Exploring the Insecticidal Potential of Boldo (Peumus boldus) Essential Oil: Toxicity to Pests and Vectors and Non-target Impact on the Microcrustacean Daphnia magna
Abstract
1. Introduction
2. Results
2.1. Composition of BEO
2.2. Insecticidal Efficacy of BEO and Impact on Non-target Organisms
2.3. Inhibitory Properties of BEO on Acetylcholinesterase (AChE)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Hydrodistillation of Essential Oil
4.3. GC-MS Analysis
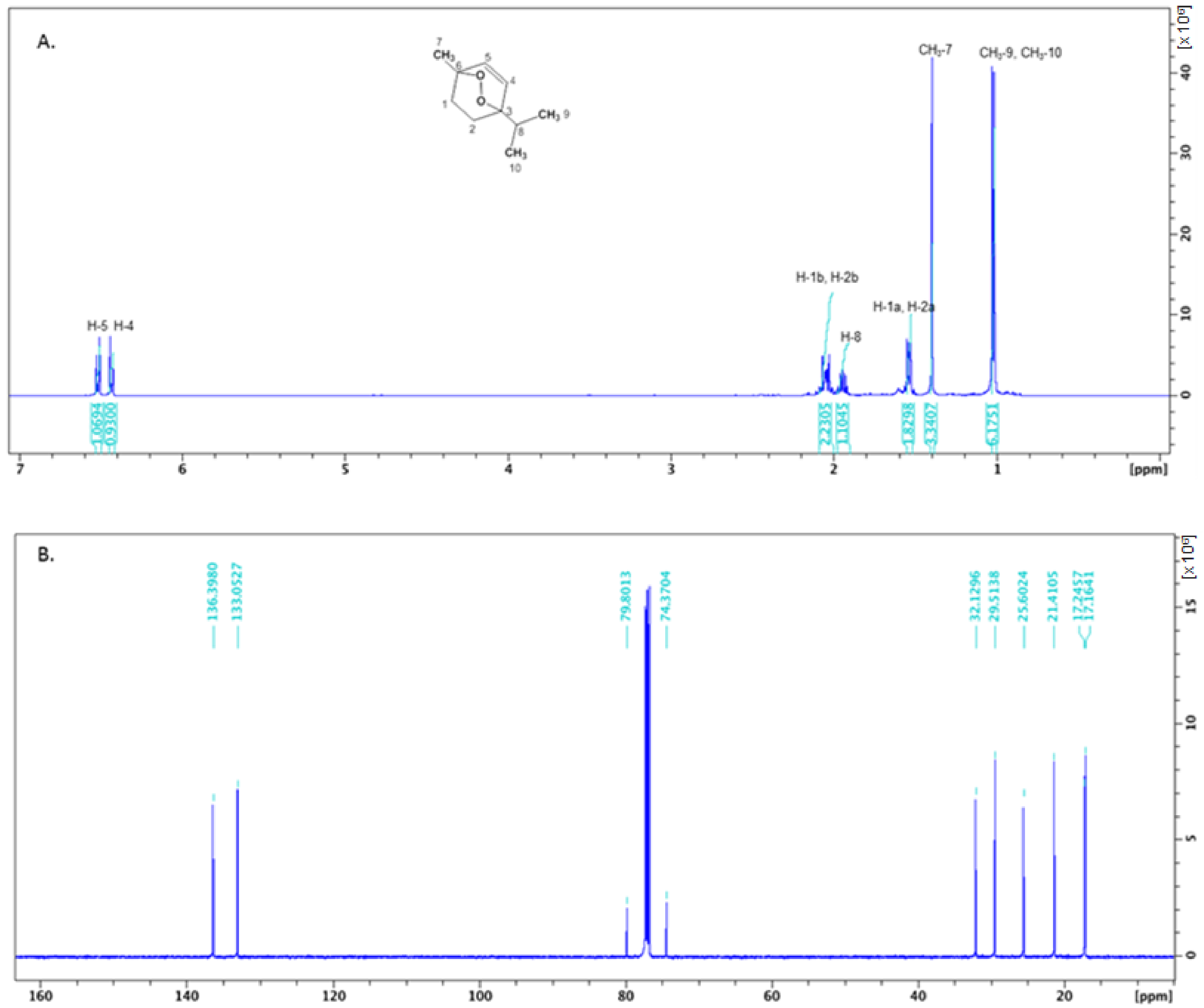
4.4. Isolation and Identification of cis-Ascaridole
4.5. Toxicity against Culex quinquefasciatus Larvae
4.6. Toxicity against Musca domestica Adults
4.7. Larval Toxicity on Spodoptera littoralis
4.8. Impact on Non-target Microcrustaceans
4.9. Acetylcholinesterase (AChE) Inhibitory Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vogel, H.; González, B.; Razmilic, I. Boldo (Peumus boldus) cultivated under different light conditions, soil humidity and plantation density. Ind. Crops Prod. 2011, 34, 1310–1312. [Google Scholar] [CrossRef]
- Palma, S.; Luján, C.; Llabot, J.M.; Barboza, G.; Manzo, R.H.; Allemandi, D.A. Design of Peumus boldus tablets by direct compression using a novel dry plant extract. Int. J. Pharm. 2002, 233, 191–198. [Google Scholar] [CrossRef]
- Leung, A.Y.; Foster, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics, 2nd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- MacDonald, D.; VanCrey, K.; Harrison, P.; Rangachari, P.K.; Rosenfeld, J.; Warren, C.; Sorger, G. Ascaridole-less infusions of Chenopodium ambrosioides contain a nematocide (s) that is (are) not toxic to mammalian smooth muscle. J. Ethnopharmacol. 2004, 92, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Lanhers, M.C.; Joyeux, M.; Soulimani, R.; Fleurentin, J.; Sayag, M.; Mot-tier, F.; Younos, C.; Pelt, J.-M. Hepatoprotective and anti-inflammatory effects of a traditional medicinal plant of Chile, Peumus holdus. Planta Med. 1991, 57, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Silano, M.; De Vincenzi, M.; De Vincenzi, A.; Silano, V. The new European legislation on traditional herbal medicines: main features and perspectives. Fitoterapia 2004, 75, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Girardi, N.S.; Passone, M.A.; García, D.; Nesci, A.; Etcheverry, M. Microencapsulation of Peumus boldus essential oil and its impact on peanut seed quality preservation. Ind. Crops Prod. 2018, 114, 108–114. [Google Scholar] [CrossRef]
- Girardi, N.S.; García, D.; Robledo, S.N.; Passone, M.A.; Nesci, A.; Etcheverry, M. Microencapsulation of Peumus boldus oil by complex coacervation to provide peanut seeds protection against fungal pathogens. Ind. Crops Prod. 2016, 92, 93–101. [Google Scholar] [CrossRef]
- Passone, M.A.; Etcheverry, M. Antifungal impact of volatile fractions of Peumus boldus and Lippia turbinata on Aspergillus section Flavi and residual levels of these oils in irradiated peanut. Int. J. Food Microbiol. 2014, 168, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; García-Rellán, D.; Boira, H.; Pérez, E.; Gandolfo, S.; Blázquez, M.A. Herbicidal activity of Peumus boldus and Drimys winterii essential oils from Chile. Molecules 2011, 16, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.A.; Carbó, E. Control of Portulaca oleracea by boldo and lemon essential oils in different soils. Ind. Crops Prod. 2015, 76, 515–521. [Google Scholar] [CrossRef]
- De Castro, D.S.B.; da Silva, D.B.; Tibúrcio, J.D.; Sobral, M.E.G.; Ferraz, V.; Taranto, A.G.; Serrão, J.E.; de Siqueira, J.M.; Alves, S.N. Larvicidal activity of essential oil of Peumus boldus Molina and its ascaridole-enriched fraction against Culex quinquefasciatus. Exp. Parasitol. 2016, 171, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Betancur, R.J.; Silva, A.G.; Rodríguez, J.M.; Fischer, G.S.; Zapata, S.M. Insecticidal activity of Peumus boldus Molina essential oil against Sitophilus zeamais Motschulsky. Chil. J. Agric. Res. 2010, 70, 399–407. [Google Scholar]
- Diana, P.; Gonzalo, S.; Maritza, T.; Rodríguez, M.; Angélica, U.; Inés, F.; Angel Lagunes, T.; Santillán-Ortega, C.; Robles-Bermúdez, A.; Aguilar-Medel, S.; et al. Essential oil from leaves of Peumus boldus Molina collected in autumn to control of maize weevil Sitophilus zeamais Motschulsky. Chil. J. Agric. Anim. Sci. 2014, 30, 171–180. [Google Scholar]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: from laboratory to the field, with insights on the modes of action. Acta Trop. 2019. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—a systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gan, J.; Schlenk, D.; Jury, W.A. Enantioselectivity in environmental safety of current chiral insecticides. Proc. Natl. Acad. Sci. 2005, 102, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Petigny, L.; Périno, S.; Minuti, M.; Visinoni, F.; Wajsman, J.; Chemat, F. Simultaneous microwave extraction and separation of volatile and non-volatile organic compounds of boldo leaves. From lab to industrial scale. Int. J. Mol. Sci. 2014, 15, 7183–7198. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Rodríguez, C.; Ramírez-Mendoza, C.; Becerra-Morales, I.; Silva-Aguayo, G.; Urbina-Parra, A.; Figueroa-Cares, I.; Martínez-Bolaños, L.; Rodríguez-Maciel, J.C.; Lagunes-Tejeda, A.; Pastene-Navarrete, E.; et al. Bioactivity of Peumus boldus Molina, Laurelia sempervirens (Ruiz & Pav.) Tul. and Laureliopsis philippiana (Looser) Schodde (Monimiacea) essential oils against Sitophilus zeamais Motschulsky. Chil. J. Agric. Res. 2015, 75, 334–340. [Google Scholar]
- Gille, L.; Monzote, L.; Stamberg, W.; Staniek, K. Toxicity of ascaridole from Chenopodium ambrosioides in mammalian mitochondria. BMC Pharmacol. 2010, 10, A10. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Tomi, F.; Bernardini, A.F.; Casanova, J. Analysis of the EO of Chenopodium ambrosioides by GC, GC–MS and 13C-NMR spectroscopy: Quantitative determination of ascaridole, a heatsensitive compound. Phytochem. Anal. 2004, 15, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Pollack, Y.; Segal, R.; Golenser, J. The effect of ascaridole on the in vitro development of Plasmodium falciparum. Parasitol. Res. 1990, 76, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.S.; Hu, J.F.; Liu, Z.L. Composition of EO of Chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais. Pest Manag. Sci. 2011, 67, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Maggi, F.; Lupidi, G.; Mbuntcha, H.; Woguem, V.; Womeni, H.M.; Barboni, L.; Tapondjou, L.A.; Benelli, G. Clausena anisata and Dysphania ambrosioides essential oils: From ethno-medicine to modern uses as effective insecticides. Environ. Sci. Pollut. Res. 2018, 25, 10493–10503. [Google Scholar] [CrossRef] [PubMed]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Opdyke, D.L.J. Monographs on fragrance raw materials, Chenopodium oil. Food Cosmet. Toxicol. 1976, 14, 713–715. [Google Scholar]
- Hassine, D.B.; Abderrabba, M.; Yvon, Y.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Bouajila, J. Chemical composition and in vitro evaluation of the antioxidant and anti- microbial activities of Eucalyptus gillii essential oil and extracts. Molecules 2012, 17, 9540–9558. [Google Scholar] [CrossRef] [PubMed]
- Qnais, E.Y.; Abdulla, F.A.; Kaddumi, E.G.; Abdalla, S.S. Antidiarrheal activity of Laurus nobilis L. leaf extract in rats. J. Med. Food 2012, 15, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Benelli, G. Essential oils from aromatic and medicinal plants as effective weapons against mosquito vectors of public health importance. In Mosquito-borne Diseases; Springer: Cham, Switzerland, 2018; pp. 69–129. [Google Scholar]
- Isman, M.B. Bridging the gap: Moving botanical insecticides from the laboratory to the farm. Ind. Crops Prod. 2017, 110, 10–14. [Google Scholar] [CrossRef]
- Isman, M.B.; Machial, C.M. Pesticides based on plant essential oils: From traditional practice to commercialization. In Naturally Occurring Bioactive Compounds; Rai, M., Carpinella, M.C., Eds.; Elsevier B.V.: Amsterdam, the Netherlands, 2006; Chapter 2; pp. 29–44. [Google Scholar]
- Koul, O. Insect Antifeedants. CRC Press: Bota Racon, FL, USA, 2004. [Google Scholar]
- Capone, D.L.; Van Leeuwen, K.; Taylor, D.K.; Jeffery, D.W.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A. Evolution and occurrence of 1,8-cineole (Eucalyptol) in Australian wine. J. Agric. Food Chem. 2011, 59, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Zorzetto, C.; Sánchez-Mateo, C.C.; Santini, G.; Canale, A.; Maggi, F. Insecticidal activity of the essential oil from Schizogyne sericea (Asteraceae) on four insect pests and two non-target species. Entomologia Generalis 2019. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Muscolo, C.; Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Quassinti, L.; Bramucci, M.; Damiano, S.; Iannarelli, R.; et al. Bioactive Secondary Metabolites from Schizogyne sericea (Asteraceae) Endemic to Canary Islands. Chem. Biodivers. 2016, 13, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono-and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. J. Clean Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Vitali, L.A.; Beghelli, D.; Nya, P.C.B.; Bistoni, O.; Cappellacci, L.; Damiano, S.; Lupidi, G.; Maggi, F.; Orsomando, G.; Papa, F.; et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab. J. Chem. 2016, 9, 775–786. [Google Scholar] [CrossRef]
- Pavela, R. Larvicidal property of essential oils against Culex quinquefasciatus Say (Diptera: Culicidae). Ind. Crops Prod. 2009, 30, 311–315. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Urzua, A.; Santander, R.; Echeverría, J.; Villalobos, C.; Palacios, S.M.; Rossi, Y. Insecticidal Properties of Peumus boldus Mol. Essential Oil on the House Fly, Musca domestica L. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2010, 9, 465–469. [Google Scholar]
- López, V.; Cascella, M.; Benelli, G.; Maggi, F.; Gómez-Rincón, C. Green drugs in the fight against Anisakis simplex—larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol. Res. 2018, 117, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of sea fennel (Crithmum maritimum L., Apiaceae) essential oils against Culex quinquefasciatus Say and Spodoptera littoralis (Boisd.). Ind. Crops Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Kamte, S.L.N.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The crop-residue of fiber hemp cv. Futura 75: From a waste product to a source of botanical insecticides. Environ. Sci. Pollut. Res. 2018, 25, 10515–10525. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan Sengottayan; Maggi, F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind. Crops Prod 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Quassinti, L.; Maggi, F.; Barboni, L.; Ricciutelli, M.; Cortese, M.; Papa, F.; Garulli, C.; Kalogris, C.; Vittori, S.; Bramucci, M. Wild celery (Smyrnium olusatrum L.) oil and isofuranodiene induce apoptosis in human colon carcinoma cells. Fitoterapia 2014, 97, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: CarolStream, IL, USA, 2007. [Google Scholar]
- NIST 17. Mass Spectral Library (NIST/EPA/NIH). National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- FFNSC 2. Flavors and Fragrances of Natural and Synthetic Compounds. MassSpectral Database. Shimadzu Corps: Kyoto, Japan, 2012. [Google Scholar]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- OECD–Organization for Economic Cooperation and Development. Guideline for testing of chemicals. Daphnia sp., acute immobilisation test. OECD 202: Paris, France, 2004. [Google Scholar]
- Pavela, R. Insecticidal properties of Pimpinella anisum essential oils against the Culex quinquefasciatus and the non-target organism Daphnia magna. J. Asia-Pacif. Entomol. 2014, 17, 287–293. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University: London, UK, 1971; pp. 68–78. [Google Scholar]
Sample Availability: Samples of cis-ascaridole are available from the authors. |
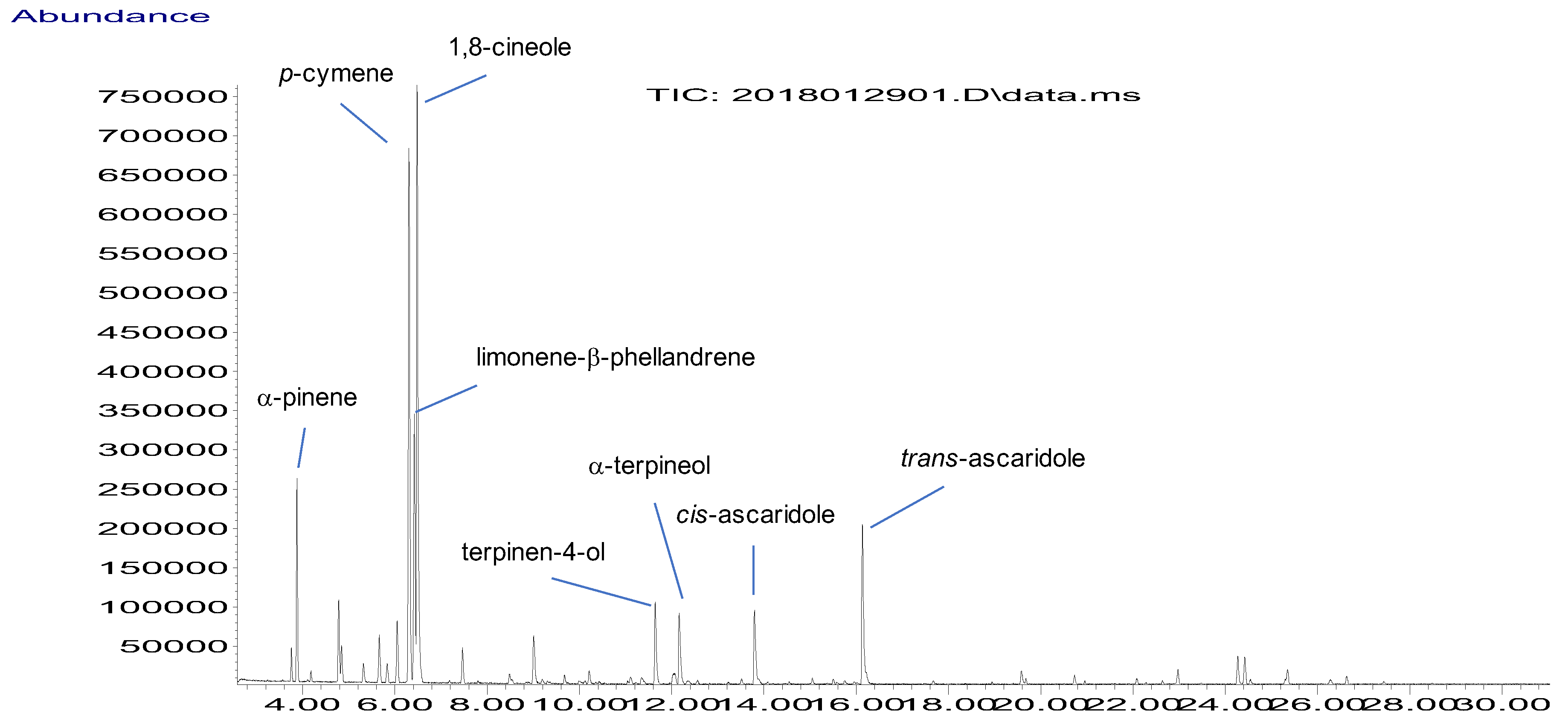
| No | Component a | RI Apolar Column | RI Polar Column | % f | ID g | ||
|---|---|---|---|---|---|---|---|
| Exp. b | Lit. c | Exp. d | Lit. e | ||||
| 1 | α-thujene | 921 | 924 | 0.8 ± 0.1 | 1,2 | ||
| 2 | α-pinene | 927 | 932 | 1020 | 1022 | 4.9 ± 0.9 | 1,2,3 |
| 3 | Camphene | 940 | 946 | 1063 | 1066 | 0.2 ± 0.0 | 1,2,3 |
| 4 | Sabinene | 966 | 969 | 1118 | 1120 | 2.4 ± 0.5 | 1,2,3 |
| 5 | β-pinene | 969 | 974 | 1107 | 1110 | 1.1 ± 0.2 | 1,2,3 |
| 6 | Myrcene | 990 | 988 | 1156 | 1156 | 0.6 ± 0.2 | 1,2,3 |
| 7 | dehydro-1,8-cineole | 990 | 988 | 1192 | 1195 | 0.6 ± 0.2 | 1,2 |
| 8 | α-phellandrene | 1003 | 1002 | 1161 | 1161 | 1.6 ± 0.4 | 1,2,3 |
| 9 | δ-3-carene | 1008 | 1008 | 1145 | 1145 | 0.6 ± 0.3 | 1,2,3 |
| 10 | α-terpinene | 1015 | 1014 | 1176 | 1176 | 2.0 ± 0.4 | 1,2,3 |
| 11 | p-cymene | 1022 | 1020 | 1266 | 1267 | 18.5 ± 2.1 | 1,2,3 |
| 12 | Limonene | 1025 | 1024 | 1196 | 1199 | 9.1 ± 1.6 | 1,2,3 |
| 13 | β-phellandrene | 1025 | 1025 | 1206 | 1206 | 6.4 ± 1.2 | 1,2,3 |
| 14 | 1,8-cineole | 1027 | 1026 | 1213 | 1212 | 20.7 ± 3.1 | 1,2,3 |
| 15 | (E)-β-ocimene | 1047 | 1044 | 1246 | 1246 | 0.1 ± 0.0 | 1,2,3 |
| 16 | γ-terpinene | 1056 | 1054 | 1242 | 1244 | 1.2 ± 0.3 | 1,2,3 |
| 17 | cis-sabinene hydrate | 1065 | 1065 | 1469 | 1469 | 0.1 ± 0.0 | 1,2 |
| 18 | Terpinolene | 1085 | 1086 | 1279 | 1278 | 0.3 ± 0.1 | 1,2,3 |
| 19 | Fenchone | 1085 | 1083 | 1400 | 0.3 ± 0.1 | 1,2 | |
| 20 | p-cymenene | 1087 | 1089 | 1432 | 1432 | 0.1 ± 0.0 | 1,2 |
| 21 | trans-sabinene hydrate | 1097 | 1098 | 1555 | 0.1 ± 0.0 | 1,2 | |
| 22 | Linalool | 1101 | 1095 | 1545 | 1545 | 1.9 ± 0.4 | 1,2,3 |
| 23 | 1,3,8-p-menthatriene | 1109 | 1108 | 1390 | 0.1 ± 0.0 | 1,2 | |
| 24 | trans-p-mentha-2,8-dien-1-ol | 1119 | 1119 | 1633 | 1637 | 0.3 ± 0.1 | 1,2 |
| 25 | trans-pinocarveol | 1134 | 1135 | 1664 | 1664 | 0.5 ± 0.1 | 1,2 |
| 26 | trans-p-menth-2-en-1-ol | 1138 | 1136 | 0.1 ± 0.0 | 1,2 | ||
| 27 | Camphor | 1140 | 1141 | 1522 | 1519 | 0.1 ± 0.0 | 1,2,3 |
| 28 | sabina ketone | 1156 | 1154 | 1641 | 1651 | 0.1 ± 0.0 | 1,2 |
| 29 | Pinocarvone | 1158 | 1160 | 1573 | 0.3 ± 0.1 | 1,2 | |
| 30 | Borneol | 1161 | 1165 | tr h | 1,2,3 | ||
| 31 | δ-terpineol | 1165 | 1162 | 0.4 ± 0.1 | 1,2 | ||
| 32 | terpinen-4-ol | 1173 | 1174 | 1606 | 1603 | 3.1 ± 0.6 | 1,2,3 |
| 33 | Cryptone | 1181 | 1183 | 1680 | 1679 | tr | 1,2 |
| 34 | trans-p-mentha-1(7),8-dien-2-ol | 1184 | 1187 | 0.8 ± 0.2 | 1,2 | ||
| 35 | α-terpineol | 1187 | 1186 | 1700 | 1700 | 2.9 ± 0.6 | 1,2,3 |
| 36 | Myrtenal | 1192 | 1195 | 1633 | 1634 | 0.3 ± 0.1 | 1,2,3 |
| 37 | Myrtenol | 1192 | 1194 | 1632 | 0.2 ± 0.0 | 1,2,3 | |
| 38 | trans-piperitol | 1205 | 1207 | tr | 1,2 | ||
| 39 | trans-carveol | 1217 | 1215 | 1841 | 1840 | 0.1 ± 0.0 | 1,2 |
| 40 | cis-p-mentha-1(7),8-dien-2-ol | 1225 | 1227 | 0.2 ± 0.0 | 1,2 | ||
| 41 | cis-ascaridole | 1233 | 1234 | 3.0 ± 0.7 | 1,2,3 | ||
| 42 | cumin aldehyde | 1236 | 1238 | 1783 | 1781 | 0.2 ± 0.0 | 1,2 |
| 43 | Carvone | 1242 | 1239 | 1740 | 1738 | 0.1 ± 0.0 | 1,2,3 |
| 44 | trans-piperitone epoxide | 1255 | 1252 | 1734 | 1733 | 0.1 ± 0.0 | 1,2 |
| 45 | p-menth-1-en-7-al | 1269 | 1273 | 0.2 ± 0.0 | 1,2 | ||
| 46 | bornyl acetate | 1282 | 1287 | 1584 | 1584 | 0.2 ± 0.0 | 1,2,3 |
| 47 | Thymol | 1289 | 1289 | 2191 | 2189 | 0.1 ± 0.0 | 1,2,3 |
| 48 | trans-ascaridole | 1301 | 1303 | 1874 | 6.1 ± 1.1 | 1,2,3 | |
| 49 | Carvacrol | 1303 | 1298 | 2203 | 2201 | 0.5 ± 0.2 | 1,2,3 |
| 50 | α-terpinyl acetate | 1347 | 1346 | 1700 | 1701 | 0.1 ± 0.0 | 1,2 |
| 51 | β-elemene | 1386 | 1389 | 1590 | 1591 | tr | 1,2,3 |
| 52 | methyl eugenol | 1406 | 1403 | 2007 | 2006 | 0.6 ± 0.2 | 1,2 |
| 53 | (E)-caryophyllene | 1409 | 1417 | 1600 | 1604 | 0.2 ± 0.0 | 1,2,3 |
| 54 | α-humulene | 1444 | 1452 | 1673 | 1680 | 0.3 ± 0.1 | 1,2,3 |
| 55 | allo-aromadendrene | 1451 | 1458 | 1650 | 1650 | 0.1 ± 0.0 | 1,2 |
| 56 | bicyclogermacrene | 1488 | 1500 | 1737 | 1735 | 0.2 ± 0.0 | 1,2 |
| 57 | α-muurolene | 1495 | 1500 | 1724 | tr | 1,2 | |
| 58 | γ-cadinene | 1506 | 1513 | 1761 | 1762 | 0.1 ± 0.0 | 1,2 |
| 59 | δ-cadinene | 1518 | 1520 | 1757 | 1757 | 0.5 ± 0.1 | 1,2 |
| 60 | α-calacorene | 1535 | 1544 | 1917 | tr | 1,2 | |
| 61 | (E)-nerolidol | 1563 | 1561 | 2040 | 2039 | 1.0 ± 0.2 | 1,2,3 |
| 62 | spathulenol | 1568 | 1577 | 2135 | 2136 | 1.0 ± 0.2 | 1,2 |
| 63 | caryophyllene oxide | 1572 | 1582 | 1997 | 1994 | 0.2 ± 0.0 | 1,2,3 |
| 64 | humulene epoxide II | 1598 | 1608 | 2056 | 2069 | 0.1 ± 0.0 | 1,2 |
| 65 | β-oplopenone | 1600 | 1607 | 2086 | 2089 | 0.5 ± 0.1 | 1,2 |
| 66 | epi-α-muurolol | 1634 | 1640 | 2191 | 2190 | 0.2 ± 0.0 | 1,2 |
| 67 | α-cadinol | 1647 | 1652 | 2242 | 2251 | 0.3 ± 0.0 | 1,2 |
| Total identified (%) | 98.9 | ||||||
| Monoterpene hydrocarbons | 51.4 | ||||||
| Oxygenated monoterpenes | 42.4 | ||||||
| Sesquiterpene hydrocarbons | 1.4 | ||||||
| Oxygenated sesquiterpenes | 3.2 | ||||||
| Others | 0.6 | ||||||
| Target Organisms | LC/LD50 ± SE | CI95 | LC/LD90 ± SE | CI95 | χ2 | d.f. | p-Value | |
|---|---|---|---|---|---|---|---|---|
| BEO | Cx. quinquefasciatus(larvae, 3rd instar) mg·L−1 | 67.9 ± 7.8 | 55.1–70.5 | 96.2 ± 7.9 | 91.5–102.3 | 5.253 | 3 | 0.395 |
| M. domestica(adults) µg·adult−1 | 98.5 ± 5.2 | 89.7–102.3 | 173.9 ± 10.9 | 165.9–198.7 | 1.452 | 3 | 0.985 | |
| S. littoralis(larvae, 3rd instar) µg·larva−1 | 268.9 ± 12.6 | 252.8–295.5 | 556.9 ± 22.9 | 621.5–708.9 | 0.187 | 3 | 0.658 | |
| Positive controlα-cypermethrin | Cx. quinquefasciatus (larvae, 3rd instar) mg·L−1 | 0.008 ± 0.001 | 0.006–0.012 | 0.025 ± 0.002 | 0.021–0.032 | 5.235 | 3 | 0.296 |
| M. domestica(adults) µg·adult−1 | 0.16 ± 0.2 | 0.16–0.22 | 0.85 ± 0.1 | 0.82–1.12 | 1.525 | 3 | 0.752 | |
| S. littoralis(larvae, 3rd instar) µg·larva−1 | 0.009 ± 0.003 | 0.007–0.012 | 0.021 ± 0.009 | 0.018–0.028 | 2.525 | 3 | 0.395 |
| Daphnia magna Mortality (%) a | ||
|---|---|---|
| After 24 h | After 48 h | |
| BEO (96.2 mg·L−1) | 46.2 ± 4.1 b | 66.2 ± 4.1 b |
| α-cypermethrin (0.025 mg·L−1) | 100.0 ± 0.0 c | 100.0 ± 0.0 d |
| Negative control | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| ANOVA F2,8; P | 1251.3; <0.0001 | 1338.5; <0.0001 |
| IC50 mg·mL−1 | mgGEIC·gr−1 a | |
|---|---|---|
| BEO | 0.45 ± 0.03 | 17.97 ± 1.0 |
| Positive control | ||
| Galantamine | 8 ± 0.2 × 10−3 |
| Origin | Main Oil Components | Reference |
|---|---|---|
| Not specified | ascaridole (46.9%), limonene (18.8%), p-cymene (12.9%) | [16] |
| Chile | ascaridole (24.4%), 1,8-cineole (14.9%), (E)-β-ocimene (12.9%) | [17] |
| Commercial | ascaridole (38.9%), p-cymene (21.6%), 1,8-cineole (12.6%) | [11] |
| Commercial | ascaridole (31.4%), 1,8-cineole (25.0%), o-ocimene (11.7%) | [12] |
| Commercial | piperitone oxide (28.1%), α-terpinene (18.8%), 1,8-cineole (12.3%) | [9] |
| Chile | 1,8-cineole (36.6%), p-cymene (29.8%), ascaridole (6.2%) | [44] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavela, R.; Benelli, G.; Petrelli, R.; Cappellacci, L.; Lupidi, G.; Sut, S.; Dall’Acqua, S.; Maggi, F. Exploring the Insecticidal Potential of Boldo (Peumus boldus) Essential Oil: Toxicity to Pests and Vectors and Non-target Impact on the Microcrustacean Daphnia magna. Molecules 2019, 24, 879. https://doi.org/10.3390/molecules24050879
Pavela R, Benelli G, Petrelli R, Cappellacci L, Lupidi G, Sut S, Dall’Acqua S, Maggi F. Exploring the Insecticidal Potential of Boldo (Peumus boldus) Essential Oil: Toxicity to Pests and Vectors and Non-target Impact on the Microcrustacean Daphnia magna. Molecules. 2019; 24(5):879. https://doi.org/10.3390/molecules24050879
Chicago/Turabian StylePavela, Roman, Giovanni Benelli, Riccardo Petrelli, Loredana Cappellacci, Giulio Lupidi, Stefania Sut, Stefano Dall’Acqua, and Filippo Maggi. 2019. "Exploring the Insecticidal Potential of Boldo (Peumus boldus) Essential Oil: Toxicity to Pests and Vectors and Non-target Impact on the Microcrustacean Daphnia magna" Molecules 24, no. 5: 879. https://doi.org/10.3390/molecules24050879
APA StylePavela, R., Benelli, G., Petrelli, R., Cappellacci, L., Lupidi, G., Sut, S., Dall’Acqua, S., & Maggi, F. (2019). Exploring the Insecticidal Potential of Boldo (Peumus boldus) Essential Oil: Toxicity to Pests and Vectors and Non-target Impact on the Microcrustacean Daphnia magna. Molecules, 24(5), 879. https://doi.org/10.3390/molecules24050879











