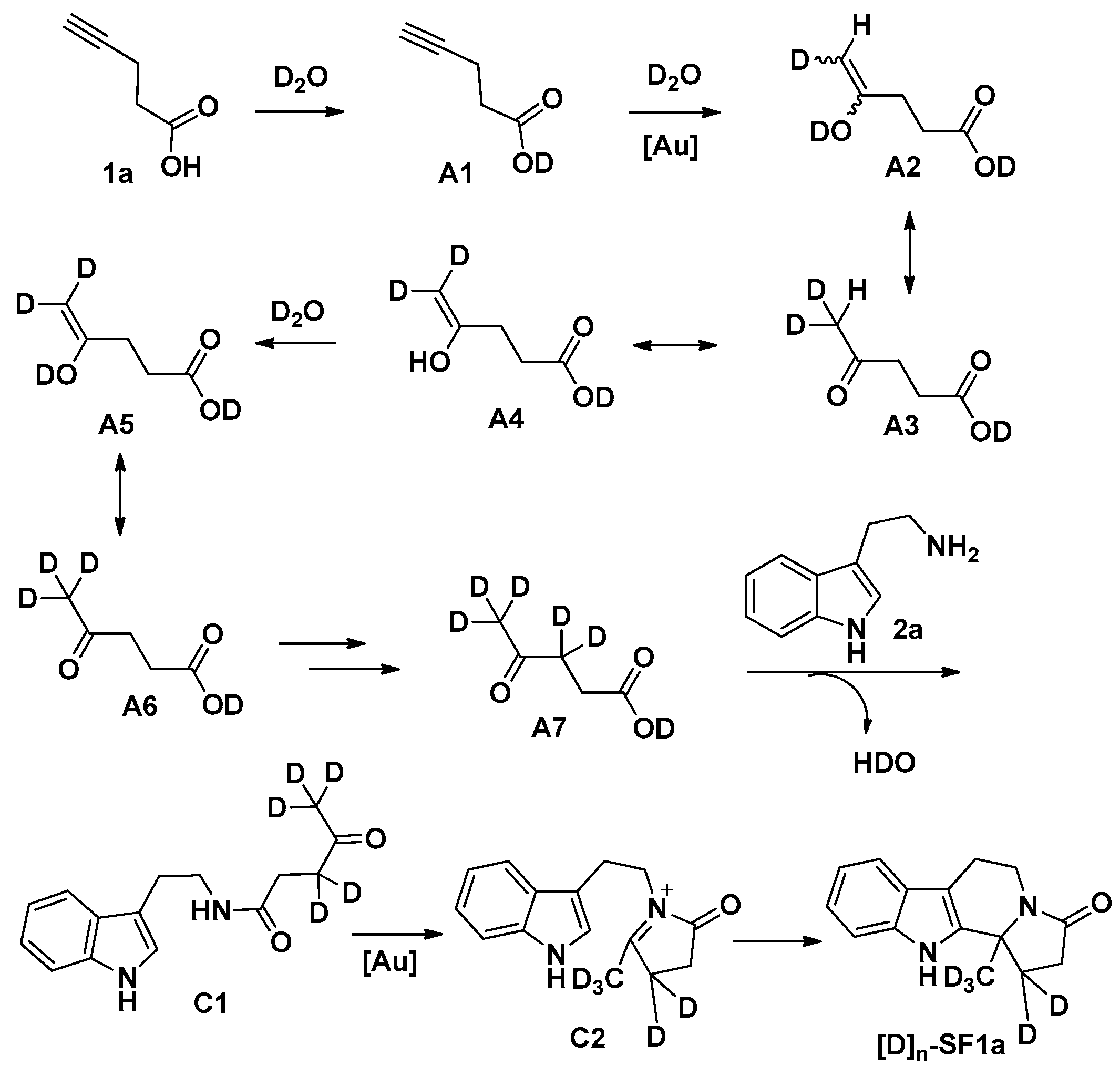
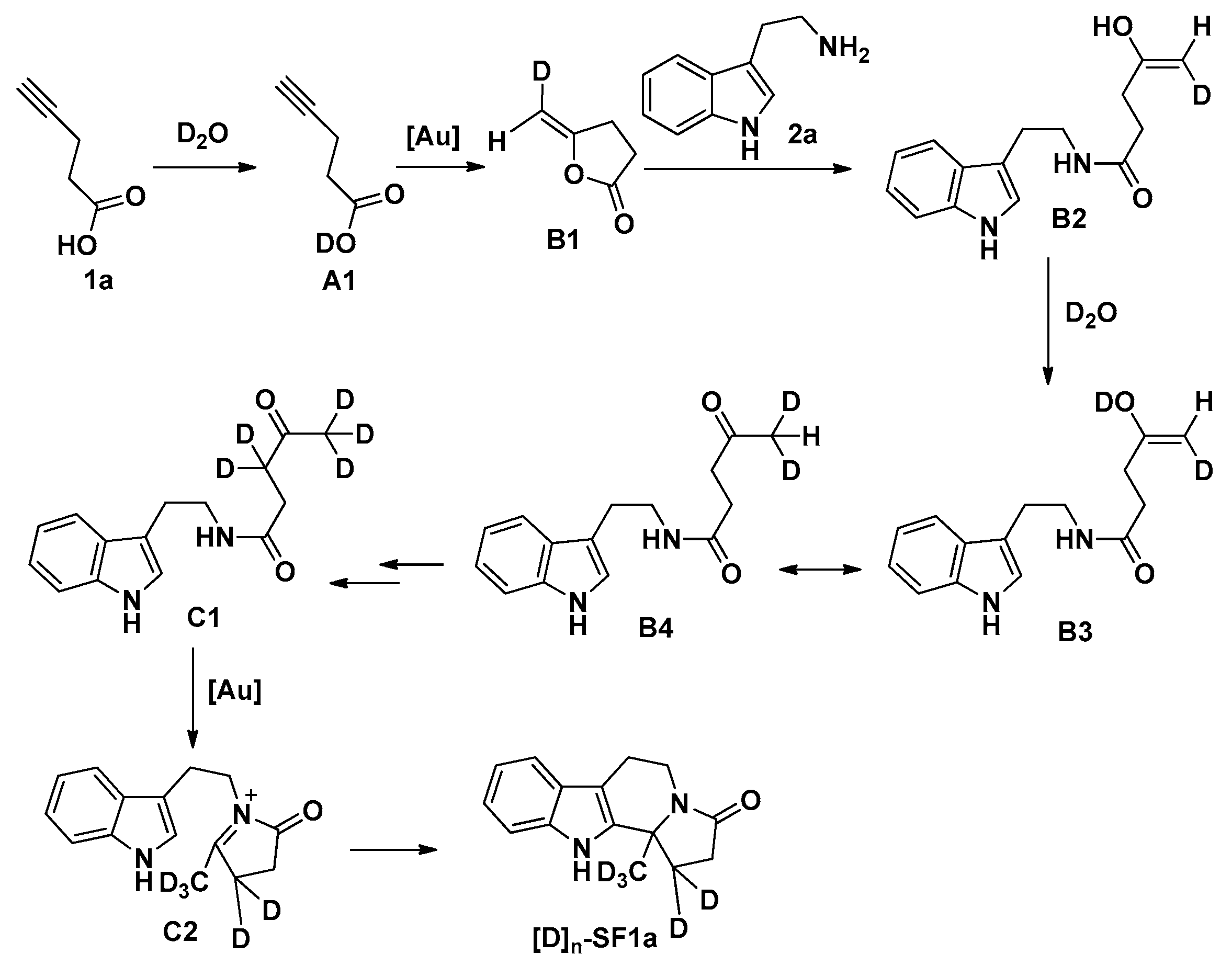
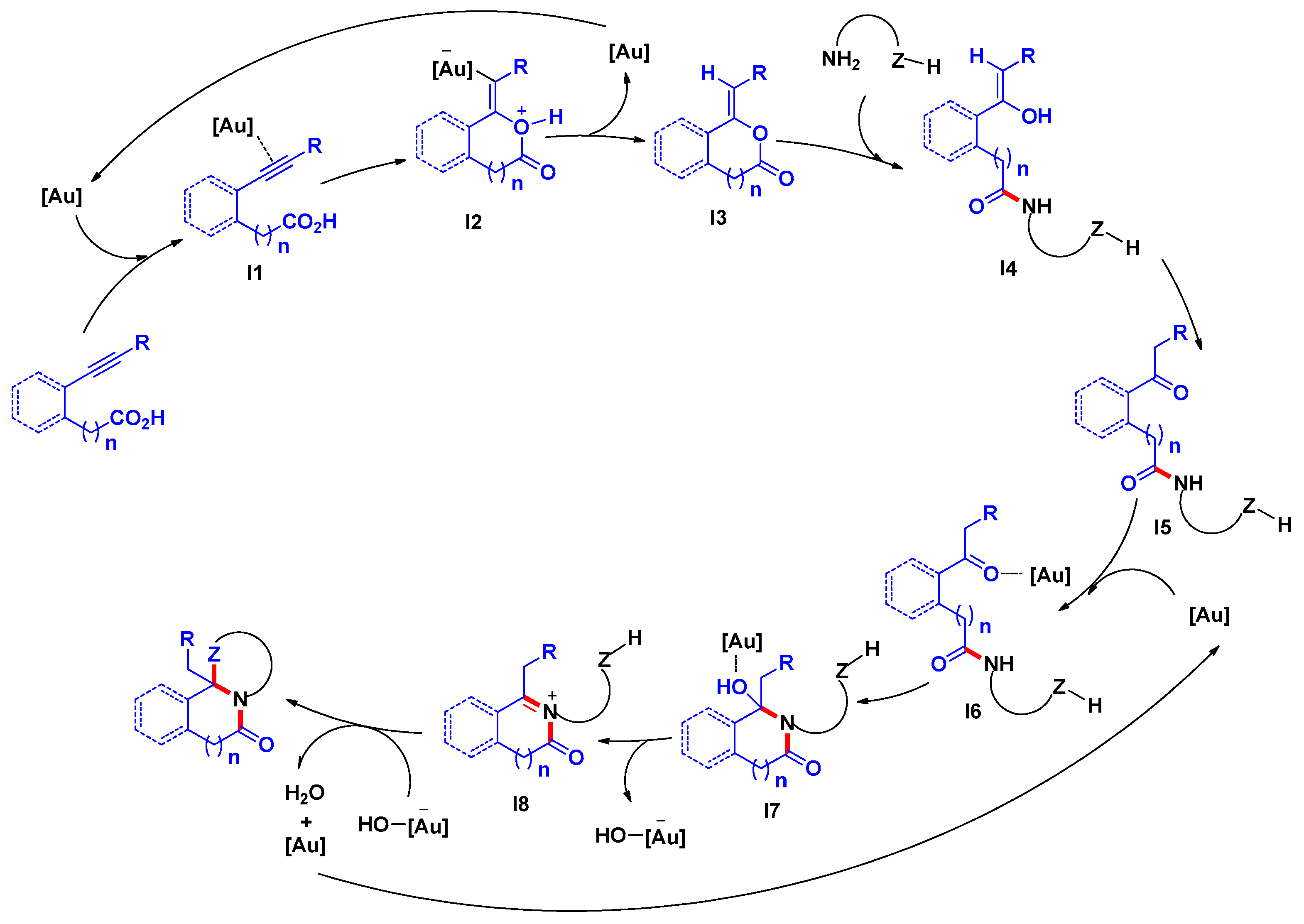
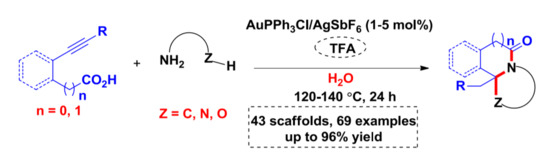
3.3. General Procedure for the Preparation of Compounds SF1c, SF2–SF4, SF5d, SF5e and SF6–SF46
A suspension of alkynoic acids 1 (0.6 mmol), amine nucleophiles 2 or 3 (0.5 mmol), and AuPPh3Cl/AgSbF6 (with the amount indicated) in H2O (4.0 mL) was stirred at the temperature indicated for 20 h. Then the reaction mixture was cooled to room temperature, and CF3COOH (0.5 mmol) was added, and the resulting mixture was stirred for another 4 h at the temperature indicated. At ambient temperature, saturated Na2CO3 solution (25.0 mL) was added to the reaction mixture. The resulting mixture was then extracted with ethyl acetate (3 × 15 mL). The combined organic layers were washed with brine, and dried over Na2SO4. After filtration and removal of the solvents in vacuo, the crude product was purified by flash chromatography on silica gel to yield the desired product.
12b-Methyl-1,2,3,6,7,12b-hexahydroindolo[2,3-a]quinolizin-4(12H)-one (
SF2a): white solid (82.6 mg, yield 65%), mp 255–256 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.60 (s, 3H), 1.80–1.68 (m, 2H), 1.97–1.86 (m, 1H), 2.32–2.21 (m, 1H), 2.45–2.32 (m, 2H), 2.61–2.53 (m, 1H), 2.69–2.62 (m, 1H), 2.99–2.87 (m, 1H), 4.90–4.81 (m, 1H), 7.00–6.93 (m, 1H), 7.10–7.02 (m, 1H), 7.32 (d,
J = 8.0 Hz, 1H), 7.39 (d,
J = 7.8 Hz, 1H), 10.92 (s, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 167.9 (CO), 139.7 (C, Ar), 136.0 (C, Ar), 126.2 (C, Ar), 120.9 (CH, Ar), 118.5 (CH, Ar), 117.9 (CH, Ar), 111.1 (CH, Ar), 105.8 (C, Ar), 56.4 (C), 35.6 (CH
2), 34.8 (CH
2), 31.8 (CH
2), 25.3 (CH
3), 21.0 (CH
2), 16.3 (CH
2); ESI-LRMS
m/
z: 255 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 255.1492, found: 255.1488. The characterization data is in accordance with that reported in [
43].
9-Methoxy-12b-methyl-1,2,3,6,7,12b-hexahydroindolo[2,3-a]quinolizin-4(12H)-one (
SF2b): pale yellow solid (95.4 mg, yield 67%), mp 190–191 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.59 (s, 3H), 1.79–1.66 (m, 2H), 1.97–1.84 (m, 1H), 2.42–2.21 (m, 3H), 2.66–2.51 (m, 2H), 2.99–2.86 (m, 1H), 3.74 (s, 3H), 4.90–4.79 (m, 1H), 6.70 (dd,
J = 8.7, 2.4 Hz, 1H), 6.89 (d,
J = 2.4 Hz, 1H), 7.20 (d,
J = 8.7 Hz, 1H), 10.73 (s, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 167.9 (CO), 153.2 (C, Ar), 140.4 (C, Ar), 131.0 (C, Ar), 126.5 (C, Ar), 111.7 (CH, Ar), 110.7 (CH, Ar), 105.7 (C, Ar), 100.1 (CH, Ar), 56.4 (C), 55.4 (OCH
3), 35.6 (CH
2), 34.8 (CH
2), 31.8 (CH
2), 25.4 (CH
3), 21.1 (CH
2), 16.3 (CH
2); ESI-LRMS
m/
z: 285 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 285.1598, found: 285.1593. The characterization data is in accordance with that reported in [
43].
13b-Methyl-7,8,13,13b-tetrahydro-5H-benzo[1,2]indolizino[8,7-b]indol-5-one (
SF3a): white solid (119.4 mg, yield 83%), mp 283–284 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.86 (s, 3H), 2.75–2.63 (m, 1H), 2.85–2.75 (m, 1H), 3.47–3.36 (m, 1H), 4.59–4.47 (m, 1H), 7.03–6.93 (m, 1H), 7.15–7.05 (m, 1H), 7.44–7.34 (m, 2H), 7.58–7.49 (m, 1H), 7.79–7.68 (m, 2H), 8.32 (d,
J = 7.9 Hz, 1H), 11.35 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 167.2 (CO), 149.3 (C, Ar), 136.2 (C, Ar), 135.2 (C, Ar), 132.2 (CH, Ar), 130.3 (C, Ar), 128.6 (CH, Ar), 126.0 (C, Ar), 123.2 (CH, Ar), 122.8 (CH, Ar), 121.6 (CH, Ar), 118.9 (CH, Ar), 118.3 (CH, Ar), 111.2 (CH, Ar), 106.3 (C, Ar), 62.0 (C), 35.4 (CH
2), 25.9 (CH
3), 21.4 (CH
2); ESI-LRMS
m/
z: 289 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 289.1335, found: 289.1330. The characterization data is in accordance with that reported in [
43].
10-Methoxy-13b-methyl-7,8,13,13b-tetrahydro-5H-benzo[1,2]indolizino[8,7-b]indol-5-one (
SF3b): white solid (146.9 mg, yield 92%), mp 164–165 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.84 (s, 3H), 2.72–2.60 (m, 1H), 2.81–2.73 (m, 1H), 3.45–3.35 (m, 1H), 3.73 (s, 3H), 4.57–4.45 (m, 1H), 6.73 (dd,
J = 8.7, 2.1 Hz, 1H), 6.89 (d,
J = 2.1 Hz, 1H), 7.26 (d,
J = 8.7 Hz, 1H), 7.58–7.47 (m, 1H), 7.77–7.67 (m, 2H), 8.29 (d,
J = 7.9 Hz, 1H), 11.18 (s, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 167.2 (CO), 153.3 (C, Ar), 149.4 (C, Ar), 135.8 (C, Ar), 132.2 (CH, Ar), 131.2 (C, Ar), 130.2 (C, Ar), 128.6 (CH, Ar), 126.3 (C, Ar), 123.2 (CH, Ar), 122.8 (CH, Ar), 111.9 (CH, Ar), 111.5 (CH, Ar), 106.2 (C, Ar), 100.3 (CH, Ar), 62.0 (C), 55.4 (OCH
3), 35.5 (CH
2), 26.0 (CH
3), 21.5 (CH
2); ESI-LRMS
m/
z: 319 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 319.1441, found: 319.1435. The characterization data is in accordance with that reported in [
43].
14b-Methyl-8,9,14,14b-tetrahydroindolo[2′,3′:3,4]pyrido[2,1-a]isoquinolin-6(5H)-one (
SF4a): pale yellow solid (120.7 mg, yield 80%), mp 137–138 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.84 (s, 3H), 2.50–2.41 (m, 1H), 2.86–2.75 (m, 1H), 2.96–2.86 (m, 1H), 3.63 (d,
J = 19.4 Hz, 1H), 4.08 (d,
J = 19.2 Hz, 1H), 4.96–4.85 (m, 1H), 7.08–6.98 (m, 1H), 7.18–7.12 (m, 1H), 7.24–7.18 (m, 1H), 7.31–7.24 (m, 2H), 7.52–7.43 (m, 3H), 11.56 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ169.0 (CO), 139.9 (C, Ar), 136.1 (C, Ar), 135.1 (C, Ar), 132.3 (C, Ar), 127.8 (CH, Ar), 127.5 (CH, Ar), 126.4 (CH, Ar), 126.0 (C, Ar), 124.1 (CH, Ar), 121.4 (CH, Ar), 118.8 (CH, Ar), 118.2 (CH, Ar), 111.3 (CH, Ar), 109.2 (C, Ar), 60.9 (C), 38.0 (CH
2), 37.9 (CH
2), 26.2 (CH
3), 21.0 (CH
2); ESI-LRMS
m/
z: 303 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 303.1492, found: 303.1487. The characterization data is in accordance with that reported in [
43].
10-Fluoro-11c-methyl-5,6,7,11c-tetrahydro-1H-indolizino[7,8-b]indol-3(2H)-one (
SF5d): pale yellow solid (95.1 mg, yield 74%), mp 104–105 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.52 (s, 3H), 1.99–1.87 (m, 1H), 2.28–2.16 (m, 1H), 2.64–2.51 (m, 2H), 2.83–2.67 (m, 2H), 3.16–3.01 (m, 1H), 4.28–4.15 (m, 1H), 6.93–6.82 (m, 1H), 7.34–7.21 (m, 2H), 11.00 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 171.5 (CO), 156.7 (d,
JC–F = 231.1 Hz, CF, Ar), 132.9 (C, Ar), 132.5 (C, Ar), 124.0 (d,
JC–F = 10.1 Hz, C, Ar), 115.7 (d,
JC–F = 4.5 Hz, C, Ar), 111.9 (d,
JC–F = 9.9 Hz, CH, Ar), 108.3 (d,
JC–F = 25.8 Hz, CH, Ar), 102.9 (d,
JC–F = 23.4 Hz, CH, Ar), 59.0 (C), 33.1 (CH
2), 32.9 (CH
2), 30.1 (CH
2), 24.8 (CH
3), 22.9 (CH
2); ESI-LRMS
m/
z: 259 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 259.1241, found: 259.1238. The characterization data is in accordance with that reported in [
43].
11c-Benzyl-5,6,7,11c-tetrahydro-1H-indolizino[7,8-b]indol-3(2H)-one (
SF5e): pale yellow oil (69.4 mg, yield 44%).
1H-NMR (500 MHz, CDCl
3) δ 1.78–1.65 (m, 1H), 2.22–2.09 (m, 2H), 2.72–2.60 (m, 2H), 2.97–2.85 (m, 1H), 3.08–2.98 (m, 1H), 3.21 (d,
J = 13.9 Hz, 1H), 3.30 (d,
J = 13.9 Hz, 1H), 4.50 (dd,
J = 12.9, 6.4 Hz, 1H), 7.12–7.06 (m, 2H), 7.22–7.13 (m, 2H), 7.26–7.23 (m, 3H), 7.35 (d,
J = 8.0 Hz, 1H), 7.52 (d,
J = 7.6 Hz, 1H), 8.33 (s, 1H);
13C-NMR (125 MHz, CDCl
3) δ 174.3 (CO), 136.7 (C, Ar), 136.2 (C, Ar), 130.6 (C, Ar), 130.2 (2 × CH, Ar), 128.5 (2 × CH, Ar), 127.0 (CH, Ar), 124.4 (C, Ar), 121.8 (CH, Ar), 119.9 (CH, Ar), 118.5 (CH, Ar), 115.9 (C, Ar), 111.3 (CH, Ar), 63.8 (C), 44.6 (CH
2), 34.3 (CH
2), 31.6 (CH
2), 31.0 (CH
2), 23.1 (CH
2); ESI-LRMS
m/
z: 317 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 317.1648, found: 317.1651. The characterization data is in accordance with that reported in [
43].
12c-Methyl-1,2,3,6,7,12c-hexahydroindolo[3,2-a]quinolizin-4(8H)-one (
SF6a): white solid (86.8 mg, yield 68%), mp 132–133 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.63 (s, 3H), 1.77–1.65 (m, 2H), 2.01–1.85 (m, 1H), 2.32–2.19 (m, 1H), 2.43–2.32 (m, 1H), 2.78–2.60 (m, 3H), 3.01–2.89 (m, 1H), 4.92–4.80 (m, 1H), 6.99–6.90 (m, 1H), 7.07–6.99 (m, 1H), 7.29 (d,
J = 8.0 Hz, 1H), 7.51 (d,
J = 7.8 Hz, 1H), 10.91 (s, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 168.1 (CO), 136.1 (C, Ar), 131.9 (C, Ar), 124.0 (C, Ar), 120.3 (CH, Ar), 118.6 (CH, Ar), 118.5 (CH, Ar), 115.7 (C, Ar), 111.1 (CH, Ar), 57.0 (C), 35.6 (CH
2), 34.7 (CH
2), 31.9 (CH
2), 25.1 (CH
3), 23.3 (CH
2), 16.5 (CH
2); ESI-LRMS
m/
z: 255 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 255.1492, found: 255.1488. The characterization data is in accordance with that reported in [
43].
12c-Benzyl-1,2,3,6,7,12c-hexahydroindolo[3,2-a]quinolizin-4(8H)-one (
SF6b): pale yellow oil (57.9 mg, yield 35%).
1H-NMR (400 MHz, DMSO-
d6) δ 1.52–1.41 (m, 1H), 1.68–1.54 (m, 1H), 1.90–1.78 (m, 1H), 2.24–2.05 (m, 2H), 2.61–2.53 (m, 1H), 2.81–2.64 (m, 3H), 3.24 (d,
J = 13.6 Hz, 1H), 3.41 (d,
J = 13.5 Hz, 1H), 4.85–4.74 (m, 1H), 6.94–6.86 (m, 1H), 7.06–6.96 (m, 3H), 7.23–7.14 (m, 3H), 7.29 (d,
J = 8.0 Hz, 1H), 7.33 (d,
J = 7.9 Hz, 1H), 10.95 (s, 1H);
13C-NMR (150 MHz, DMSO-
d6) δ 169.2 (CO), 137.8 (C, Ar), 136.0 (C, Ar), 133.0 (C, Ar), 130.4 (2 × CH, Ar), 127.9 (2 × CH, Ar), 126.4 (CH, Ar), 124.3 (C, Ar), 120.2 (CH, Ar), 119.0 (CH, Ar), 118.5 (CH, Ar), 114.2 (C, Ar), 111.1 (CH, Ar), 60.9 (C), 44.3 (CH
2), 35.0 (CH
2), 33.9 (CH
2), 31.7 (CH
2), 23.0 (CH
2), 16.3 (CH
2); ESI-LRMS
m/
z: 331 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 331.1805, found: 331.1812. The characterization data is in accordance with that reported in [
43].
13b-Methyl-6,7-dihydro-5H-benzo[1,2]indolizino[7,8-b]indol-9(13bH)-one (
SF7a): pale yellow solid (116.4 mg, yield 81%), mp 235–236 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.90 (s, 3H), 2.80–2.70 (m, 1H), 2.91–2.80 (m, 1H), 3.45–3.37 (m, 1H), 4.56–4.46 (m, 1H), 7.12–7.03 (m, 2H), 7.34–7.26 (m, 1H), 7.52–7.44 (m, 1H), 7.69–7.64 (m, 1H), 7.71 (d,
J = 7.5 Hz, 1H), 8.12–8.03 (m, 1H), 8.27 (d,
J = 7.8 Hz, 1H), 11.10 (s, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 167.3 (CO), 151.4 (C, Ar), 135.9 (C, Ar), 132.3 (C, Ar), 132.0 (CH, Ar), 130.2 (C, Ar), 128.1 (CH, Ar), 124.3 (C, Ar), 123.2 (CH, Ar), 123.1 (CH, Ar), 120.7 (CH, Ar), 119.0 (CH, Ar), 119.0 (CH, Ar), 111.6 (C, Ar), 111.3 (CH, Ar), 63.6 (C), 34.6 (CH
2), 25.7 (CH
3), 23.4 (CH
2); ESI-LRMS
m/
z: 289 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 289.1335, found: 289.1330. The characterization data is in accordance with that reported in [
43].
13b-Benzyl-6,7-dihydro-5H-benzo[1,2]indolizino[7,8-b]indol-9(13bH)-one (
SF7b): white solid (121.1 mg, yield 66%), mp 281–282 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 2.91–2.72 (m, 2H), 3.49–3.38 (m, 1H), 3.57 (d,
J = 13.8 Hz, 1H), 3.85 (d,
J = 13.9 Hz, 1H), 4.53 (dd,
J = 13.0, 5.6 Hz, 1H), 6.95–6.87 (m, 2H), 7.08–6.98 (m, 3H), 7.20–7.08 (m, 2H), 7.39–7.30 (m, 2H), 7.46 (d,
J = 7.4 Hz, 1H), 7.66–7.58 (m, 1H), 8.32 (d,
J = 7.8 Hz, 1H), 8.43 (d,
J = 7.8 Hz, 1H), 11.14 (s, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 167.7 (CO), 148.8 (C, Ar), 135.9 (C, Ar), 135.7 (C, Ar), 132.8 (C, Ar), 131.4 (CH, Ar), 131.0 (C, Ar), 130.0 (2 × CH, Ar), 127.8 (CH, Ar), 127.3 (2 × CH, Ar), 126.2 (CH, Ar), 124.4 (C, Ar), 123.9 (CH, Ar), 122.6 (CH, Ar), 120.7 (CH, Ar), 119.4 (CH, Ar), 119.1 (CH, Ar), 111.3 (CH, Ar), 111.1 (C, Ar), 67.1 (C), 42.7 (CH
2), 34.8 (CH
2), 23.4 (CH
2); ESI-LRMS
m/
z: 365 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 365.1648, found: 365.1649. The characterization data is in accordance with that reported in [
43].
13b-Pentyl-6,7-dihydro-5H-benzo[1,2]indolizino[7,8-b]indol-9(13bH)-one (
SF7c): pale yellow oil (68.7 mg, yield 40%).
1H-NMR (400 MHz, CDCl
3) δ 0.77 (t,
J = 6.7 Hz, 3H), 0.89–0.83 (m, 1H), 1.20–1.02 (m, 5H), 2.24–2.10 (m, 1H), 2.76–2.58 (m, 2H), 3.13–2.98 (m, 1H), 3.38–3.24 (m, 1H), 4.77 (dd,
J = 13.1, 6.1 Hz, 1H), 7.23–7.12 (m, 2H), 7.31 (d,
J = 7.8 Hz, 1H), 7.45–7.38 (m, 1H), 7.62–7.54 (m, 1H), 7.85 (d,
J = 7.5 Hz, 1H), 7.99 (d,
J = 7.7 Hz, 1H), 8.06 (d,
J = 7.7 Hz, 1H), 8.23 (s, 1H);
13C-NMR (150 MHz, CDCl
3) δ 169.4 (CO), 149.6 (C, Ar), 136.1 (C, Ar), 132.0 (CH, Ar), 131.9 (C, Ar), 131.8 (C, Ar), 128.1 (CH, Ar), 124.9 (C, Ar), 123.9 (CH, Ar), 122.9 (CH, Ar), 121.7 (CH, Ar), 120.0 (CH, Ar), 119.4 (CH, Ar), 113.5 (C, Ar), 111.4 (CH, Ar), 67.4 (C), 38.2 (CH
2), 35.3 (CH
2), 31.8 (CH
2), 24.1 (CH
2), 23.1 (CH
2), 22.5 (CH
2), 14.1 (CH
3); ESI-LRMS
m/
z: 345 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 345.1961, found: 345.1966. The characterization data is in accordance with that reported in [
43].
14c-Methyl-8,9,10,14c-tetrahydroindolo[3′,2’:3,4]pyrido[2,1-a]isoquinolin-6(5H)-one (
SF8a): yellow solid (129.8 mg, yield 86%), mp 250–251 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.89 (s, 3H), 2.72–2.60 (m, 1H), 2.84–2.73 (m, 1H), 3.02–2.89 (m, 1H), 3.62 (d,
J = 19.4 Hz, 1H), 4.10 (d,
J = 19.2 Hz, 1H), 4.95–4.86 (m, 1H), 7.15–7.04 (m, 3H), 7.30–7.19 (m, 2H), 7.44–7.38 (m, 2H), 7.59 (d,
J = 7.6 Hz, 1H), 11.26 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 169.3 (CO), 141.4 (C, Ar), 136.0 (C, Ar), 134.5 (C, Ar), 132.4 (C, Ar), 127.7 (CH, Ar), 127.1 (CH, Ar), 126.2 (C, Ar), 126.1 (CH, Ar), 124.7 (CH, Ar), 120.5 (CH, Ar), 120.2 (CH, Ar), 119.2 (CH, Ar), 111.5 (CH, Ar), 111.2 (C, Ar), 61.5 (C), 38.2 (CH
2), 36.9 (CH
2), 25.7 (CH
3), 23.3 (CH
2); ESI-LRMS
m/
z: 303 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 303.1492, found: 303.1486. The characterization data is in accordance with that reported in [
43].
14c-Benzyl-8,9,10,14c-tetrahydroindolo[3’,2’:3,4]pyrido[2,1-a]isoquinolin-6(5H)-one (
SF8b): pale yellow oil (66.5 mg, yield 35%).
1H-NMR (500 MHz, CDCl
3) δ 1.84–1.73 (m, 1H), 2.48–2.38 (m, 1H), 2.67–2.56 (m, 1H), 3.40 (d,
J = 13.8 Hz, 1H), 3.96–3.76 (m, 3H), 4.79 (dd,
J = 12.7, 4.3 Hz, 1H), 6.80–6.71 (m, 2H), 7.08–7.00 (m, 2H), 7.16–7.08 (m, 2H), 7.28–7.20 (m, 2H), 7.33–7.28 (m, 2H), 7.50–7.42 (m, 1H), 7.66 (d,
J = 7.9 Hz, 1H), 7.96–7.88 (m, 1H), 8.36 (s, 1H);
13C-NMR (125 MHz, CDCl
3) δ 170.6 (CO), 141.0 (C, Ar), 136.4 (C, Ar), 136.1 (C, Ar), 135.7 (C, Ar), 132.3 (C, Ar), 130.4 (2 × CH, Ar), 128.2 (2 × CH, Ar), 128.0 (CH, Ar), 127.7 (CH, Ar), 127.3 (C, Ar), 126.9 (CH, Ar), 126.8 (CH, Ar), 126.0 (CH, Ar), 121.6 (CH, Ar), 121.4 (CH, Ar), 120.5 (CH, Ar), 111.7 (CH, Ar), 109.9 (C, Ar), 66.5 (C), 45.0 (CH
2), 39.1 (CH
2), 38.7 (CH
2), 23.3 (CH
2); ESI-LRMS
m/
z: 379 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 379.1805, found: 379.1815. The characterization data is in accordance with that reported in [
43].
12b-Methyl-1,5,6,12b-tetrahydropyrrolo[2’,1’:3,4]pyrazino[1,2-a]indol-3(2H)-one (
SF9a): white solid (107.8 mg, yield 90%), mp 121–122 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.59 (s, 3H), 2.33–2.17 (m, 2H), 2.47–2.36 (m, 1H), 2.65–2.53 (m, 1H), 3.49–3.38 (m, 1H), 3.81–3.68 (m, 1H), 4.34–4.22 (m, 2H), 6.34 (s, 1H), 7.08–7.01 (m, 1H), 7.15–7.09 (m, 1H), 7.38 (d,
J = 8.1 Hz, 1H), 7.51 (d,
J = 7.7 Hz, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 172.1 (CO), 142.1 (C, Ar), 135.2 (C, Ar), 127.6 (C, Ar), 120.7 (CH, Ar), 119.9 (CH, Ar), 119.8 (CH, Ar), 109.6 (CH, Ar), 95.5 (CH, Ar), 58.6 (C), 40.9 (CH
2), 34.3 (CH
2), 33.6 (CH
2), 29.7 (CH
2), 27.2 (CH
3); ESI-LRMS
m/
z: 241 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 241.1335, found: 241.1330. The characterization data is in accordance with that reported in [
43].
11,12b-Dimethyl-1,5,6,12b-tetrahydropyrrolo[2’,1’:3,4]pyrazino[1,2-a]indol-3(2H)-one (
SF9b): pale yellow oil (111.3 mg, yield 88%).
1H-NMR (300 MHz, CDCl
3) δ 1.65 (s, 3H), 2.49–2.37 (m, 3H), 2.54 (s, 3H), 2.73–2.57 (m, 1H), 3.47–3.32 (m, 1H), 3.96–3.81 (m, 1H), 4.20 (dd,
J = 11.7, 4.7 Hz, 1H), 4.52 (dd,
J = 13.6, 5.1 Hz, 1H), 6.30 (s, 1H), 6.98–6.89 (m, 1H), 7.15–7.09 (m, 2H);
13C-NMR (100 MHz, CDCl
3) δ 173.2 (CO), 141.1 (C, Ar), 135.4 (C, Ar), 130.0 (C, Ar), 127.9 (C, Ar), 121.8 (CH, Ar), 120.7 (CH, Ar), 106.8 (CH, Ar), 94.8 (CH, Ar), 59.5 (C), 41.3 (CH
2), 35.0 (CH
2), 34.3 (CH
2), 30.4 (CH
2), 27.8 (CH
3), 18.8 (CH
3); ESI-LRMS
m/
z: 255 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 255.1492, found: 255.1488. The characterization data is in accordance with that reported in [
45].
12b-Methyl-3-oxo-1,2,3,5,6,12b-hexahydropyrrolo[2’,1’:3,4]pyrazino[1,2-a]indole-11-carbonitrile (
SF9c): yellow oil (104.3 mg, yield 79%).
1H-NMR (300 MHz, CDCl
3) δ 1.66 (s, 3H), 2.54–2.32 (m, 3H), 2.73–2.59 (m, 1H), 3.49–3.33 (m, 1H), 4.02–3.88 (m, 1H), 4.26 (dd,
J = 11.7, 4.8 Hz, 1H), 4.56 (dd,
J = 13.7, 5.2 Hz, 1H), 6.52 (s, 1H), 7.26–7.19 (m, 1H), 7.52–7.44 (m, 2H);
13C-NMR (125 MHz, CDCl
3) δ 173.1 (CO), 144.6 (C, Ar), 135.4 (C, Ar), 129.7 (C, Ar), 125.7 (CH, Ar), 121.2 (CH, Ar), 118.7 (CN), 113.9 (CH, Ar), 102.8 (C, Ar), 95.6 (CH, Ar), 59.3 (C), 41.5 (CH
2), 34.8 (CH
2), 33.9 (CH
2), 30.2 (CH
2), 27.6 (CH
3); ESI-LRMS
m/
z: 266 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 266.1288, found: 266.1282. The characterization data is in accordance with that reported in [
45].
9-Fluoro-12b-methyl-1,5,6,12b-tetrahydropyrrolo[2’,1’:3,4]pyrazino[1,2-a]indol-3(2H)-one (SF9d): pale yellow solid (43.6 mg, yield 68%), mp 71–72 °C. 1H-NMR (500 MHz, DMSO-d6) δ 1.58 (s, 3H), 2.30–2.18 (m, 2H), 2.44–2.36 (m, 1H), 2.63–2.52 (m, 1H), 3.49–3.38 (m, 1H), 3.77–3.67 (m, 1H), 4.32–4.22 (m, 2H), 6.36 (s, 1H), 6.94–6.86 (m, 1H), 7.26 (dd, J = 10.2, 2.2 Hz, 1H), 7.50 (dd, J = 8.6, 5.4 Hz, 1H); 13C-NMR (125 MHz, DMSO-d6) δ 172.1 (CO), 158.5 (d, JC–F = 234.3 Hz, CF, Ar), 142.9 (d, JC–F = 3.7 Hz, C, Ar), 135.2 (d, JC–F = 12.5 Hz, C, Ar), 124.2 (C, Ar), 120.9 (d, JC–F = 10.0 Hz, CH, Ar), 108.1 (d, JC–F = 24.3 Hz, CH, Ar), 96.3 (d, JC–F = 26.3 Hz, CH, Ar), 95.6 (CH, Ar), 58.6 (C), 41.1 (CH2), 34.3 (CH2), 33.5 (CH2), 29.6 (CH2), 27.3 (CH3); ESI-LRMS m/z: 259 [M + H]+; ESI-HRMS m/z calcd for M + H+ 259.1241, found: 259.1238.
10-Chloro-12b-methyl-1,5,6,12b-tetrahydropyrrolo[2’,1’:3,4]pyrazino[1,2-a]indol-3(2H)-one (
SF9e): pale yellow oil (87.5 mg, yield 64%).
1H-NMR (300 MHz, CDCl
3) δ 1.63 (s, 3H), 2.52–2.32 (m, 3H), 2.71–2.56 (m, 1H), 3.45–3.32 (m, 1H), 3.94–3.81 (m, 1H), 4.17 (dd,
J = 11.6, 4.6 Hz, 1H), 4.52 (dd,
J = 13.7, 5.0 Hz, 1H), 6.23 (s, 1H), 7.20–7.11 (m, 2H), 7.52 (s, 1H);
13C-NMR (100 MHz, CDCl
3) δ 173.1 (CO), 143.0 (C, Ar), 134.1 (C, Ar), 129.1 (C, Ar), 126.1 (C, Ar), 121.8 (CH, Ar), 119.9 (CH, Ar), 110.1 (CH, Ar), 96.0 (CH, Ar), 59.4 (C), 41.3 (CH
2), 34.8 (CH
2), 34.0 (CH
2), 30.2 (CH
2), 27.7 (CH
3); ESI-LRMS
m/
z: 277 ([M + H]
+, Cl
37), 275 ([M + H]
+, Cl
35); ESI-HRMS
m/
z calcd for M + H
+ 275.0946, found: 275.0943. The characterization data is in accordance with that reported in [
45].
13b-Methyl-2,3,6,7-tetrahydro-1H-pyrido[2’,1’:3,4]pyrazino[1,2-a]indol-4(13bH)-one (
SF10a): white solid (73.5 mg, yield 58%), mp 113–114 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.67 (s, 3H), 1.78–1.69 (m, 1H), 1.98–1.88 (m, 1H), 2.07–1.99 (m, 1H), 2.42–2.26 (m, 3H), 3.40–3.25 (m, 1H), 3.81–3.70 (m, 1H), 4.33–4.24 (m, 1H), 4.95–4.85 (m, 1H), 6.34 (s, 1H), 7.06–7.01 (m, 1H), 7.14–7.07 (m, 1H), 7.37 (d,
J = 8.1 Hz, 1H), 7.50 (d,
J = 7.8 Hz, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 168.1 (CO), 142.4 (C, Ar), 135.1 (C, Ar), 127.7 (C, Ar), 120.6 (CH, Ar), 119.8 (CH, Ar), 119.7 (CH, Ar), 109.4 (CH, Ar), 95.2 (CH, Ar), 56.7 (C), 41.2 (CH
2), 36.6 (CH
2), 34.5 (CH
2), 31.7 (CH
2), 28.2 (CH
3), 16.9 (CH
2); ESI-LRMS
m/
z: 255 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 255.1492, found: 255.1488. The characterization data is in accordance with that reported in [
43].
12,13b-Dimethyl-2,3,6,7-tetrahydro-1H-pyrido[2’,1’:3,4]pyrazino[1,2-a]indol-4(13bH)-one (
SF10b): colorless oil (95.4 mg, yield 71%).
1H-NMR (300 MHz, CDCl
3) δ 1.73 (s, 3H), 2.06–1.86 (m, 2H), 2.26–2.11 (m, 1H), 2.50–2.32 (m, 2H), 2.65–2.51 (m, 4H), 3.39–3.23 (m, 1H), 4.01–3.87 (m, 1H), 4.22–4.10 (m, 1H), 5.16 (dd,
J = 13.7, 4.5 Hz, 1H), 6.26 (s, 1H), 6.99–6.88 (m, 1H), 7.18–7.07 (m, 2H);
13C-NMR (100 MHz, CDCl
3) δ 169.5 (CO), 141.4 (C, Ar), 135.2 (C, Ar), 129.9 (C, Ar), 127.9 (C, Ar), 121.6 (CH, Ar), 120.5 (CH, Ar), 106.6 (CH, Ar), 94.3 (CH, Ar), 57.4 (C), 41.7 (CH
2), 37.5 (CH
2), 35.3 (CH
2), 32.2 (CH
2), 28.9 (CH
3), 18.8 (CH
3), 17.4 (CH
2); ESI-LRMS
m/
z: 269 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 269.1648, found: 269.1645. The characterization data is in accordance with that reported in [
45].
11-Chloro-13b-methyl-2,3,6,7-tetrahydro-1H-pyrido[2’,1’:3,4]pyrazino[1,2-a]indol-4(13bH)-one (
SF10c): pale yellow oil (75.5 mg, yield 52%).
1H-NMR (300 MHz, CDCl
3) δ 1.70 (s, 3H), 2.20–1.83 (m, 3H), 2.63–2.27 (m, 3H), 3.37–3.22 (m, 1H), 3.98–3.86 (m, 1H), 4.14 (dd,
J = 11.6, 4.2 Hz, 1H), 5.16 (dd,
J = 13.8, 4.2 Hz, 1H), 6.18 (s, 1H), 7.22–7.09 (m, 2H), 7.55–7.48 (m, 1H);
13C-NMR (100 MHz, CDCl
3) δ 169.4 (CO), 143.4 (C, Ar), 134.0 (C, Ar), 129.1 (C, Ar), 126.0 (C, Ar), 121.6 (CH, Ar), 119.8 (CH, Ar), 110.0 (CH, Ar), 95.5 (CH, Ar), 57.3 (C), 41.7 (CH
2), 37.4 (CH
2), 35.1 (CH
2), 32.1 (CH
2), 28.7 (CH
3), 17.3 (CH
2); ESI-LRMS
m/
z: 291 ([M + H]
+, Cl
37), 289 ([M + H]
+, Cl
35); ESI-HRMS
m/
z calcd for M + H
+ 289.1102, found: 289.1099. The characterization data is in accordance with that reported in [
45].
13b-Methyl-6,7-dihydroisoindolo[1’,2’:3,4]pyrazino[1,2-a]indol-9(13bH)-one (
SF11a): pale yellow solid (95.6 mg, yield 66%), mp 149–150 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.91 (s, 3H), 3.90–3.75 (m, 2H), 4.45–4.31 (m, 1H), 4.68–4.57 (m, 1H), 6.84 (s, 1H), 7.08–7.00 (m, 1H), 7.17–7.09 (m, 1H), 7.38 (d,
J = 8.1 Hz, 1H), 7.59–7.48 (m, 2H), 7.80–7.69 (m, 2H), 8.24 (d,
J = 7.6 Hz, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 166.6 (CO), 150.3 (C, Ar), 137.8 (C, Ar), 135.2 (C, Ar), 132.8 (CH, Ar), 129.9 (C, Ar), 128.7 (CH, Ar), 127.3 (C, Ar), 123.1 (2 × CH, Ar), 121.2 (CH, Ar), 120.0 (2 × CH, Ar), 109.9 (CH, Ar), 97.8 (CH, Ar), 61.4 (C), 41.1 (CH
2), 34.2 (CH
2), 28.2 (CH
3); ESI-LRMS
m/
z: 289 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 289.1335, found: 289.1332. The characterization data is in accordance with that reported in [
43].
15b-Methyl-8,9-dihydro-5H-indolo[2’,1’:3,4]pyrazino[2,1-a]isoquinolin-6(15bH)-one (
SF12a): yellow solid (77.6 mg, yield 51%), mp 235–236 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.89 (s, 3H), 3.20–3.08 (m, 1H), 3.68 (d,
J = 19.5 Hz, 1H), 3.81–3.72 (m, 1H), 4.10 (d,
J = 19.4 Hz, 1H), 4.40–4.31 (m, 1H), 4.99–4.90 (m, 1H), 6.83 (s, 1H), 7.15–7.08 (m, 1H), 7.23–7.15 (m, 2H), 7.33–7.25 (m, 2H), 7.44–7.38 (m, 2H), 7.67 (d,
J = 7.7 Hz, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 168.3 (CO), 138.6 (C, Ar), 136.9 (C, Ar), 136.5 (C, Ar), 132.1 (C, Ar), 128.0 (CH, Ar), 127.6 (CH, Ar), 127.2 (C, Ar), 126.5 (CH, Ar), 124.2 (CH, Ar), 121.4 (CH, Ar), 120.3 (CH, Ar), 119.9 (CH, Ar), 109.7 (CH, Ar), 101.4 (CH, Ar), 61.8 (C), 42.8 (CH
2), 37.7 (CH
2), 37.4 (CH
2), 28.6 (CH
3); ESI-LRMS
m/
z: 303 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 303.1492, found: 303.1487. The characterization data is in accordance with that reported in [
43].
13b-Methyl-5,6,7,13b-tetrahydro-1H-pyrrolo[2’,1’:3,4][1,4]diazepino[1,2-a]indol-3(2H)-one (
SF13a): colorless oil (91.6 mg, yield 72%).
1H-NMR (400 MHz, CDCl
3) δ 1.72 (s, 3H), 2.14–1.88 (m, 3H), 2.53–2.42 (m, 2H), 2.80–2.70 (m, 1H), 3.17–3.04 (m, 1H), 4.20 (ddd,
J = 14.8, 9.1, 2.5 Hz, 1H), 4.56–4.33 (m, 2H), 6.45 (s, 1H), 7.13–7.06 (m, 1H), 7.24–7.19 (m, 1H), 7.30 (d,
J = 8.3 Hz, 1H), 7.57 (d,
J = 7.8 Hz, 1H);
13C-NMR (100 MHz, CDCl
3) δ 174.2 (CO), 142.5 (C, Ar), 137.7 (C, Ar), 126.9 (C, Ar), 122.0 (CH, Ar), 120.7 (CH, Ar), 119.9 (CH, Ar), 109.0 (CH, Ar), 99.6 (CH, Ar), 62.4 (C), 43.4 (CH
2), 38.5 (CH
2), 35.0 (CH
2), 30.5 (CH
2), 28.0 (CH
2), 26.4 (CH
3); ESI-LRMS
m/
z: 255 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 255.1492, found: 255.1489. The characterization data is in accordance with that reported in [
45].
11-Bromo-13b-methyl-5,6,7,13b-tetrahydro-1H-pyrrolo[2’,1’:3,4][1,4]diazepino[1,2-a]indol-3(2H)-one (
SF13b): pale yellow oil (89.3 mg, yield 54%).
1H-NMR (300 MHz, CDCl
3) δ 1.71 (s, 3H), 2.15–1.91 (m, 3H), 2.51–2.40 (m, 2H), 2.77–2.66 (m, 1H), 3.16–3.03 (m, 1H), 4.18 (ddd,
J = 14.8, 8.8, 2.7 Hz, 1H), 4.46–4.32 (m, 2H), 6.37 (s, 1H), 7.14 (d,
J = 8.8 Hz, 1H), 7.30–7.26 (m, 1H), 7.66 (d,
J = 1.9 Hz, 1H);
13C-NMR (100 MHz, CDCl
3) δ 174.1 (CO), 143.7 (C, Ar), 136.4 (C, Ar), 128.5 (C, Ar), 124.8 (CH, Ar), 123.1 (CH, Ar), 112.9 (C, Ar), 110.6 (CH, Ar), 99.1 (CH, Ar), 62.3 (C), 43.7 (CH
2), 38.5 (CH
2), 34.9 (CH
2), 30.4 (CH
2), 27.9 (CH
2), 26.3 (CH
3); ESI-LRMS
m/
z: 335 ([M + H]
+, Br
81), 333 ([M + H]
+, Br
79); ESI-HRMS
m/
z calcd for M + H
+ 333.0597, found: 333.0595. The characterization data is in accordance with that reported in [
45].
12,13b-Dimethyl-5,6,7,13b-tetrahydro-1H-pyrrolo[2’,1’:3,4][1,4]diazepino[1,2-a]indol-3(2H)-one (
SF13c): colorless oil (107.1 mg, yield 80%).
1H-NMR (300 MHz, CDCl
3) δ 1.73 (s, 3H), 2.03–1.82 (m, 2H), 2.17–2.07 (m, 1H), 2.54–2.44 (m, 2H), 2.55 (s, 3H), 2.85–2.70 (m, 1H), 3.17–3.04 (m, 1H), 4.25–4.13 (m, 1H), 4.52–4.33 (m, 2H), 6.46 (s, 1H), 6.95–6.88 (m, 1H), 7.18–7.12 (m, 2H);
13C-NMR (100 MHz, CDCl
3) δ 174.0 (CO), 141.8 (C, Ar), 137.3 (C, Ar), 130.1 (C, Ar), 126.5 (C, Ar), 122.1 (CH, Ar), 120.0 (CH, Ar), 106.6 (CH, Ar), 97.9 (CH, Ar), 62.3 (C), 43.5 (CH
2), 38.4 (CH
2), 34.8 (CH
2), 30.4 (CH
2), 28.0 (CH
2), 26.3 (CH
3), 18.6 (CH
3); ESI-LRMS
m/
z: 269 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 269.1648, found: 269.1644. The characterization data is in accordance with that reported in [
45].
14b-Methyl-1,14b-dihydroindolo[1,2-a]pyrrolo[2,1-c]quinoxalin-3(2H)-one (
SF14a): pale yellow solid (123.5 mg, yield 86%), mp 179–180 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.39 (s, 3H), 2.69–2.45 (m, 3H), 2.95–2.81 (m, 1H), 6.64 (s, 1H), 7.25–7.17 (m, 1H), 7.35–7.26 (m, 2H), 7.46–7.39 (m, 1H), 7.67 (d,
J = 7.7 Hz, 1H), 8.13–8.04 (m, 2H), 8.16 (d,
J = 8.1 Hz, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 172.2 (CO), 142.7 (C, Ar), 133.1 (C, Ar), 129.3 (C, Ar), 128.8 (C, Ar), 125.8 (CH, Ar), 125.7 (C, Ar), 124.1 (CH, Ar), 123.0 (CH, Ar), 122.7 (CH, Ar), 121.4 (CH, Ar), 121.2 (CH, Ar), 117.2 (CH, Ar), 111.8 (CH, Ar), 97.6 (CH, Ar), 59.4 (C), 31.2 (CH
2), 30.0 (CH
2), 25.9 (CH
3); ESI-LRMS
m/
z: 289 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 289.1335, found: 289.1331. The characterization data is in accordance with that reported in [
43].
2-Hexyl-14b-methyl-1,14b-dihydroindolo[1,2-a]pyrrolo[2,1-c]quinoxalin-3(2H)-one (SF14b): pale yellow oil (159.8 mg, yield 86% (dr = 1.5:1)), and the two diastereomers were inseparable by chromatography. 1H-NMR (600 MHz, CDCl3) δ 1.00–0.80 (m, 3.02H), 1.53–1.22 (m, 11.93H), 2.09–1.96 (m, 1.03H), 2.14–2.08 (m, 0.34H), 2.35–2.27 (m, 0.53H), 2.62–2.52 (m, 0.31H), 2.81–2.73 (m, 0.53H), 2.91–2.82 (m, 0.53H), 3.03–2.94 (m, 0.32H), 6.44 (s, 0.51H), 6.47 (s, 0.34H), 7.25–7.15 (m, 1.61H), 7.36–7.27 (m, 1.91H), 7.43–7.37 (m, 0.41H), 7.68–7.61 (m, 0.92H), 7.86 (dd, J = 7.9, 1.3 Hz, 0.34H), 8.06–7.95 (m, 1.91H), 8.28 (dd, J = 8.1, 1.4 Hz, 0.53H); 13C-NMR (150 MHz, CDCl3) δ 175.3 (CO), 174.1 (CO), 143.4 (C, Ar), 141.9 (C, Ar), 134.4 (C, Ar), 133.6 (C, Ar), 130.6 (C, Ar), 129.8 (C, Ar), 129.5 (C, Ar), 129.4 (C, Ar), 126.4 (CH, Ar), 126.2 (C, Ar), 125.6 (CH, Ar), 125.4 (CH, Ar), 124.3 (2 × CH, Ar), 123.1 (2 × CH, Ar), 122.8 (CH, Ar), 121.6 (CH, Ar), 121.3 (2 × CH, Ar), 117.2 (CH, Ar), 117.1 (CH, Ar), 111.9 (CH, Ar), 111.7 (CH, Ar), 97.4 (CH, Ar), 97.1 (CH, Ar), 58.3 (C), 41.9 (CH), 41.5 (CH), 39.5 (CH2), 37.7 (CH2), 32.1 (CH2), 31.9 (CH2), 31.8 (CH2), 30.9 (CH2), 29.3 (CH2), 29.3 (CH2), 28.9 (CH3), 27.6 (CH2), 27.2 (CH2), 26.2 (CH3), 22.8 (CH2), 22.7 (CH2), 14.2 (2 × CH3); ESI-LRMS m/z: 373 [M + H]+; ESI-HRMS m/z calcd for M + H+ 373.2274, found: 373.2273.
15b-Methyl-2,3-dihydro-1H-indolo[1,2-a]pyrido[2,1-c]quinoxalin-4(15bH)-one (
SF15): pale yellow oil (89.4 mg, yield 59%).
1H-NMR (400 MHz, DMSO-
d6) δ 1.29 (s, 3H), 1.97–1.75 (m, 2H), 2.42–2.28 (m, 2H), 2.71–2.56 (m, 2H), 6.60 (s, 1H), 7.23–7.17 (m, 1H), 7.33–7.24 (m, 2H), 7.47–7.40 (m, 1H), 7.71–7.64 (m, 2H), 8.08–8.01 (m, 2H);
13C-NMR (100 MHz, DMSO-
d6) δ 168.2 (CO), 142.5 (C, Ar), 133.2 (C, Ar), 130.5 (C, Ar), 129.1 (C, Ar), 128.4 (CH, Ar), 127.8 (C, Ar), 126.6 (CH, Ar), 123.4 (CH, Ar), 122.8 (CH, Ar), 121.2 (2 × CH, Ar), 116.9 (CH, Ar), 111.5 (CH, Ar), 96.8 (CH, Ar), 57.3 (C), 33.3 (CH
2), 32.9 (CH
2), 27.7 (CH
3), 17.1 (CH
2); ESI-LRMS
m/
z: 303 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 303.1492, found: 303.1486. The characterization data is in accordance with that reported in [
43].
15b-Mmethylindolo[1,2-a]isoindolo[1,2-c]quinoxalin-11(15bH)-one (
SF16): white solid (100.5 mg, yield 60%), mp 149–150 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.65 (s, 3H), 6.77 (s, 1H), 7.24–7.18 (m, 1H), 7.35–7.29 (m, 1H), 7.47–7.40 (m, 1H), 7.57–7.51 (m, 1H), 7.65 (d,
J = 7.5 Hz, 1H), 7.76–7.68 (m, 1H), 7.96–7.87 (m, 2H), 8.04 (dd,
J = 7.9, 1.5 Hz, 1H), 8.15 (d,
J = 8.4 Hz, 1H), 8.31–8.26 (m, 1H), 8.35 (d,
J = 7.6 Hz, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 164.1 (CO), 146.4 (C, Ar), 137.1 (C, Ar), 133.9 (C, Ar), 133.5 (CH, Ar), 129.7 (C, Ar), 129.5 (CH, Ar), 129.3 (C, Ar), 128.9 (C, Ar), 126.5 (CH, Ar), 124.6 (C, Ar), 124.4 (CH, Ar), 124.0 (CH, Ar), 123.9 (CH, Ar), 123.7 (CH, Ar), 123.5 (CH, Ar), 121.6 (CH, Ar), 121.3 (CH, Ar), 117.5 (CH, Ar), 111.9 (CH, Ar), 99.3 (CH, Ar), 61.2 (C), 26.3 (CH
3); ESI-LRMS
m/
z: 337 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 337.1335, found: 337.1329. The characterization data is in accordance with that reported in [
43].
11b-Methyl-4,5-dihydro-3H-pyrrolo[3’,2’:3,4]pyrido[2,1-a]isoindol-7(11bH)-one (
SF17): pale yellow solid (77.1 mg, yield 65%), mp 237–238 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.66 (s, 3H), 2.66–2.54 (m, 2H), 3.41–3.27 (m, 1H), 4.48–4.37 (m, 1H), 6.31–6.26 (m, 1H), 6.62–6.55 (m, 1H), 7.49–7.42 (m, 1H), 7.68–7.60 (m, 2H), 7.97 (d,
J = 7.6 Hz, 1H), 10.56 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 166.8 (CO), 152.2 (C, Ar), 131.9 (CH, Ar), 130.0 (C, Ar), 127.8 (CH, Ar), 122.7 (CH, Ar), 122.7 (C, Ar), 122.3 (CH, Ar), 119.9 (C, Ar), 116.8 (CH, Ar), 104.0 (CH, Ar), 62.3 (C), 34.5 (CH
2), 27.7 (CH
3), 22.6 (CH
2); ESI-LRMS
m/
z: 239 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 239.1179, found: 239.1175. The characterization data is in accordance with that reported in [
43].
12b-Methyl-5,6-dihydropyrrolo[2’,1’:3,4]pyrazino[2,1-a]isoindol-8(12bH)-one (
SF18): pale yellow solid (110.9 mg, yield 93%), mp 156–157 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.76 (s, 3H), 3.69–3.60 (m, 1H), 3.78–3.70 (m, 1H), 4.08 (dd,
J = 12.0, 3.6 Hz, 1H), 4.45 (dd,
J = 13.3, 4.2 Hz, 1H), 6.06–5.98 (m, 1H), 6.39–6.31 (m, 1H), 6.67–6.60 (m, 1H), 7.55–7.47 (m, 1H), 7.75–7.65 (m, 2H), 8.06 (d,
J = 7.9 Hz, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 166.8 (CO), 151.1 (C, Ar), 132.6 (CH, Ar), 130.0 (C, Ar), 129.6 (C, Ar), 128.4 (CH, Ar), 122.9 (CH, Ar), 122.8 (CH, Ar), 119.4 (CH, Ar), 107.8 (CH, Ar), 104.2 (CH, Ar), 61.3 (C), 43.8 (CH
2), 34.9 (CH
2), 28.5 (CH
3); ESI-LRMS
m/
z: 239 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 239.1179, found: 239.1175. The characterization data is in accordance with that reported in [
43].
9a-Methyl-4,5,9,9a-tetrahydrothieno[2,3-g]indolizin-7(8H)-one (
SF19): pale yellow solid (65.1 mg, yield 63%), mp 128–129 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.42 (s, 3H), 1.90–1.79 (m, 1H), 2.31–2.15 (m, 2H), 2.61–2.52 (m, 1H), 2.74–2.62 (m, 1H), 2.85–2.76 (m, 1H), 3.09–2.98 (m, 1H), 4.15 (dd,
J = 13.2, 5.6 Hz, 1H), 6.99 (d,
J = 5.2 Hz, 1H), 7.37 (d,
J = 5.1 Hz, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 171.6 (CO), 141.8 (C, Ar), 131.3 (C, Ar), 124.2 (CH, Ar), 124.1 (CH, Ar), 60.4 (C), 33.7 (CH
2), 33.4 (CH
2), 30.1 (CH
2), 25.8 (CH
3), 24.3 (CH
2); ESI-LRMS
m/
z: 208 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 208.0791, found: 208.0788. The characterization data is in accordance with that reported in [
43].
11b-Methyl-4,5-dihydrothieno[3’,2’:3,4]pyrido[2,1-a]isoindol-7(11bH)-one (
SF20): yellow solid (105.6 mg, yield 83%), mp 199–200 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.75 (s, 3H), 2.82–2.71 (m, 1H), 2.93–2.84 (m, 1H), 3.46–3.38 (m, 1H), 4.47 (dd,
J = 13.4, 5.7 Hz, 1H), 7.39 (d,
J = 5.3 Hz, 1H), 7.56–7.48 (m, 2H), 7.74–7.63 (m, 2H), 8.16 (d,
J = 7.6 Hz, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 166.8 (CO), 150.2 (C, Ar), 137.8 (C, Ar), 132.8 (C, Ar), 132.3 (CH, Ar), 130.1 (C, Ar), 128.4 (CH, Ar), 125.3 (CH, Ar), 124.0 (CH, Ar), 123.0 (2 × CH, Ar), 63.4 (C), 34.7 (CH
2), 27.0 (CH
3), 24.8 (CH
2); ESI-LRMS
m/
z: 256 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 256.0791, found: 256.0785. The characterization data is in accordance with that reported in [
43].
9a-Methyl-4,5,9,9a-tetrahydrothieno[3,2-g]indolizin-7(8H)-one (
SF21): colorless oil (93.3 mg, yield 90%).
1H-NMR (500 MHz, DMSO-
d6) δ 1.50 (s, 3H), 2.03–1.92 (m, 1H), 2.32–2.19 (m, 2H), 2.61–2.51 (m, 2H), 2.72–2.63 (m, 1H), 3.07–2.96 (m, 1H), 4.11 (dd,
J = 13.3, 6.2 Hz, 1H), 6.80 (d,
J = 5.1 Hz, 1H), 7.40 (d,
J = 5.0 Hz, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 171.7 (CO), 141.8 (C, Ar), 132.3 (C, Ar), 127.1 (CH, Ar), 123.5 (CH, Ar), 60.4 (C), 35.1 (CH
2), 33.6 (CH
2), 30.2 (CH
2), 27.9 (CH
3), 25.1 (CH
2); ESI-LRMS
m/
z: 208 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 208.0791, found: 208.0788. The characterization data is in accordance with that reported in [
43].
11b-Methyl-4,5-dihydrothieno[2’,3’:3,4]pyrido[2,1-a]isoindol-7(11bH)-one (
SF22): pale yellow solid (108.6 mg, yield 85%), mp 137–138 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.82 (s, 3H), 2.62 (ddd,
J = 16.2, 11.7, 6.5 Hz, 1H), 2.76 (dd,
J = 16.2, 4.5 Hz, 1H), 3.40 (ddd,
J = 13.4, 12.0, 4.7 Hz, 1H), 4.43 (dd,
J = 13.5, 6.2 Hz, 1H), 6.82 (d,
J = 5.1 Hz, 1H), 7.45 (d,
J = 5.1 Hz, 1H), 7.57–7.49 (m, 1H), 7.76–7.67 (m, 2H), 7.97–7.90 (m, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 166.5 (CO), 150.2 (C, Ar), 138.0 (C, Ar), 133.4 (C, Ar), 132.5 (CH, Ar), 130.0 (C, Ar), 128.7 (CH, Ar), 127.1 (CH, Ar), 124.4 (CH, Ar), 123.1 (CH, Ar), 122.4 (CH, Ar), 63.1 (C), 34.3 (CH
2), 28.8 (CH
3), 25.6 (CH
2); ESI-LRMS
m/
z: 256 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 256.0791, found: 256.0789. The characterization data is in accordance with that reported in [
43].
12b-Methyl-8,12b-dihydro-4H-thieno[2’,3’:3,4]pyrido[2,1-a]isoquinolin-7(5H)-one (
SF23): pale yellow solid (63.4 mg, yield 47%), mp 155–156 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.87 (s, 3H), 2.50–2.44 (m, 1H), 2.76–2.68 (m, 1H), 3.10–2.99 (m, 1H), 3.64 (d,
J = 20.3 Hz, 1H), 3.97 (d,
J = 20.2 Hz, 1H), 4.90–4.79 (m, 1H), 6.91 (d,
J = 5.1 Hz, 1H), 7.32–7.22 (m, 3H), 7.55 (d,
J = 5.1 Hz, 1H), 7.77–7.71 (m, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 167.9 (CO), 139.0 (C, Ar), 138.8 (C, Ar), 136.4 (C, Ar), 131.3 (C, Ar), 128.0 (CH, Ar), 127.6 (CH, Ar), 127.4 (CH, Ar), 126.5 (CH, Ar), 124.8 (CH, Ar), 124.5 (CH, Ar), 62.6 (C), 37.0 (CH
2), 36.7 (CH
2), 31.7 (CH
3), 25.2 (CH
2); ESI-LRMS
m/
z: 270 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 270.0947, found: 270.0942. The characterization data is in accordance with that reported in [
43].
8,9-Dimethoxy-10b-methyl-1,5,6,10b-tetrahydropyrrolo[2,1-a]isoquinolin-3(2H)-one (
SF24a): white solid (114.5 mg, yield 88%), mp 53–54 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.45 (s, 3H), 1.93–1.82 (m, 1H), 2.25–2.15 (m, 1H), 2.45–2.37 (m, 1H), 2.59–2.47 (m, 1H), 2.70–2.62 (m, 2H), 3.05–2.96 (m, 1H), 3.71 (s, 3H), 3.75 (s, 3H), 4.08–4.00 (m, 1H), 6.66 (s, 1H), 6.78 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 171.3 (CO), 147.7 (C, Ar), 147.4 (C, Ar), 134.9 (C, Ar), 124.1 (C, Ar), 111.9 (CH, Ar), 108.7 (CH, Ar), 60.3 (C), 55.8 (OCH
3), 55.5 (OCH
3), 34.3 (CH
2), 33.4 (CH
2), 30.2 (CH
2), 27.7 (CH
2), 26.8 (CH
3); ESI-LRMS
m/
z: 262 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 262.1438, found: 262.1434. The characterization data is in accordance with that reported in [
43].
2-Hexyl-8,9-dimethoxy-10b-methyl-1,5,6,10b-tetrahydropyrrolo[2,1-a]isoquinolin-3(2H)-one (SF24b): white solid (81.2 mg, yield 47% (dr = 4:1)), mp 65–67 °C, and the two diastereomers were inseparable by chromatography. 1H-NMR (400 MHz, DMSO-d6) δ 0.85–0.72 (m, 3H), 1.37–0.99 (m, 10H), 1.47–1.35 (m, 3H), 1.73–1.57 (m, 1H), 2.43–2.35 (m, 1H), 2.52–2.44 (m, 1H), 2.70–2.52 (m, 2H), 3.12–2.88 (m, 1H), 3.65–3.59 (m, 3H), 3.68 (s, 3H), 4.00–3.89 (m, 1H), 6.61–6.51 (m, 1H), 6.77–6.69 (m, 1H); 13C-NMR (100 MHz, DMSO-d6) δ 174.9 (CO), 147.5 (C, Ar), 147.4 (C, Ar), 134.7 (C, Ar), 124.4 (C, Ar), 112.0 (CH, Ar), 108.8 (CH, Ar), 59.5 (C, Ar), 55.7 (OCH3), 55.4 (OCH3), 41.2 (CH), 39.5 (CH2), 34.2 (CH2), 31.8 (CH2), 31.2 (CH2), 30.8 (CH3), 28.6 (CH2), 27.0 (CH2), 26.9 (CH2), 22.0 (CH2), 14.0 (CH3); ESI-LRMS m/z: 346 [M + H]+; ESI-HRMS m/z calcd for M + H+ 346.2377, found: 346.2375.
9,10-Dimethoxy-11b-methyl-2,3,6,7-tetrahydro-1H-pyrido[2,1-a]isoquinolin-4(11bH)-one (
SF25): white solid (84.4 mg, yield 61%), mp 75–76 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.55 (s, 3H), 1.64–1.57 (m, 1H), 1.75–1.64 (m, 1H), 1.96–1.81 (m, 1H), 2.37–2.16 (m, 2H), 2.49–2.41 (m, 1H), 2.68–2.53 (m, 2H), 2.87–2.75 (m, 1H), 3.71 (s, 3H), 3.74 (s, 3H), 4.75–4.63 (m, 1H), 6.65 (s, 1H), 6.84 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 167.7 (CO), 147.4 (C, Ar), 147.2 (C, Ar), 135.2 (C, Ar), 125.5 (C, Ar), 111.7 (CH, Ar), 109.3 (CH, Ar), 58.2 (C), 55.8 (OCH
3), 55.4 (OCH
3), 36.7 (CH
2), 34.8 (CH
2), 31.5 (CH
2), 28.5 (CH
2), 27.3 (CH
3), 16.6 (CH
2); ESI-LRMS
m/
z: 276 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 276.1594, found: 276.1589. The characterization data is in accordance with that reported in [
43].
2,3-Dimethoxy-12b-methyl-5,6-dihydroisoindolo[1,2-a]isoquinolin-8(12bH)-one (
SF26): white solid (118.8 mg, yield 77%), mp 187–188 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.82 (s, 3H), 2.83–2.67 (m, 2H), 3.42–3.34 (m, 1H), 3.69 (s, 3H), 3.83 (s, 3H), 4.39–4.31 (m, 1H), 6.69 (s, 1H), 7.39 (s, 1H), 7.53–7.47 (m, 1H), 7.72–7.65 (m, 2H), 8.30 (d,
J = 7.7 Hz, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 166.5 (CO), 151.1 (C, Ar), 147.8 (C, Ar), 147.5 (C, Ar), 132.2 (CH, Ar), 130.8 (C, Ar), 130.3 (C, Ar), 128.3 (CH, Ar), 125.3 (C, Ar), 123.4 (CH, Ar), 122.8 (CH, Ar), 112.2 (CH, Ar), 110.2 (CH, Ar), 63.4 (C), 56.1 (OCH
3), 55.5 (OCH
3), 34.6 (CH
2), 28.7 (CH
2), 28.2 (CH
3); ESI-LRMS
m/
z: 310 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 310.1438, found: 310.1432. The characterization data is in accordance with that reported in [
43].
3a-Methyl-2,3,3a,4-tetrahydropyrrolo[1,2-a]quinazoline-1,5-dione (
SF27a): white solid (91.2 mg, yield 84%), mp 177–178 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.42 (s, 3H), 2.30–2.19 (m, 2H), 2.56–2.49 (m, 1H), 2.78–2.68 (m, 1H), 7.32–7.25 (m, 1H), 7.65–7.57 (m, 1H), 7.91 (dd,
J = 7.7, 1.4 Hz, 1H), 8.07 (d,
J = 8.0 Hz, 1H), 8.94 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 171.9 (CO), 161.2 (CO), 135.7 (C, Ar), 133.2 (CH, Ar), 127.7 (CH, Ar), 124.5 (CH, Ar), 119.9 (CH, Ar), 119.8 (C, Ar), 74.0 (C), 32.4 (CH
2), 29.6 (CH
2), 26.4 (CH
3); ESI-LRMS
m/
z: 217 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 217.0972, found: 217.0969. The characterization data is in accordance with that reported in [
43].
3a,4-Dimethyl-2,3,3a,4-tetrahydropyrrolo[1,2-a]quinazoline-1,5-dione (
SF27b): white solid (97.5 mg, yield 85%), mp 105–107 °C.
1H-NMR (600 MHz, CDCl
3) δ 1.46 (s, 3H), 2.30 (ddd,
J = 12.0, 6.1, 4.0 Hz, 1H), 2.48–2.39 (m, 1H), 2.70–2.63 (m, 2H), 3.07 (s, 3H), 7.27–7.24 (m, 1H), 7.56–7.51 (m, 1H), 8.08 (dd,
J = 7.8, 1.6 Hz, 1H), 8.26 (dd,
J = 8.2, 0.8 Hz, 1H);
13C-NMR (150 MHz, CDCl
3) δ 171.3 (CO), 162.1 (CO), 135.2 (C, Ar), 133.4 (CH, Ar), 128.6 (CH, Ar), 125.0 (CH, Ar), 119.7 (CH, Ar), 119.4 (C, Ar), 78.5 (C), 32.4 (CH
2), 30.3 (CH
2), 27.8 (CH
3), 21.8 (CH
3); ESI-LRMS
m/
z: 231 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 231.1128, found: 231.1127. The characterization data is in accordance with that reported in [
43].
4a-Methyl-3,4,4a,5-tetrahydro-1H-pyrido[1,2-a]quinazoline-1,6(2H)-dione (
SF28a): white solid (70.7 mg, yield 61%), mp 198–199 °C.
1H-NMR (500 MHz, DMSO-
d6) δ 1.35 (s, 3H), 1.90–1.74 (m, 2H), 2.09–2.02 (m, 1H), 2.17–2.09 (m, 1H), 2.48–2.39 (m, 1H), 2.62–2.54 (m, 1H), 7.36–7.28 (m, 1H), 7.60–7.52 (m, 1H), 7.66 (d,
J = 8.0 Hz, 1H), 7.86 (dd,
J = 7.7, 1.4 Hz, 1H), 8.85 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 168.8 (CO), 162.3 (CO), 138.1 (C, Ar), 131.8 (CH, Ar), 126.5 (CH, Ar), 126.2 (CH, Ar), 125.2 (CH, Ar), 123.7 (C, Ar), 71.1 (C), 34.7 (CH
2), 33.1 (CH
2), 28.3 (CH
3), 16.6 (CH
2); ESI-LRMS
m/
z: 231 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 231.1128, found: 231.1126. The characterization data is in accordance with that reported in [
43].
4a,5-Dimethyl-3,4,4a,5-tetrahydro-1H-pyrido[1,2-a]quinazoline-1,6(2H)-dione (
SF28b): white solid (78.3 mg, yield 64%), mp 138–139 °C.
1H-NMR (600 MHz, CDCl
3) δ 1.39 (s, 3H), 1.98–1.86 (m, 2H), 2.17–2.10 (m, 1H), 2.44–2.36 (m, 1H), 2.63–2.55 (m, 1H), 2.74–2.65 (m, 1H), 3.12 (s, 3H), 7.32–7.28 (m, 1H), 7.54–7.49 (m, 1H), 7.62 (dd,
J = 8.1, 0.9 Hz, 1H), 8.02 (dd,
J = 7.8, 1.6 Hz, 1H);
13C-NMR (150 MHz, CDCl
3) δ 169.3 (CO), 163.6 (CO), 137.4 (C, Ar), 132.0 (CH, Ar), 127.8 (CH, Ar), 126.2 (CH, Ar), 126.0 (CH, Ar), 123.9 (C, Ar), 75.5 (C), 34.4 (CH
2), 33.7 (CH
2), 27.5 (CH
3), 24.6 (CH
3), 16.9 (CH
2); ESI-LRMS
m/
z: 245 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 245.1285, found: 245.1284. The characterization data is in accordance with that reported in [
43].
6a-Methyl-6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-dione (
SF29a): white solid (99.3 mg, yield 75%), mp 219–220 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.72 (s, 3H), 7.42–7.36 (m, 1H), 7.70–7.64 (m, 1H), 7.77–7.71 (m, 1H), 7.85–7.78 (m, 1H), 7.89 (d,
J = 7.5 Hz, 1H), 7.95 (d,
J = 7.6 Hz, 1H), 8.04–7.98 (m, 2H), 9.48 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 164.4 (CO), 162.5 (CO), 146.0 (C, Ar), 135.2 (C, Ar), 133.7 (CH, Ar), 133.6 (CH, Ar), 130.1 (CH, Ar), 129.4 (C, Ar), 128.0 (CH, Ar), 125.1 (CH, Ar), 124.0 (CH, Ar), 122.9 (CH, Ar), 121.2 (CH, Ar), 119.8 (C, Ar), 74.2 (C), 27.3 (CH
3); ESI-LRMS
m/
z: 265 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 265.0972, found: 265.0966. The characterization data is in accordance with that reported in [
43].
6,6a-Dimethyl-6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-dione (
SF29b): white solid (97.1 mg, yield 70%), mp 182–184 °C.
1H-NMR (600 MHz, CDCl
3) δ 1.72 (s, 3H), 3.25 (s, 3H), 7.37–7.32 (m, 1H), 7.67–7.63 (m, 2H), 7.76–7.70 (m, 2H), 8.05–7.99 (m, 2H), 8.15 (dd,
J = 8.1, 1.0 Hz, 1H);
13C-NMR (150 MHz, CDCl
3) δ 165.0 (CO), 163.0 (CO), 143.3 (C, Ar), 134.8 (C, Ar), 133.5 (CH, Ar), 132.8 (CH, Ar), 131.4 (C, Ar), 130.5 (CH, Ar), 128.9 (CH, Ar), 125.5 (CH, Ar), 125.2 (CH, Ar), 124.6 (CH, Ar), 121.8 (CH, Ar), 120.6 (C, Ar), 78.1 (C), 29.6 (CH
3), 23.3 (CH
3); ESI-LRMS
m/
z: 279 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 279.1128, found: 279.1127. The characterization data is in accordance with that reported in [
43].
4b-Methyl-4bH-isoquinolino[2,1-a]quinazoline-6,12(5H,13H)-dione (
SF30a): yellow solid (91.5 mg, yield 66%), mp 225–226 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.71 (s, 3H), 3.84 (d,
J = 21.2 Hz, 1H), 4.16 (d,
J = 21.1 Hz, 1H), 7.30–7.23 (m, 1H), 7.45–7.36 (m, 3H), 7.68–7.61 (m, 1H), 7.79–7.71 (m, 1H), 7.82 (d,
J = 7.8 Hz, 1H), 7.93 (dd,
J = 7.7, 1.5 Hz, 1H), 8.89 (s, 1H);
13C-NMR (100 MHz, DMSO-
d6) δ 165.5 (CO), 161.9 (CO), 137.6 (C, Ar), 133.1 (C, Ar), 132.1 (CH, Ar), 128.8 (CH, Ar), 128.6 (C, Ar), 127.5 (CH, Ar), 127.2 (CH, Ar), 126.6 (CH, Ar), 126.2 (CH, Ar), 126.1 (CH, Ar), 125.7 (CH, Ar), 123.3 (C, Ar), 73.9 (C), 35.2 (CH
2), 30.5 (CH
3); ESI-LRMS
m/
z: 279 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 279.1128, found: 279.1122. The characterization data is in accordance with that reported in [
43].
4b,5-Dimethyl-4bH-isoquinolino[2,1-a]quinazoline-6,12(5H,13H)-dione (SF30b): yellow oil (29.1 mg, yield 20%). 1H-NMR (600 MHz, CDCl3) δ 1.86 (s, 3H), 2.77 (s, 3H), 3.91 (s, 2H), 7.28 (d, J = 7.5 Hz, 1H), 7.45–7.39 (m, 2H), 7.49–7.45 (m, 1H), 7.61–7.55 (m, 2H), 7.63 (d, J = 8.0 Hz, 1H), 8.08 (dd, J = 7.9, 1.3 Hz, 1H); 13C-NMR (150 MHz, DMSO-d6) δ 169.2 (CO), 162.1 (CO), 138.6 (C, Ar), 131.9 (CH, Ar), 131.8 (C, Ar), 130.6 (C, Ar), 129.9 (CH, Ar), 128.9 (CH, Ar), 128.0 (CH, Ar), 127.7 (CH, Ar), 127.0 (CH, Ar), 126.9 (CH, Ar), 126.7 (CH, Ar), 125.2 (C, Ar), 76.7 (C), 36.2 (CH2), 29.9 (CH3), 25.7 (CH3); ESI-LRMS m/z: 293 [M + H]+; ESI-HRMS m/z calcd for M + H+ 293.1285, found: 293.1284.
3a-Methyl-2,3,3a,4-tetrahydropyrrolo[2,1-b]quinazolin-1(9H)-one (
SF31): white solid (69.4 mg, yield 69%), mp 141–143 °C.
1H-NMR (600 MHz, CDCl
3) δ 1.54 (s, 3H), 2.15–2.05 (m, 2H), 2.52–2.45 (m, 1H), 2.60–2.52 (m, 1H), 4.17 (d,
J = 16.7 Hz, 1H), 5.02 (d,
J = 16.8 Hz, 1H), 6.58 (dd,
J = 8.0, 0.8 Hz, 1H), 6.81–6.76 (m, 1H), 7.07–6.99 (m, 2H);
13C-NMR (150 MHz, CDCl
3) δ 174.3 (CO), 141.9 (C, Ar), 127.6 (CH, Ar), 127.0 (CH, Ar), 119.3 (CH, Ar), 117.4 (C, Ar), 116.5 (CH, Ar), 71.9 (C), 38.6 (CH
2), 33.0 (CH
2), 29.6 (CH
2), 25.6 (CH
3); ESI-LRMS
m/
z: 203 [M + H]
+; ESI-HRMS
m/
z calcd for M+H
+ 203.1179, found: 203.1178. The characterization data is in accordance with that reported in [
43].
4b-Methyl-4b,5-dihydroisoindolo[1,2-b]quinazolin-12(10H)-one (
SF32): pale yellow solid (41.1 mg, yield 33%), mp 222–223 °C.
1H-NMR (600 MHz, CDCl
3) δ 1.71 (s, 3H), 4.24 (s, 1H), 4.45 (d,
J = 16.9 Hz, 1H), 5.32 (d,
J = 17.0 Hz, 1H), 6.69 (d,
J = 8.3 Hz, 1H), 6.90–6.85 (m, 1H), 7.12–7.08 (m, 1H), 7.14 (d,
J = 7.5 Hz, 1H), 7.56–7.52 (m, 1H), 7.63 (d,
J = 3.9 Hz, 2H), 7.88 (d,
J = 7.6 Hz, 1H);
13C-NMR (150 MHz, CDCl
3) δ 165.8 (CO), 147.8 (C, Ar), 140.2 (C, Ar), 132.3 (CH, Ar), 131.5 (C, Ar), 129.6 (CH, Ar), 127.9 (CH, Ar), 127.2 (CH, Ar), 124.4 (CH, Ar), 120.7 (CH, Ar), 120.5 (CH, Ar), 118.7 (C, Ar), 118.1 (CH, Ar), 71.5 (C), 38.0 (CH
2), 23.9 (CH
3); ESI-LRMS
m/
z: 251 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 251.1179, found: 251.1178. The characterization data is in accordance with that reported in [
43].
3a-Methyl-2,3,3a,4-tetrahydro-1H-benzo[d]pyrrolo[1,2-a]imidazol-1-one (
SF33): colorless oil (45.1 mg, yield 48%).
1H-NMR (600 MHz, CDCl
3) δ 1.51 (s, 3H), 2.44–2.33 (m, 2H), 2.54 (ddd,
J = 16.8, 8.5, 1.6 Hz, 1H), 2.78 (ddd,
J = 16.8, 11.7, 8.5 Hz, 1H), 6.68 (dd,
J = 7.7, 0.7 Hz, 1H), 6.84–6.79 (m, 1H), 6.98–6.93 (m, 1H), 7.43 (dd,
J = 7.6, 1.1 Hz, 1H);
13C-NMR (150 MHz, CDCl
3) δ 173.9 (CO), 142.8 (C, Ar), 128.7 (C, Ar), 125.4 (CH, Ar), 120.3 (CH, Ar), 115.5 (CH, Ar), 110.7 (CH, Ar), 85.7 (C), 37.8 (CH
2), 33.7 (CH
2), 26.3 (CH
3); ESI-LRMS
m/
z: 189 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 189.1022, found: 189.1023. The characterization data is in accordance with that reported in [
34].
12a-Methyl-12,12a-dihydrobenzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one (
SF34): colorless oil (26.1 mg, yield 21%).
1H-NMR (600 MHz, CDCl
3) δ 1.69 (s, 3H), 3.71 (d,
J = 19.0 Hz, 1H), 3.88 (d,
J = 19.0 Hz, 1H), 4.48 (s, 1H), 6.85 (dd,
J = 7.6, 0.5 Hz, 1H), 6.93–6.89 (m, 1H), 7.02–6.97 (m, 1H), 7.24 (d,
J = 7.5 Hz, 1H), 7.33–7.30 (m, 1H), 7.37–7.33 (m, 1H), 7.41 (dd,
J = 7.6, 0.9 Hz, 1H), 8.02–7.98 (m, 1H);
13C-NMR (150 MHz, CDCl
3) δ 165.5 (CO), 139.3 (C, Ar), 139.1 (C, Ar), 131.3 (C, Ar), 129.8 (C, Ar), 128.4 (CH, Ar), 128.0 (CH, Ar), 127.6 (CH, Ar), 125.0 (CH, Ar), 123.2 (CH, Ar), 121.6 (CH, Ar), 116.6 (CH, Ar), 112.0 (CH, Ar), 82.3 (C), 38.7 (CH
2), 29.5 (CH
3); ESI-LRMS
m/
z: 251 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 251.1179, found: 251.1178. The characterization data is in accordance with that reported in [
43].
3a-Methyl-3,3a-dihydro-1H-benzo[d]pyrrolo[2,1-b][1,3]oxazine-1,5(2H)-dione (
SF35a): white solid (98.9 mg, yield 91%), mp 114–115 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.63 (s, 3H), 2.48–2.41 (m, 2H), 2.68–2.59 (m, 1H), 2.82–2.71 (m, 1H), 7.44–7.36 (m, 1H), 7.83–7.76 (m, 1H), 8.08–7.92 (m, 2H);
13C-NMR (125 MHz, DMSO-
d6) δ 171.7 (CO), 161.1 (CO), 136.1 (C, Ar), 135.7 (CH, Ar), 129.9 (CH, Ar), 125.5 (CH, Ar), 120.5 (CH, Ar), 115.7 (C, Ar), 95.4 (C), 31.7 (CH
2), 29.1 (CH
2), 24.0 (CH
3); ESI-LRMS
m/
z: 218 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 218.0812, found: 218.0809. The characterization data is in accordance with that reported in [
43].
2-Hexyl-3a-methyl-3,3a-dihydro-1H-benzo[d]pyrrolo[2,1-b][1,3]oxazine-1,5(2H)-dione (SF35b): colorless oil (75.4 mg, yield 50% (dr = 5.5:1)), and the two diastereomers were inseparable by chromatography. 1H-NMR (600 MHz, DMSO-d6) for the major isomer: δ 0.91–0.84 (m, 3H), 1.42–1.21 (m, 9H), 1.65–1.61 (m, 3H), 1.85–1.78 (m, 1H), 2.13–2.07 (m, 1H), 2.71–2.62 (m, 1H), 2.91–2.83 (m, 1H), 7.41–7.37 (m, 1H), 7.81–7.78 (m, 1H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H), 8.10–8.07 (m, 1H); 13C-NMR (150 MHz, DMSO-d6) for major isomer: δ 173.0 (CO), 161.0 (CO), 136.0 (CH, Ar), 135.8 (CH, Ar), 129.9 (CH, Ar), 125.3 (CH, Ar), 119.8 (CH, Ar), 115.1 (C, Ar), 93.5 (C, Ar), 39.8 (CH), 38.5 (CH2), 31.2 (CH2), 29.6 (CH2), 28.6 (CH2), 26.3 (CH2), 23.5 (CH3), 22.1 (CH2), 14.0 (CH3); ESI-LRMS m/z: 302 [M + H]+; ESI-HRMS m/z calcd for M + H+ 302.1751, found: 302.1750.
4a-Methyl-2,3,4,4a-tetrahydrobenzo[d]pyrido[2,1-b][1,3]oxazine-1,6-dione (
SF36): colorless oil (88.5 mg, yield 77%).
1H-NMR (500 MHz, DMSO-
d6) δ 1.55 (s, 3H), 1.86–1.77 (m, 1H), 1.95–1.86 (m, 1H), 2.31–2.21 (m, 2H), 2.55–2.48 (m, 1H), 2.68–2.58 (m, 1H), 7.46–7.39 (m, 1H), 7.77–7.70 (m, 2H), 7.98–7.93 (m, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 169.0 (CO), 161.8 (CO), 138.6 (C, Ar), 134.1 (CH, Ar), 128.7 (CH, Ar), 126.1 (2 × CH, Ar), 119.9 (C, Ar), 92.7 (C), 34.9 (CH
2), 32.9 (CH
2), 25.9 (CH
3), 16.1 (CH
2); ESI-LRMS
m/
z: 232 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 232.0968, found: 232.0965. The characterization data is in accordance with that reported in ref.43. The characterization data is in accordance with that reported in [
43].
6a-Methyl-5H-benzo[4,5][1,3]oxazino[2,3-a]isoindole-5,11(6aH)-dione (
SF37): pale yellow solid (83.6 mg, yield 63%), mp 138–139 °C.
1H-NMR (400 MHz, DMSO-
d6) δ 1.94 (s, 3H), 7.51–7.45 (m, 1H), 7.79–7.73 (m, 1H), 7.93–7.85 (m, 2H), 7.95 (d,
J = 7.5 Hz, 1H), 8.06–8.00 (m, 2H), 8.08 (dd,
J = 7.8, 1.2 Hz, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 164.1 (CO), 161.3 (CO), 143.8 (C, Ar), 136.0 (CH, Ar), 135.7 (C, Ar), 134.4 (CH, Ar), 131.3 (CH, Ar), 130.2 (CH, Ar), 129.4 (C, Ar), 125.7 (CH, Ar), 124.2 (CH, Ar), 123.2 (CH, Ar), 121.2 (CH, Ar), 115.3 (C, Ar), 92.5 (C), 23.4 (CH
3); ESI-LRMS
m/
z: 266 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 266.0812, found: 266.0807. The characterization data is in accordance with that reported in [
43].
3a-Methyl-3,3a-dihydro-1H-naphtho[2,3-d]pyrrolo[2,1-b][1,3]oxazine-1,5(2H)-dione (
SF38): yellow solid (128.1 mg, yield 96%), mp 228–230 °C.
1H-NMR (600 MHz, CDCl
3) δ 1.72 (s, 3H), 2.50–2.42 (m, 1H), 2.68–2.61 (m, 1H), 2.76–2.68 (m, 1H), 2.84–2.77 (m, 1H), 7.56–7.51 (m, 1H), 7.67–7.62 (m, 1H), 7.91 (d,
J = 8.3 Hz, 1H), 7.95 (d,
J = 8.2 Hz, 1H), 8.44 (s, 1H), 8.72 (s, 1H);
13C-NMR (150 MHz, CDCl
3) δ 171.7 (CO), 162.3 (CO), 136.7 (C, Ar), 133.2 (CH, Ar), 131.1 (C, Ar), 130.5 (C, Ar), 129.9 (CH, Ar), 129.7 (CH, Ar), 128.0 (CH, Ar), 126.9 (CH, Ar), 119.4 (CH, Ar), 115.7 (C, Ar), 95.7 (C), 32.3 (CH
2), 29.6 (CH
2), 25.7 (CH
3); ESI-LRMS
m/
z: 268 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 268.0968, found: 268.0962. The characterization data is in accordance with that reported in [
35].
4a-Methyl-2,3,4,4a-tetrahydronaphtho[2,3-d]pyrido[2,1-b][1,3]oxazine-1,6-dione (
SF39): pale yellow oil (103.7 mg, yield 74%).
1H-NMR (600 MHz, CDCl
3) δ 1.64 (s, 3H), 1.94–1.87 (m, 1H), 2.22–2.12 (m, 2H), 2.52–2.46 (m, 1H), 2.74–2.61 (m, 2H), 7.57–7.53 (m, 1H), 7.66–7.61 (m, 1H), 7.90 (d,
J = 8.3 Hz, 1H), 7.96 (d,
J = 8.2 Hz, 1H), 8.22 (s, 1H), 8.67 (s, 1H);
13C-NMR (150 MHz, CDCl
3) δ 169.3 (CO), 163.3 (CO), 135.9 (C, Ar), 133.0 (C, Ar), 131.8 (CH, Ar), 130.8 (C, Ar), 129.6 (CH, Ar), 129.5 (CH, Ar), 128.2 (CH, Ar), 127.1 (CH, Ar), 124.9 (CH, Ar), 119.2 (C, Ar), 92.5 (C), 36.3 (CH
2), 33.7 (CH
2), 27.7 (CH
3), 16.7 (CH
2); ESI-LRMS
m/
z: 282 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 282.1125, found: 282.1117. The characterization data is in accordance with that reported in [
43].
4b-Methyl-4bH-naphtho[2’,3’:4,5][1,3]oxazino[2,3-a]isoindole-6,14-dione (
SF40): white solid (113.2 mg, yield 72%), mp 210–212 °C.
1H-NMR (600 MHz, CDCl
3) δ 1.98 (s, 3H), 7.58–7.54 (m, 1H), 7.69–7.64 (m, 2H), 7.78–7.74 (m, 1H), 7.81–7.79 (m, 1H), 8.02–7.95 (m, 3H), 8.49 (s, 1H), 8.79 (s, 1H);
13C-NMR (150 MHz, CDCl
3) δ 164.7 (CO), 162.4 (CO), 144.0 (C, Ar), 136.9 (C, Ar), 133.9 (CH, Ar), 133.5 (CH, Ar), 131.1 (CH, Ar), 130.9 (C, Ar), 130.4 (C, Ar), 130.3 (C, Ar), 130.0 (CH, Ar), 129.9 (CH, Ar), 128.0 (CH, Ar), 126.8 (CH, Ar), 124.8 (CH, Ar), 122.6 (CH, Ar), 119.4 (CH, Ar), 115.0 (C, Ar), 92.7 (C), 25.0 (CH
3); ESI-LRMS
m/
z: 316 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 316.0968, found: 316.0959. The characterization data is in accordance with that reported in [
43].
6a-Methyl-7,8-dihydro-5H-pyrido[2,3-d]pyrrolo[2,1-b][1,3]oxazine-5,9(6aH)-dione (
SF41): colorless oil (65.1 mg, yield 60%).
1H-NMR (600 MHz, CDCl
3) δ 1.72 (s, 3H), 2.52–2.41 (m, 1H), 2.68–2.60 (m, 1H), 2.86–2.69 (m, 2H), 7.36 (dd,
J = 7.7, 4.9 Hz, 1H), 8.42 (dd,
J = 7.7, 1.9 Hz, 1H), 8.81 (dd,
J = 4.9, 1.9 Hz, 1H);
13C-NMR (150 MHz, CDCl
3) δ 171.6 (CO), 161.3 (CO), 155.1 (CH, Ar), 149.1 (C, Ar), 139.4 (CH, Ar), 122.0 (CH, Ar), 113.0 (C, Ar), 96.1 (C), 32.3 (CH
2), 29.8 (CH
2), 25.4 (CH
3); ESI-LRMS
m/
z: 219 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 219.0764, found: 219.0763. The characterization data is in accordance with that reported in [
35].
3a-Methyl-2,3,3a,5-tetrahydro-1H-benzo[d]pyrrolo[2,1-b][1,3]oxazin-1-one (
SF42): pale yellow oil (33.8 mg, yield 33%).
1H-NMR (500 MHz, DMSO-
d6) δ 1.45 (s, 3H), 2.07–1.97 (m, 1H), 2.29–2.19 (m, 1H), 2.43 (ddd,
J = 17.1, 10.0, 2.1 Hz, 1H), 2.76–2.65 (m, 1H), 4.88 (d,
J = 16.0 Hz, 1H), 4.99 (d,
J = 16.0 Hz, 1H), 7.16–7.10 (m, 1H), 7.21–7.16 (m, 1H), 7.32–7.25 (m, 1H), 8.20 (d,
J = 8.1 Hz, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 170.9 (CO), 132.6 (C, Ar), 127.1 (CH, Ar), 124.7 (CH, Ar), 123.7 (CH, Ar), 123.2 (C, Ar), 119.2 (CH, Ar), 89.6 (C), 62.0 (CH
2), 32.5 (CH
2), 29.7 (CH
2), 20.7 (CH
3); ESI-LRMS
m/
z: 204 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 204.1019, found: 204.1017. The characterization data is in accordance with that reported in [
43].
6a-Methyl-5H-benzo[4,5][1,3]oxazino[2,3-a]isoindol-11(6aH)-one (
SF43): colorless oil (22.4 mg, yield 18%).
1H-NMR (600 MHz, CDCl
3) δ 1.74 (s, 3H), 4.98 (d,
J = 15.3 Hz, 1H), 5.20 (d,
J = 15.3 Hz, 1H), 7.14–7.10 (m, 1H), 7.19–7.15 (m, 1H), 7.40–7.35 (m, 1H), 7.59–7.54 (m, 1H), 7.68–7.60 (m, 2H), 7.91 (d,
J = 7.5 Hz, 1H), 8.25 (d,
J = 8.2 Hz, 1H);
13C-NMR (150 MHz, CDCl
3) δ 165.2 (CO), 146.1 (C, Ar), 133.1 (CH, Ar), 132.6 (C, Ar), 131.2 (C, Ar), 130.2 (CH, Ar), 127.9 (CH, Ar), 124.3 (2 × CH, Ar), 124.2 (CH, Ar), 123.0 (C, Ar), 121.8 (CH, Ar), 121.5 (CH, Ar), 88.1 (C), 63.3 (CH
2), 20.8 (CH
3); ESI-LRMS
m/
z: 252 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 252.1019, found: 252.1018. The characterization data is in accordance with that reported in [
43].
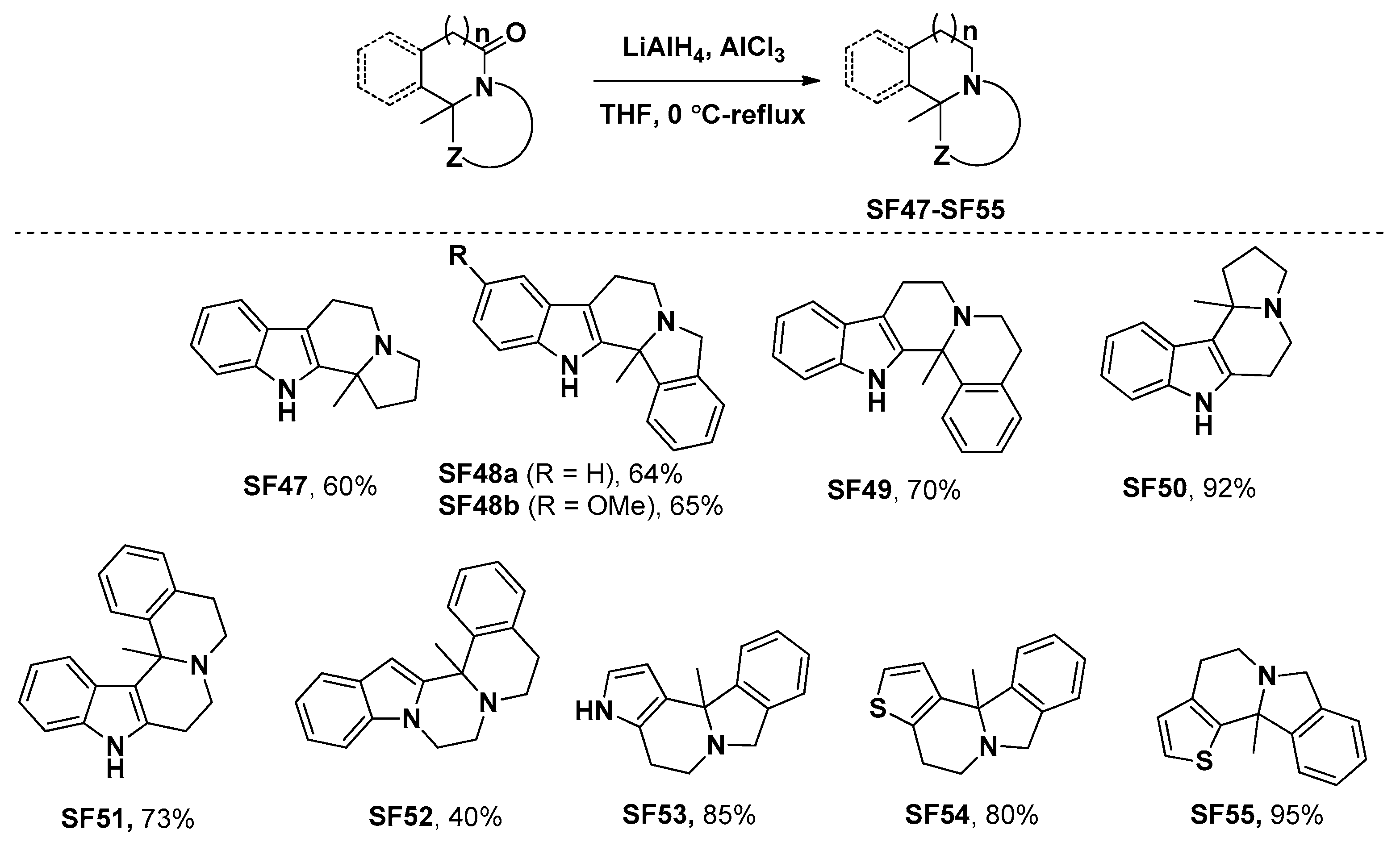
3.4. General Procedure of the Reductive Preparation of Compounds SF47–SF55
To a solution of substrates (0.3 mmol) in dry THF was added AlCl3 (0.6 mmol), then LiAlH4 (0.6 mmol) was added portionwise at 0 °C. After that, the mixture was heated to reflux for 4 h. After the reaction was cooled, the reaction mixture was diluted with dichloromethane (120.0 mL), and then water was added dropwise at 0 °C to quench the reaction under vigorous stirring conditions. The solid which precipitated out was removed by filtration, and the organic layer obtained was dried over Na2SO4. After the removal of the solvents in vacuo, the residue was purified to give SF47–SF55.
11b-Methyl-2,3,5,6,11,11b-hexahydro-1H-indolizino[8,7-b]indole (SF47): pale yellow oil (40.5 mg, yield 60%). 1H-NMR (400 MHz, DMSO-d6) δ 1.67–1.54 (m, 1H), 1.80 (s, 3H), 2.04–1.93 (m, 1H), 2.20–2.08 (m, 1H), 2.94–2.85 (m, 1H), 3.07–2.96 (m, 1H), 3.30–3.22 (m, 2H), 3.56–3.47 (m, 3H), 7.05–6.99 (m, 1H), 7.15–7.09 (m, 1H), 7.35 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 7.8 Hz, 1H), 11.28 (s, 1H); 13C-NMR (125 MHz, DMSO-d6) δ 136.3 (C, Ar), 125.7 (C, Ar), 121.8 (CH, Ar), 119.0 (CH, Ar), 118.3 (CH, Ar), 111.3 (CH, Ar), 104.5 (C, Ar), 48.9 (CH2), 42.5 (CH2), 36.4 (CH2), 24.1 (CH3), 21.0 (CH2), 15.3 (CH2); ESI-LRMS m/z: 227 [M + H]+; ESI-HRMS m/z calcd for M + H+ 227.1543, found: 227.1540.
13b-Methyl-7,8,13,13b-tetrahydro-5H-benzo[1,2]indolizino[8,7-b]indole (
SF48a): pale yellow solid (52.3 mg, yield 64%), mp 204–206 °C.
1H-NMR (400 MHz, CDCl
3) δ 1.95 (s, 3H), 2.62–2.53 (m, 1H), 3.28–3.13 (m, 1H), 3.54–3.38 (m, 2H), 4.27–4.18 (m, 2H), 7.13–7.03 (m, 2H), 7.21–7.13 (m, 2H), 7.31–7.22 (m, 2H), 7.85–7.74 (m, 2H), 7.93 (s, 1H);
13C-NMR (125 MHz, DMSO-
d6) δ 135.5 (C, Ar), 131.8 (C, Ar), 126.5 (CH, Ar), 126.4 (CH, Ar), 124.9 (C, Ar), 122.8 (CH, Ar), 122.4 (CH, Ar), 120.2 (CH, Ar), 119.0 (CH, Ar), 118.3 (CH, Ar), 110.8 (CH, Ar), 52.7 (CH
2), 41.0 (CH
2), 26.2 (CH
3), 17.1 (CH
2); ESI-LRMS
m/
z: 275 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 275.1543, found: 275.1541. The characterization data is in accordance with that reported in [
43].
10-Methoxy-13b-methyl-7,8,13,13b-tetrahydro-5H-benzo[1,2]indolizino[8,7-b]indole (
SF48b): pale yellow oil (59.2 mg, yield 65%).
1H-NMR (400 MHz, CDCl
3) δ 1.86 (s, 3H), 2.60 (ddd,
J = 15.8, 4.3, 1.4 Hz, 1H), 3.13 (ddd,
J = 16.2, 11.1, 6.4 Hz, 1H), 3.54–3.43 (m, 2H), 3.84 (s, 3H), 4.26–4.15 (m, 2H), 6.78 (dd,
J = 8.7, 2.5 Hz, 1H), 6.93 (d,
J = 2.4 Hz, 1H), 7.15 (d,
J = 8.7 Hz, 1H), 7.25–7.18 (m, 2H), 7.33–7.27 (m, 1H), 7.45 (d,
J = 7.4 Hz, 1H), 7.60 (s, 1H);
13C-NMR (100 MHz, CDCl
3) δ 154.2 (C, Ar), 144.9 (C, Ar), 139.0 (C, Ar), 137.8 (C, Ar), 131.3 (C, Ar), 127.7 (CH, Ar), 127.7 (C, Ar), 127.4 (CH, Ar), 123.4 (CH, Ar), 121.1 (CH, Ar), 111.7 (CH, Ar), 111.7 (CH, Ar), 106.9 (C, Ar), 100.9 (CH, Ar), 65.0 (C), 56.1 (OCH
3), 53.2 (CH
2), 41.7 (CH
2), 26.0 (CH
3), 16.4 (CH
2); ESI-LRMS
m/
z: 305 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 305.1648, found: 305.1645. The characterization data is in accordance with that reported in [
43].
14b-Methyl-5,6,8,9,14,14b-hexahydroindolo[2’,3’:3,4]pyrido[2,1-a]isoquinoline (
SF49): pale yellow oil (60.2 mg, yield 70%).
1H-NMR (400 MHz, CDCl
3) δ 1.95 (s, 3H), 2.85–2.66 (m, 2H), 3.07–2.91 (m, 2H), 3.14–3.07 (m, 1H), 3.19–3.15 (m, 1H), 3.26 (ddd,
J = 13.8, 6.1, 2.6 Hz, 1H), 3.76 (ddd,
J = 13.9, 10.0, 5.8 Hz, 1H), 7.19–7.05 (m, 3H), 7.25–7.20 (m, 1H), 7.31–7.27 (m, 1H), 7.37–7.31 (m, 1H), 7.49 (d,
J = 7.6 Hz, 1H), 7.65 (d,
J = 7.8 Hz, 1H), 7.87 (s, 1H);
13C-NMR (100 MHz, CDCl
3) δ140.0 (C, Ar), 137.5 (C, Ar), 135.8 (C, Ar), 134.5 (C, Ar), 130.1 (CH, Ar), 127.7 (C, Ar), 126.8 (CH, Ar), 126.6 (CH, Ar), 126.0 (CH, Ar), 121.8 (CH, Ar), 119.6 (CH, Ar), 118.5 (CH, Ar), 111.0 (CH, Ar), 105.9 (C, Ar), 58.3 (C), 46.8 (CH
2), 45.5 (CH
2), 30.4 (CH
3), 28.8 (CH
2), 17.9 (CH
2); ESI-LRMS
m/
z: 289 [M + H]
+; ESI-HRMS
m/
z calcd for M + H
+ 289.1699, found: 289.1695. The characterization data is in accordance with that reported in [
43].
11c-Methyl-2,3,5,6,7,11c-hexahydro-1H-indolizino[7,8-b]indole (SF50): pale yellow solid (62.2 mg, yield 92%), mp 249–250 °C. 1H-NMR (400 MHz, DMSO-d6) δ 1.66–1.51 (m, 1H), 1.82 (s, 3H), 2.04–1.92 (m, 1H), 2.27–2.15 (m, 1H), 2.61–2.52 (m, 1H), 3.02–2.87 (m, 1H), 3.24–3.13 (m, 1H), 3.31–3.25 (m, 1H), 3.60–3.44 (m, 3H), 7.04–6.97 (m, 1H), 7.13–7.07 (m, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 7.9 Hz, 1H), 11.24 (s, 1H); 13C-NMR (125 MHz, DMSO-d6) δ 136.2 (C, Ar), 129.5 (C, Ar), 123.3 (C, Ar), 121.3 (CH, Ar), 119.0 (CH, Ar), 118.0 (CH, Ar), 111.4 (CH, Ar), 48.7 (CH2), 41.8 (CH2), 36.1 (CH2), 24.3 (CH3), 21.2 (CH2), 16.8 (CH2); ESI-LRMS m/z: 227 [M + H]+; ESI-HRMS m/z calcd for M + H+ 227.1543, found: 227.1541.
14c-Methyl-5,6,8,9,10,14c-hexahydroindolo[3’,2’:3,4]pyrido[2,1-a]isoquinoline (SF51): pale yellow oil (63.5 mg, yield 73%). 1H-NMR (600 MHz, CDCl3) δ 2.21 (s, 3H), 2.82–2.75 (m, 1H), 2.97 (dd, J = 17.9, 6.5 Hz, 1H), 3.23–3.17 (m, 1H), 3.32–3.27 (m, 2H), 3.42–3.38 (m, 1H), 3.50–3.43 (m, 1H), 4.15–4.07 (m, 1H), 7.10–7.05 (m, 2H), 7.14–7.10 (m, 2H), 7.20–7.16 (m, 1H), 7.31 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 7.9 Hz, 1H), 7.79 (d, J = 7.9 Hz, 1H), 9.64 (s, 1H); 13C-NMR (150 MHz, CDCl3) δ 142.9 (C, Ar), 136.6 (C, Ar), 132.7 (C, Ar), 131.2 (C, Ar), 129.2 (CH, Ar), 128.1 (CH, Ar), 127.3 (C, Ar), 126.3 (2 × CH, Ar), 120.8 (CH, Ar), 120.7 (CH, Ar), 119.5 (CH, Ar), 115.3 (C, Ar), 111.2 (CH, Ar), 59.8 (C), 45.8 (CH2), 45.3 (CH2), 29.9 (CH3), 23.6 (CH2), 23.1 (CH2); ESI-LRMS m/z: 289 [M + H]+; ESI-HRMS m/z calcd for M + H+ 289.1699, found: 289.1698.
15b-Methyl-6,8,9,15b-tetrahydro-5H-indolo[2’,1’:3,4]pyrazino[2,1-a]isoquinoline (SF52): pale yellow oil (34.3 mg, yield 40%). 1H-NMR (600 MHz, CDCl3) δ 1.94 (s, 3H), 2.92–2.83 (m, 1H), 3.07–2.99 (m, 1H), 3.17–3.09 (m, 1H), 3.40–3.32 (m, 1H), 3.55–3.47 (m, 1H), 3.63–3.56 (m, 1H), 4.08–4.01 (m, 1H), 4.15–4.10 (m, 1H), 6.55 (s, 1H), 7.15–7.10 (m, 2H), 7.20–7.15 (m, 3H), 7.27 (d, J = 8.0 Hz, 1H), 7.63–7.57 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 140.9 (C, Ar), 139.8 (C, Ar), 136.7 (C, Ar), 132.8 (C, Ar), 129.5 (CH, Ar), 128.0 (C, Ar), 127.8 (CH, Ar), 126.6 (CH, Ar), 126.1 (CH, Ar), 121.2 (CH, Ar), 120.3 (CH, Ar), 120.1 (CH, Ar), 109.1 (CH, Ar), 100.6 (CH, Ar), 59.2 (C), 45.7 (CH2), 45.6 (CH2), 40.4 (CH2), 31.5 (CH3), 25.3 (CH2); ESI-LRMS m/z: 289 [M + H]+; ESI-HRMS m/z calcd for M + H+ 289.1699, found: 289.1696.
11b-Methyl-4,5,7,11b-tetrahydro-3H-pyrrolo[3’,2’:3,4]pyrido[2,1-a]isoindole (SF53): pale yellow oil (57.2 mg, yield 85%). 1H-NMR (600 MHz, CDCl3) δ 1.78 (s, 3H), 3.07–2.97 (m, 1H), 3.49–3.36 (m, 2H), 3.72–3.65 (m, 1H), 4.18–4.11 (m, 1H), 4.28–4.20 (m, 1H), 6.08–6.04 (m, 1H), 6.62–6.58 (m, 1H), 7.19–7.14 (m, 2H), 7.29–7.23 (m, 1H), 7.45–7.40 (m, 1H), 7.83 (s, 1H); 13C-NMR (150 MHz, CDCl3) δ 148.1 (C, Ar), 137.8 (C, Ar), 127.2 (CH, Ar), 126.8 (CH, Ar), 122.9 (C, Ar), 122.7 (CH, Ar), 121.9 (1CH + 1C, Ar), 116.6 (CH, Ar), 105.2 (CH, Ar), 65.8 (C), 53.5 (CH2), 42.1 (CH2), 28.2 (CH3), 17.2 (CH2); ESI-LRMS m/z: 225 [M + H]+; ESI-HRMS m/z calcd for M + H+ 225.1386, found: 225.1385.
11b-Methyl-4,5,7,11b-tetrahydrothieno[3’,2’:3,4]pyrido[2,1-a]isoindole (SF54): pale yellow solid (57.9 mg, yield 80%), mp 73–74 °C. 1H-NMR (600 MHz, CDCl3) δ 1.76 (s, 3H), 2.60 (ddd, J = 16.5, 4.4, 1.5 Hz, 1H), 3.22–3.13 (m, 1H), 3.49–3.38 (m, 2H), 4.20–4.12 (m, 2H), 6.94 (d, J = 5.2 Hz, 1H), 7.00 (dd, J = 5.3, 0.7 Hz, 1H), 7.21–7.17 (m, 2H), 7.28–7.25 (m, 1H), 7.45 (d, J = 7.6 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 146.7 (C, Ar), 139.5 (C, Ar), 138.6 (C, Ar), 132.4 (C, Ar), 127.1 (2 × CH, Ar), 125.6 (CH, Ar), 122.8 (CH, Ar), 122.3 (CH, Ar), 122.1 (CH, Ar), 67.0 (C, Ar), 53.6 (CH2), 42.1 (CH2), 27.9 (CH3), 19.2 (CH2); ESI-LRMS m/z: 242 [M + H]+; ESI-HRMS m/z calcd for M + H+ 242.0998, found: 242.0997.
11b-Methyl-4,5,7,11b-tetrahydrothieno[2’,3’:3,4]pyrido[2,1-a]isoindole (SF55): yellow oil (68.6 mg, yield 95%). 1H-NMR (600 MHz, CDCl3) δ 1.85 (s, 3H), 2.47 (ddd, J = 16.4, 4.5, 1.4 Hz, 1H), 3.07–2.97 (m, 1H), 3.45–3.30 (m, 2H), 4.22–4.12 (m, 2H), 6.69 (d, J = 5.1 Hz, 1H), 7.11 (d, J = 5.0 Hz, 1H), 7.18–7.16 (m, 1H), 7.21–7.18 (m, 1H), 7.30–7.26 (m, 1H), 7.46 (d, J = 7.6 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 147.2 (C, Ar), 142.4 (C, Ar), 138.4 (C, Ar), 132.5 (C, Ar), 127.3 (2 × CH, Ar), 126.9 (CH, Ar), 123.1 (CH, Ar), 122.8 (CH, Ar), 122.1 (CH, Ar), 67.0 (C), 53.7 (CH2), 41.8 (CH2), 29.8 (CH3), 20.2 (CH2); ESI-LRMS m/z: 242 [M + H]+; ESI-HRMS m/z calcd for M + H+ 242.0998, found: 242.0997.