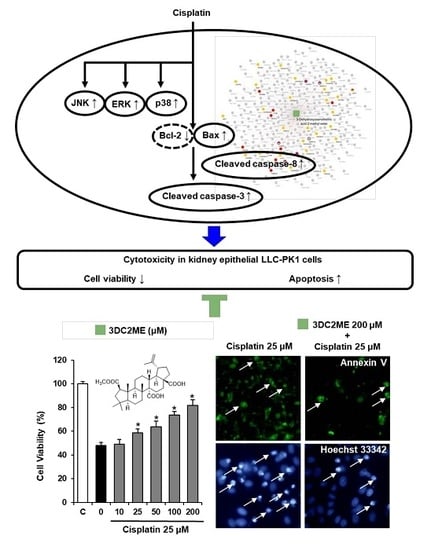
Multiple Targets of 3-Dehydroxyceanothetric Acid 2-Methyl Ester to Protect Against Cisplatin-Induced Cytotoxicity in Kidney Epithelial LLC-PK1 Cells
Abstract
1. Introduction
2. Results
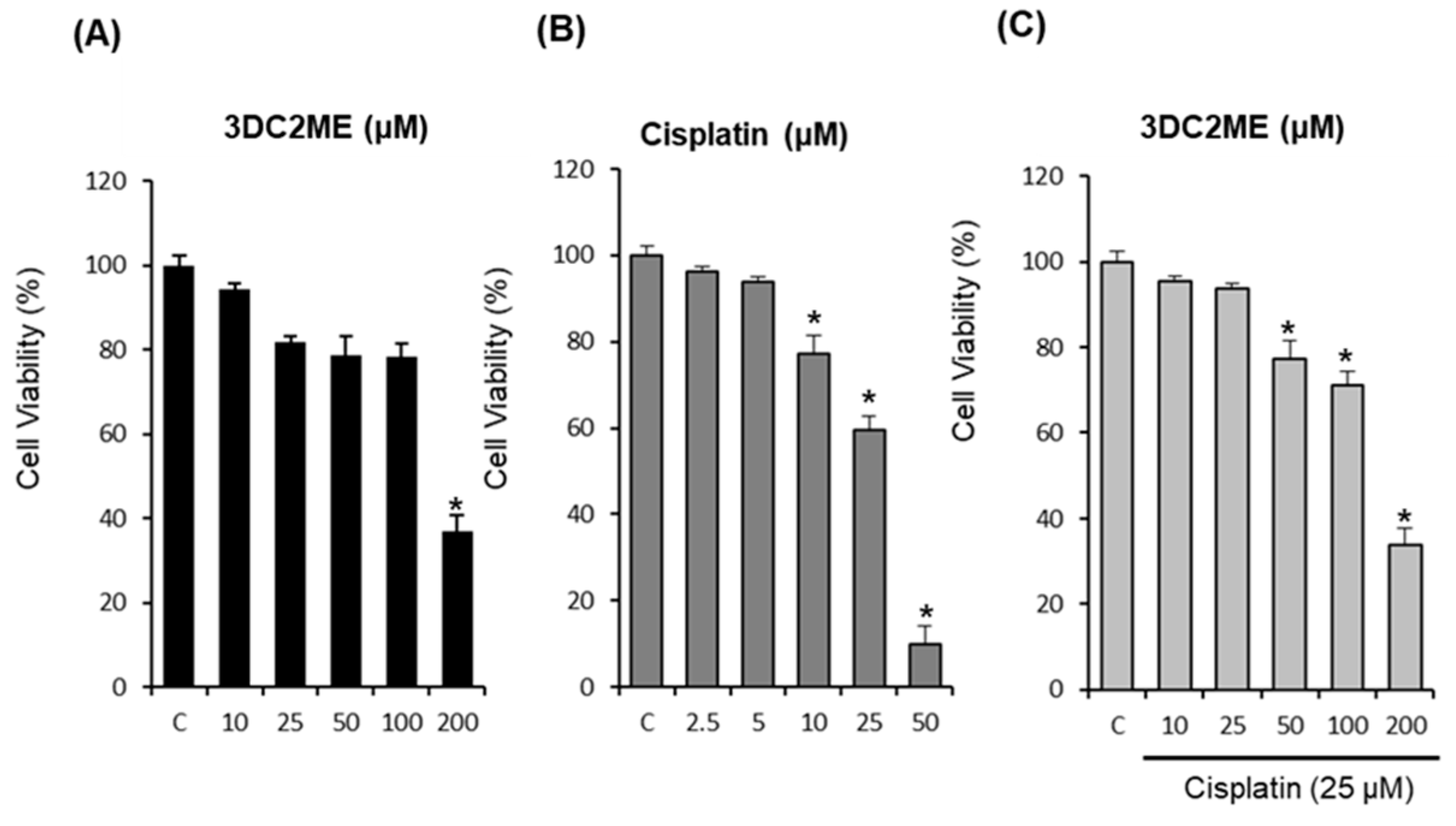
2.1. Protective Effects of Nine Triterpenoids from Z. jujuba Against Cisplatin-Induced LLC-PK1 Cell Death in LLC-PK1 Cells
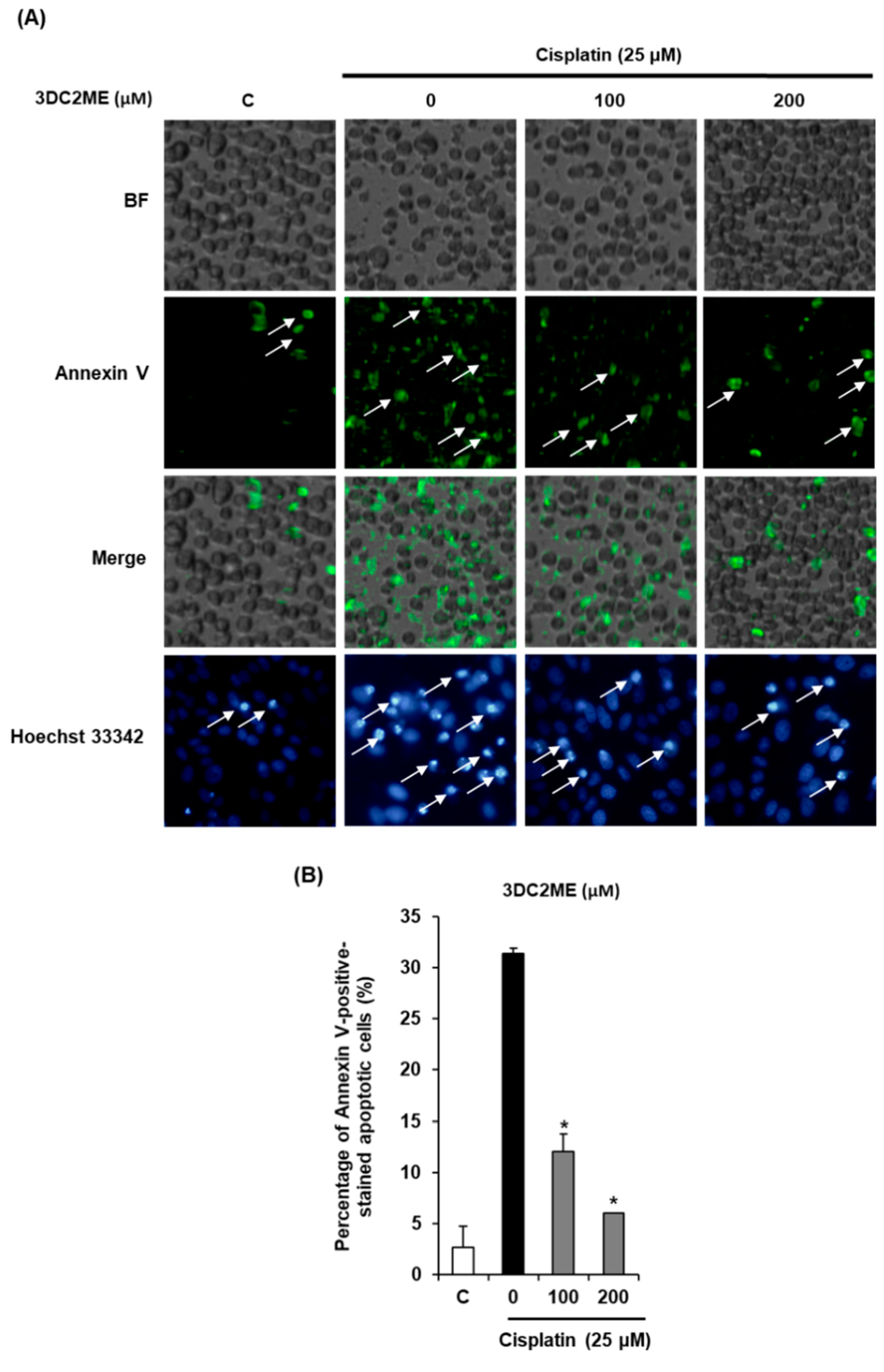
2.2. Protective Effects of 3DC2ME Against Cisplatin-Induced Apoptosis in LLC-PK1 Cells
2.3. Protective Effects of 3DC2ME on Expression of MAPK and Apoptosis Proteins in Cisplatin-Induced Damage in LLC-PK1 Cells
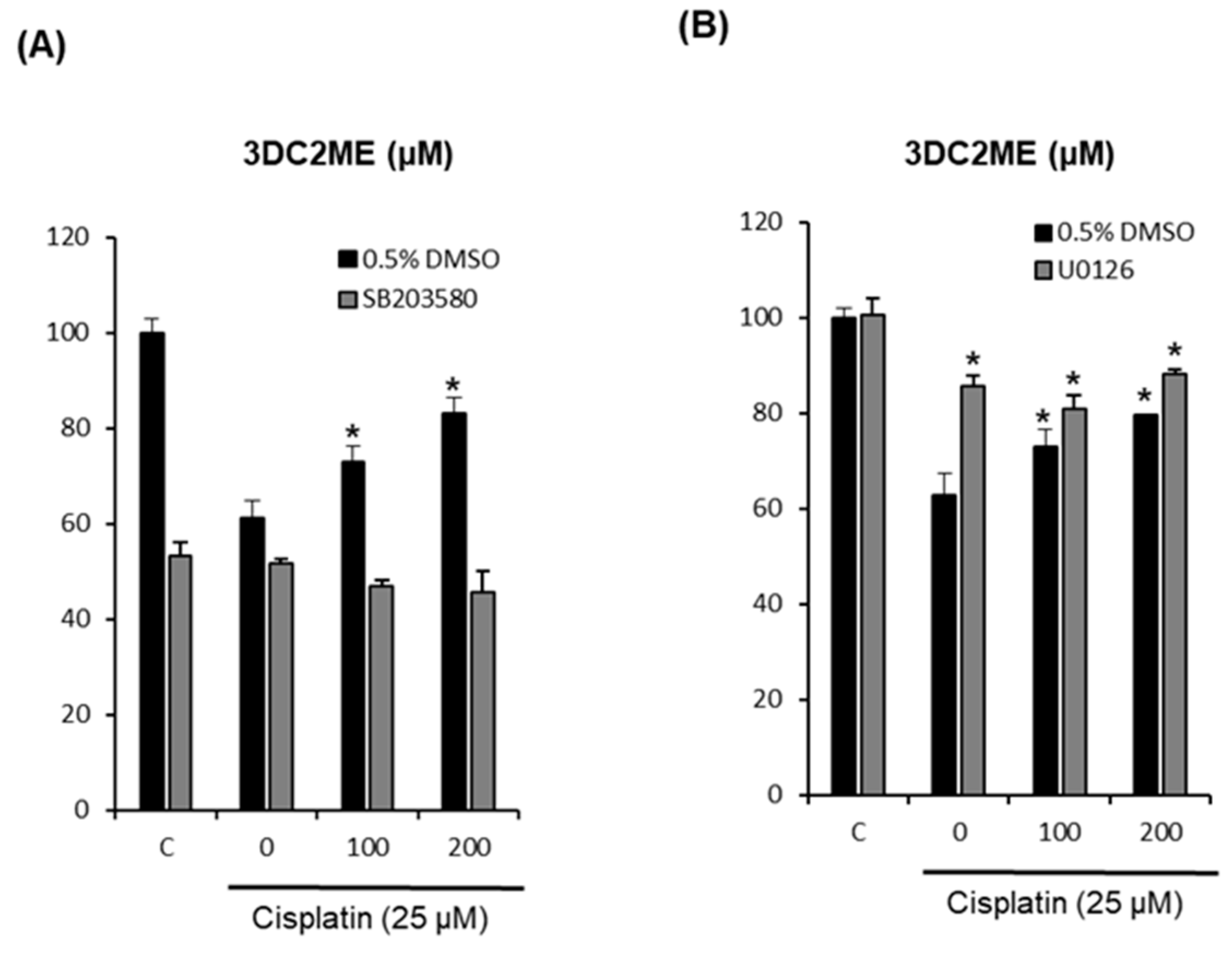
2.4. Effects of Combined Treatment with Inhibitors of MAPK Pathways (SB203580 and U0126) and 3DC2ME on Cisplatin-Induced LLC-PK1 Cell Death
2.5. Effect of Co-Treatment with 3DC2ME and Cisplatin in HeLa Human Cervical Carcinoma Cells
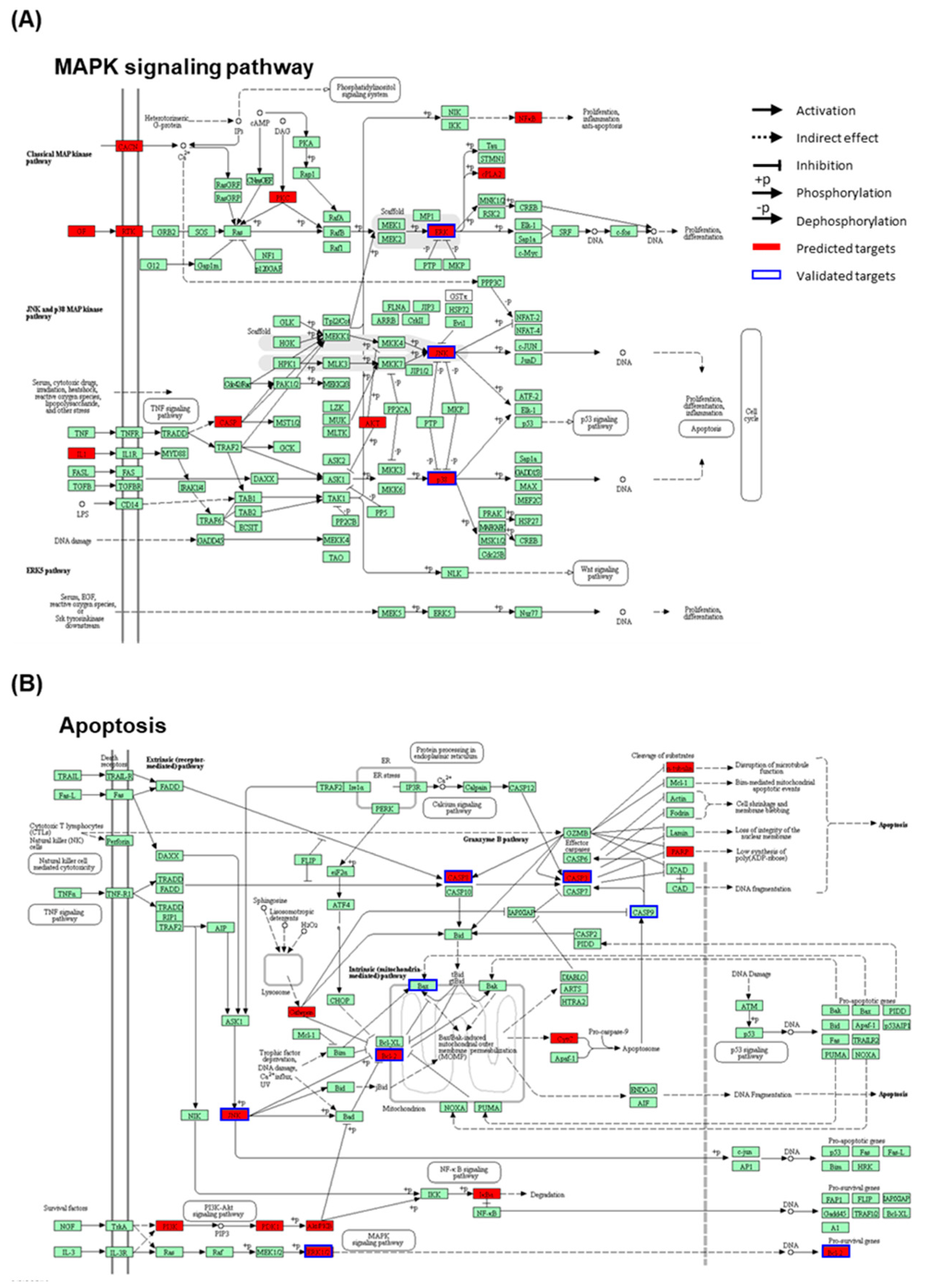
2.6. Network Pharmacological Approach
3. Discussion
4. Materials and Methods
4.1. Preparation of Triterpenoids from Z. jujuba
4.2. Cell Culture
4.3. Measurement of Cell Viability
4.4. Image-based Cytometric Assay
4.5. Cell Staining with Hoechst 33342
4.6. Western Blotting Analysis
4.7. Network Pharmacological Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Much, W.E.; Wilcox, C.S. Disorders of body fluids, sodium and potassium in chronic renal failure. Am. J. Med. 1982, 72, 536–550. [Google Scholar] [CrossRef]
- Schrier, R.W.; Wang, W.; Poole, B.; Mitra, A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J. Clin. Invest. 2004, 114, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Zhang, M.; Chen, L. 5′-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharm. Sin. B 2016, 6, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Peres, G.B.; Juliano, M.A.; Simoes, M.J.; Michelacci, Y.M. Lysosomal enzymes are decreased in the kidney of diabetic rats. Mol. Basis Dis. 2013, 1832, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Soreshjani, S.; Asadi-Samani, M.; Yang, Q.; Saeedi-Boroujeni, A. Phytotherapy of nephrotoxicity-induced by cancer drugs: An updated review. J. Nephropathol. 2017, 6, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.A.; Youssef, M.I.; El-Shennawy, L.K. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: the protective effect of grape seed proanthocyanidin extract. Food Chem. Toxicol. 2009, 47, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Thomale, J.; Stojanović, N.; Osmak, M.; Henninger, C.; Bormann, S.; Fritz, G. Platinum-induced kidney damage: Unraveling the DNA damage response (DDR) of renal tubular epithelial and glomerular endothelial cells following platinum injury. Biochim. Biophys. Acta 2015, 1853, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pabla, N.; Tang, C.; He, L.; Dong, Z. DNA damage response in cisplatin-induced nephrotoxicity. Archives Toxicol. 2015, 89, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Rivera, G. Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radical Bio. Med. 2002, 33, 409–422. [Google Scholar] [CrossRef]
- Pérez-Rojas, J.M.; Cruz, C.; García-López, P.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Ceballos, G.; Espinosa, M.; Meléndez-Zajgla, J.; Pedraza-Chaverri, J. Renoprotection by α-mangostin is related to the attenuation in renal oxidative/nitrosative stress induced by cisplatin nephrotoxicity. Free Radical Res. 2009, 43, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. Role of oxidative stress in drug-induced kidney injury. Internat. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Chirino, Y.I.; Pedraza-Chaverri, J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009, 61, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-K.; Cho, W.Y.; Sung, S.A.; Kim, H.K.; Won, N.H. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005, 67, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horváth, B.; Kechrid, M.; Tanchian, G.; Rajesh, M.; Naura, A.S.; Boulares, A.H.; Pacher, P. Poly (ADP-ribose) polymerase-1 is a key mediator of cisplatin-induced kidney inflammation and injury. Free Radical Bio. Med. 2011, 51, 1774–1788. [Google Scholar] [CrossRef] [PubMed]
- El Daly, E.S. Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extracts on cisplatin-induced toxicity in rats. J. Pharm. Belg. 1998, 53, 87–93. [Google Scholar] [PubMed]
- Gaikwad, K.; Dagle, P.; Choughule, P.; Joshi, Y.; Kadam, V. A review on some nephroprotective medicinal plants. Inter. J. Pharm. Sci. Res. 2012, 3, 2451–2454. [Google Scholar]
- Prabhu, V.V.; Kannan, N.; Guruvayoorappan, C. 1,2-Diazole prevents cisplatin-induced nephrotoxicity in experimental rats. Pharmacol. Rep. 2013, 65, 980–990. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, H.; Wang, X.Z.; Yang, X.Z.; Wu, S.N.; Wang, H.G.; Shen, P.; Ma, T.H. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Surendran, A.; Chandraprabha, V.R.; Pettamanna, A.; Nair, H.N.R. Betulinic acid, natural pentacyclic triterpenoid prevents arsenic-induced nephrotoxicity in male Wistar rats. Comp. Clin. Pathol. 2018, 27, 37–44. [Google Scholar] [CrossRef]
- Kuang, Q.T.; Zhao, J.J.; Ye, C.L.; Wang, J.R.; Ye, K.H.; Zhang, X.Q.; Wang, Y.; Ye, W.C. Nephro-protective effects of total triterpenoids from Psidium guajava leaves on type 2 diabetic rats. J. Chin. Med. Mat. 2012, 35, 94–97. [Google Scholar]
- Mapanga, R.F.; Tufts, M.A.; Shode, F.O.; Musabayane, C.T. Renal effects of plant-derived oleanolic acid in streptozotocin-induced diabetic rats. Renal Fail. 2009, 31, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Madlala, H.P.; Van Heerden, F.R.; Mubagwa, K.; Musabayane, C.T. Changes in renal function and oxidative status associated with the hypotensive effects of oleanolic acid and related synthetic derivatives in experimental animals. PloS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Yang, J.; Yang, H.; Li, X.; Wang, G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti-inflammatory, and anti-apoptotic activities. Mol. Med. Rep. 2016, 13, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Stidham, R.D.; Bumeister, R.; Trevino, I.; Winters, A.; Sprouse, M.; Ding, M.; Ferguson, D.A.; Meyer, C.J.; Wigley, W.C.; et al. The synthetic triterpenoid, RTA 405, increases the glomerular filtration rate and reduces angiotensin II-induced contraction of glomerular mesangial cells. Kidney Int. 2013, 83, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Qin, Z.Q.; Tang, J.Y.; Xu, Z.; Li, R.; Jiang, X.P.; Yang, C.D.; Xing, Q.W.; Qi, X.K.; Tang, M.; et al. RTA-408 protects kidney from ischemia-reperfusion injury in mice via activating Nrf2 and downstream GSH biosynthesis gene. Oxid. Med. Cell Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, J.A.; Qian, D.; Tang, Y.; Wu, D.; Su, S.; Wang, H.; Zhao, Y. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem. 2015, 167, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kitamura, K.; Irie, K.; Naruse, S.; Matsuura, T.; Uemae, T.; Taira, S.; Ohigashi, H.; Murakami, S.; Takahashi, M.; et al. Triterpenoids Isolated from Ziziphus jujuba Enhance Glucose Uptake Activity in Skeletal Muscle Cells. J. Nutr. Sci. Vitaminol. 2017, 63, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, P.; Li, L.; Huang, Y.; Pu, Y.; Hou, X.; Song, L. Identification and antioxidant activity of flavonoids extracted from Xinjiang Jujube (Ziziphus jujuba Mill.) leaves with ultra-high pressure extraction technology. Molecules 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, J.; Xu, X.; Li, Y.; Zhao, H.; Fang, Y.; Li, X.; Zhou, W.; Wang, W.; Wang, Y. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PloS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, D.; Kang, K.S.; Song, J.H.; Choi, Y.K. Inhibition of intracellular ROS accumulation by formononetin attenuates cisplatin-mediated apoptosis in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acid Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, H.J.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, J.M. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J. Applied Toxicol. 2005, 25, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, K.; Lee, D.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Protective effect and mechanism of action of lupane triterpenes from Cornus walteri in cisplatin-induced nephrotoxicity. Bioorgan. Med. Chem. Lett. 2015, 25, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Shim, S.H.; Lee, H.J.; Choi, Y.; Jang, T.S.; Kim, K.H.; Kang, K.S. Protective effect of lanostane triterpenoids from the sclerotia of Poria cocos Wolf against cisplatin-induced apoptosis in LLC-PK1 cells. Bioorgan. Med. Chem. Lett. 2017, 27, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Kaushal, V.; Hong, X.; Shah, S.V. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001, 60, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; De Leon, M.; Devarajan, P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J. Am. Soc. Nephrol. 2002, 13, 858–865. [Google Scholar] [PubMed]
- Wei, Q.; Dong, G.; Franklin, J.; Dong, Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007, 72, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.A.G.; Rodrigues, M.A.C.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Archives Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef] [PubMed]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Koopman, G.; Reutelingsperger, C.; Kuijten, G.; Keehnen, R.; Pals, S.; Van Oers, M. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Di Mari, J.F.; Davis, R.; Safirstein, R.L. MAPK activation determines renal epithelial cell survival during oxidative injury. Am. J. Physiol. 1999, 277, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, Z.; Cohen, D.M. MAPK signaling and the kidney. Am. J. Physiol. 2000, 279, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.J.; Jeong, J.Y.; Chang, Y.K.; Na, K.R.; Lee, K.W.; Shin, Y.T.; Choi, D.E. C-phycocyanin attenuates cisplatin-induced nephrotoxicity in mice. Ren. Fail 2012, 34, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.G.; Yan, Y.; Daubert, R.A.; Han, J.; Schnellmann, R.G. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am. J. Physiol. 2007, 292, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Feng, L.; Pabla, N.; Liu, K.; Smith, S.; Dong, Z. Effects of targeted Bcl-2 expression in mitochondria or endoplasmic reticulum on renal tubular cell apoptosis. Am. J. Physiol. 2008, 294, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Servais, H.; Ortiz, A.; Devuyst, O.; Denamur, S.; Tulkens, P.M.; Mingeot-Leclercq, M.-P. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 2008, 13, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; van de Water, B.; Wang, Y.; Stevens, J.L. The roles of caspase-3 and bcl-2 in chemically-induced apoptosis but not necrosis of renal epithelial cells. Oncogene 1999, 18, 6505–6512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, B.; Johnson, T.S.; Thomas, G.L.; Watson, P.F.; Wagner, B.; Furness, P.N.; El Nahas, A.M. A shift in the Bax/Bcl-2 balance may activate caspase-3 and modulate apoptosis in experimental glomerulonephritis. Kidney Int. 2002, 62, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Kaushal, V.; Herzog, C.; Yang, C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 2008, 4, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Kim, J.W.; Oh, W.K.; Kim, J.; Sung, S.H. Cytotoxic ceanothane- and lupane-Type triterpenoids from the roots of Ziziphus jujuba. J. Nat. Prod. 2016, 79, 2364–2375. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Hong, Y.D.; Baek, K.S.; Yoo, S.; Hong, Y.H.; Kim, J.H.; Lee, J.O.; Kim, D.; Park, J.; Cho, J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.; Park, J.Y.; Kang, K.S.; Park, J.H.; Hwang, G.S. Processed Panax ginseng, sun ginseng, inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans. J. Ginseng Res. 2017, 41, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Guon, T.; Chung, H.S. Induction of apoptosis with Moringa oleifera fruits in HCT116 human colon cancer cells via intrinsic pathway. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef]
- Yoon, D.H.; Han, C.; Fang, Y.; Gundeti, S.; Lee, I.-S.H.; Song, W.O.; Hwang, K.-C.; Kim, T.W.; Sung, G.-H.; Park, H. Inhibitory activity of Cordyceps bassiana extract on LPS-induced inflammation in RAW 264.7 cells by suppressing NF-κB activation. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acid Res. 2018, 46, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tao, L.; Zeng, X.; Qin, C.; Chen, S.Y.; Zhu, F.; Li, Z.R.; Jiang, Y.Y.; Chen, W.P.; Chen, Y.Z. A protein network descriptor server and its use in studying protein, disease, metabolic and drug targeted networks. Brief Bioinform. 2017, 18, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.N.; Wang, Z.C.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acid Res. 2016, 44, 90–97. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, K.H.; Lee, W.Y.; Kim, C.-E.; Sung, S.H.; Kang, K.B.; Kang, K.S. Multiple Targets of 3-Dehydroxyceanothetric Acid 2-Methyl Ester to Protect Against Cisplatin-Induced Cytotoxicity in Kidney Epithelial LLC-PK1 Cells. Molecules 2019, 24, 878. https://doi.org/10.3390/molecules24050878
Lee D, Kim KH, Lee WY, Kim C-E, Sung SH, Kang KB, Kang KS. Multiple Targets of 3-Dehydroxyceanothetric Acid 2-Methyl Ester to Protect Against Cisplatin-Induced Cytotoxicity in Kidney Epithelial LLC-PK1 Cells. Molecules. 2019; 24(5):878. https://doi.org/10.3390/molecules24050878
Chicago/Turabian StyleLee, Dahae, Ki Hyun Kim, Won Yung Lee, Chang-Eop Kim, Sang Hyun Sung, Kyo Bin Kang, and Ki Sung Kang. 2019. "Multiple Targets of 3-Dehydroxyceanothetric Acid 2-Methyl Ester to Protect Against Cisplatin-Induced Cytotoxicity in Kidney Epithelial LLC-PK1 Cells" Molecules 24, no. 5: 878. https://doi.org/10.3390/molecules24050878
APA StyleLee, D., Kim, K. H., Lee, W. Y., Kim, C.-E., Sung, S. H., Kang, K. B., & Kang, K. S. (2019). Multiple Targets of 3-Dehydroxyceanothetric Acid 2-Methyl Ester to Protect Against Cisplatin-Induced Cytotoxicity in Kidney Epithelial LLC-PK1 Cells. Molecules, 24(5), 878. https://doi.org/10.3390/molecules24050878