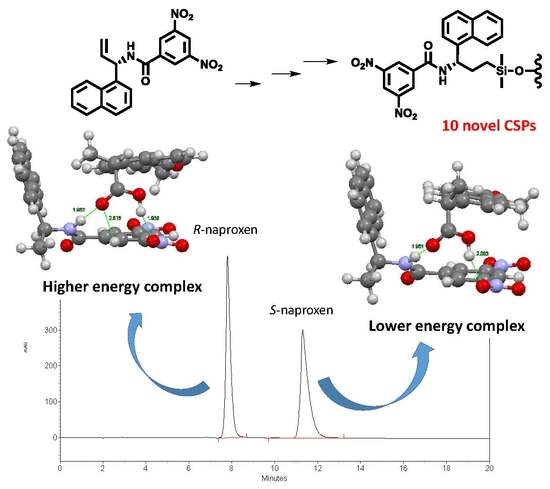
New Brush-Type Chiral Stationary Phases for Enantioseparation of Pharmaceutical Drugs
Abstract
:1. Introduction
2. Results and Discussion
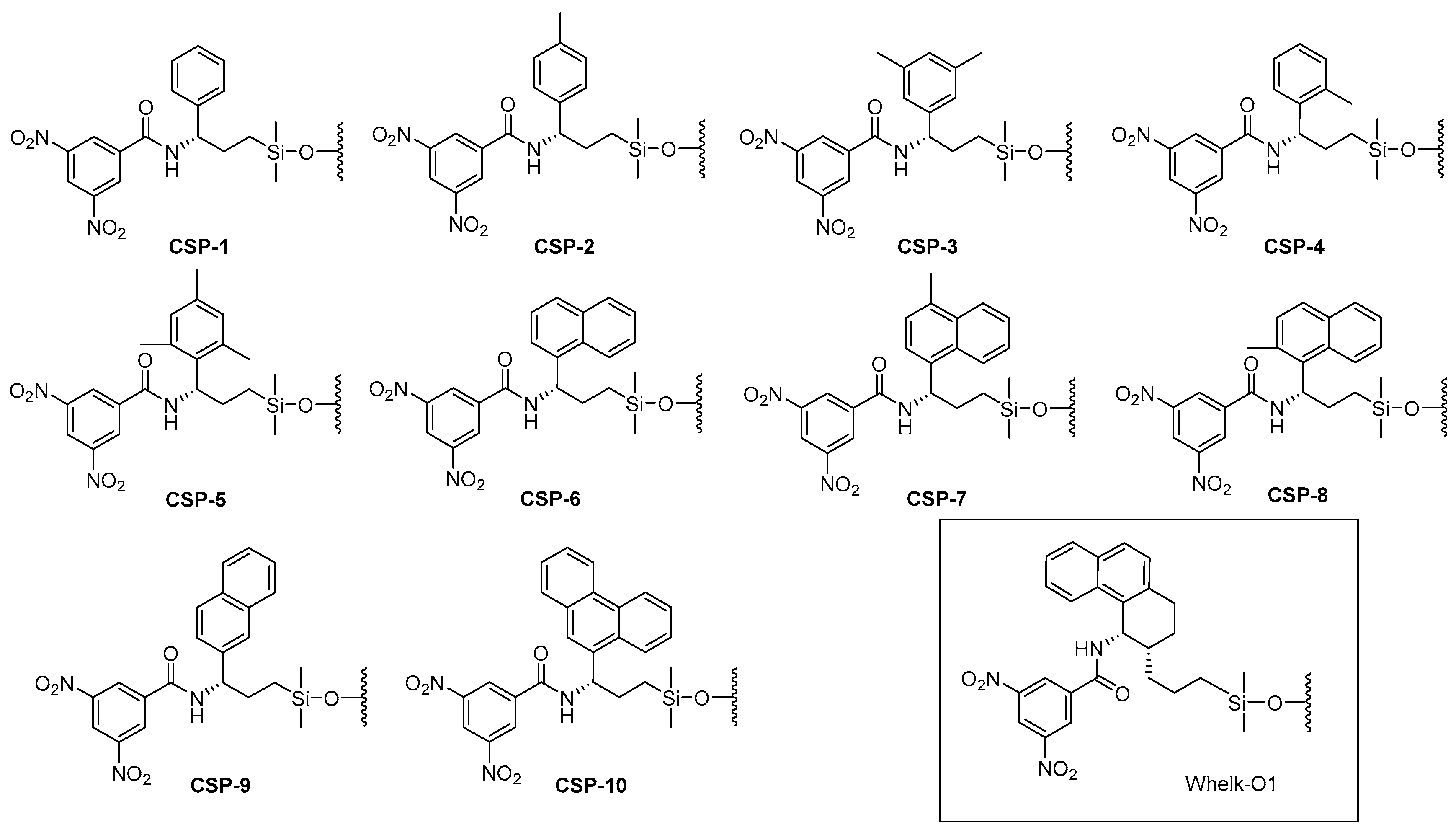
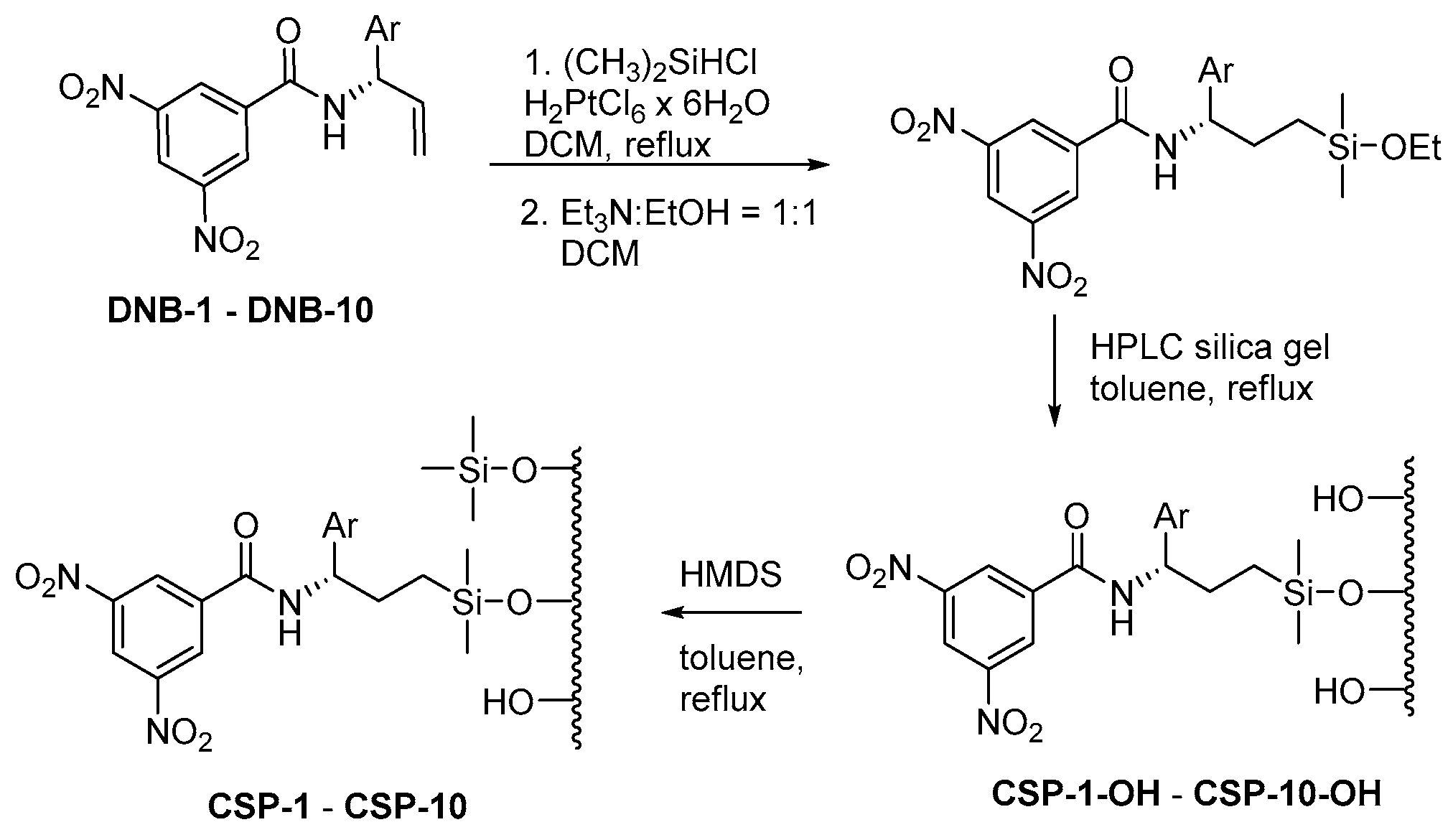
2.1. Preparation of New Brush-Type Chiral Stationary Phases (CSPs)
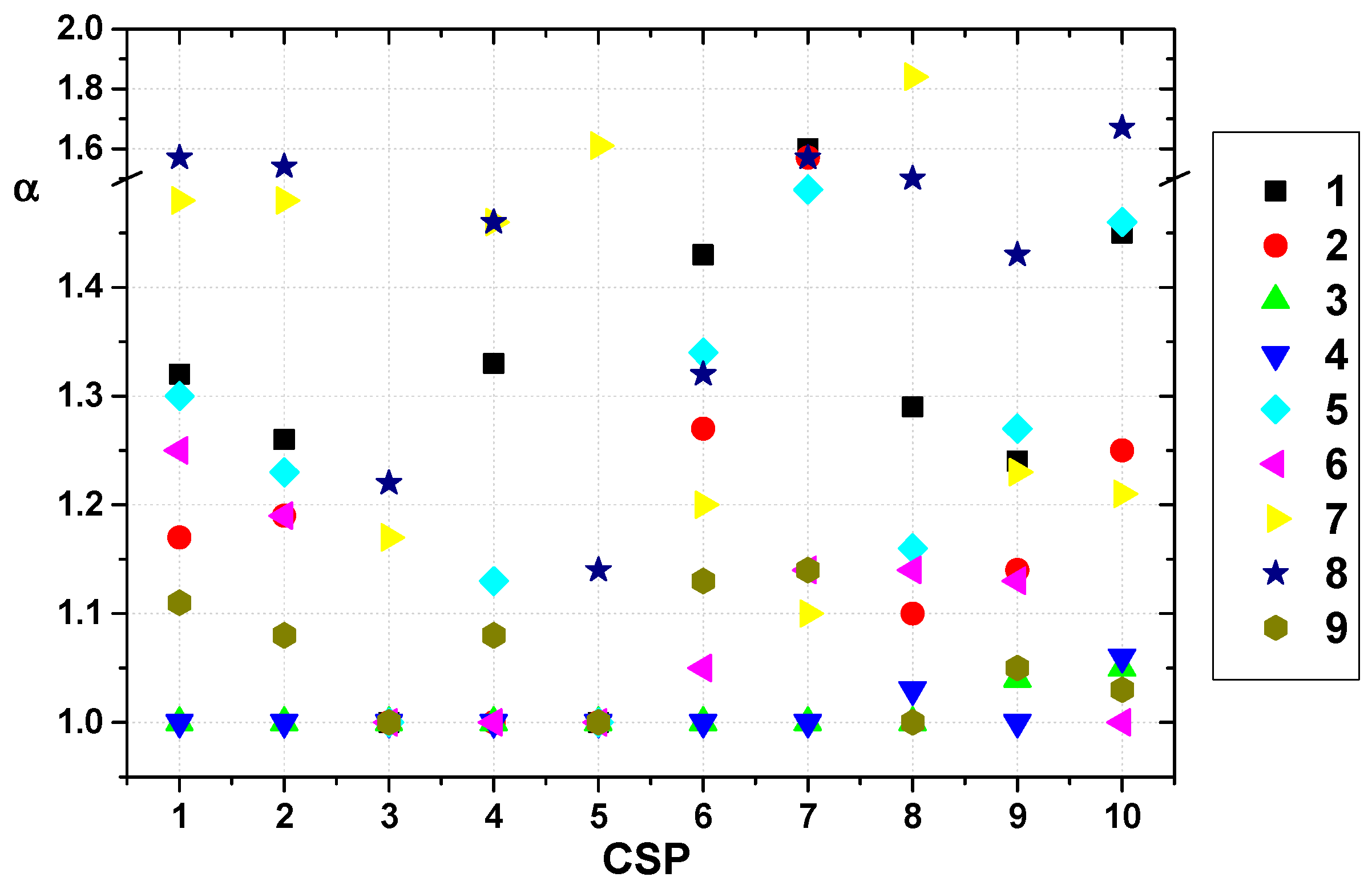
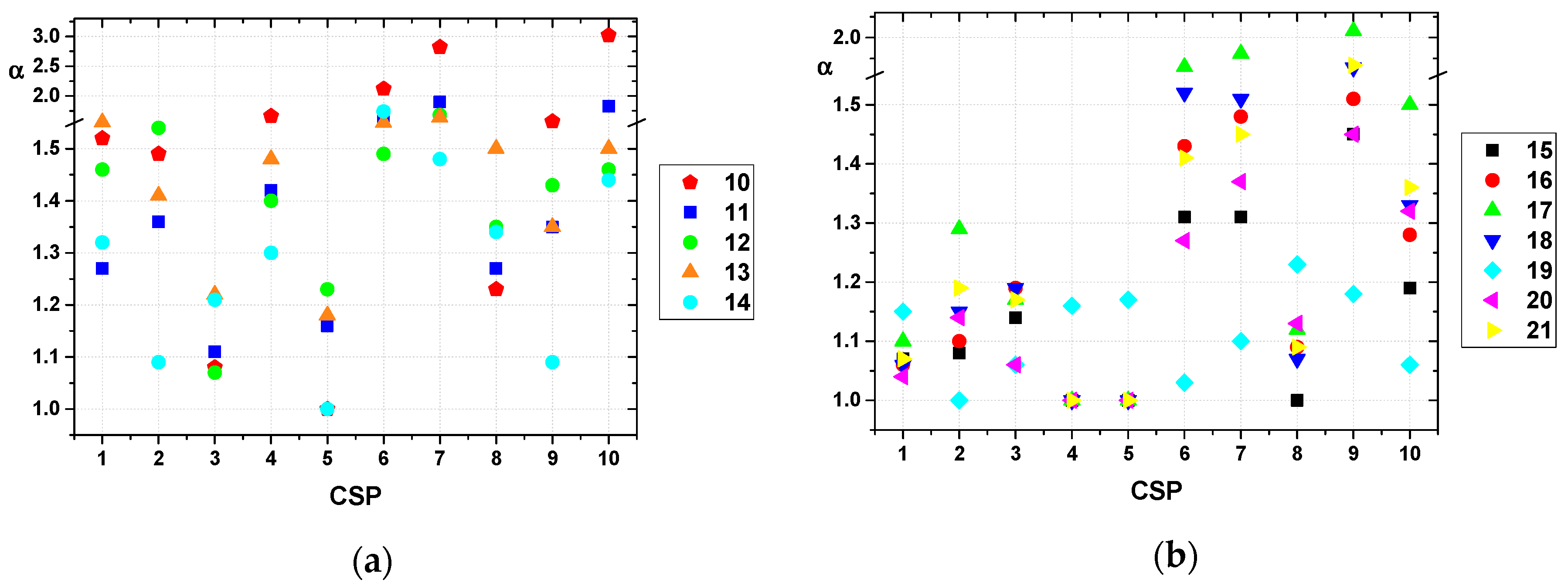
2.2. Evaluation of Prepared CSPs
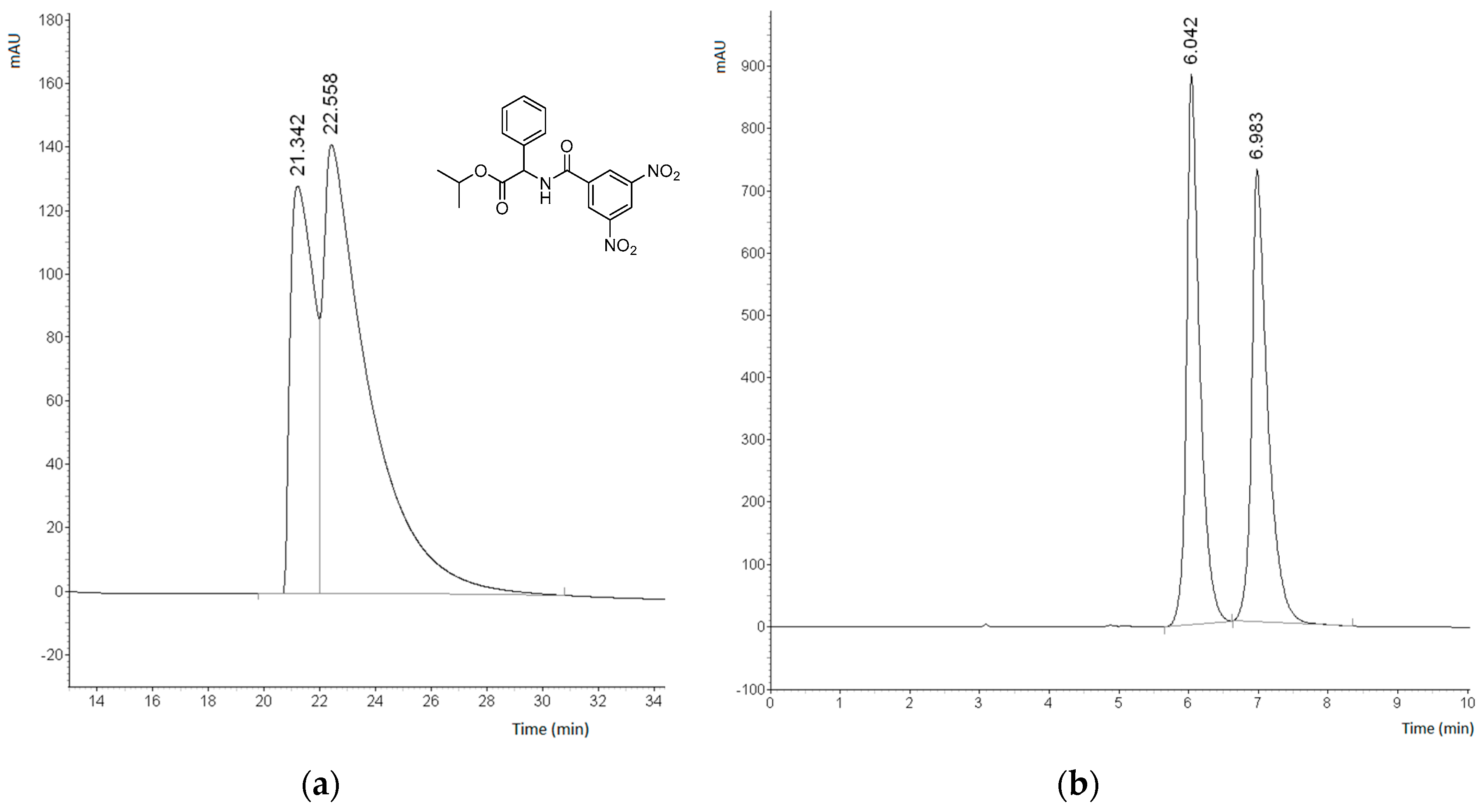
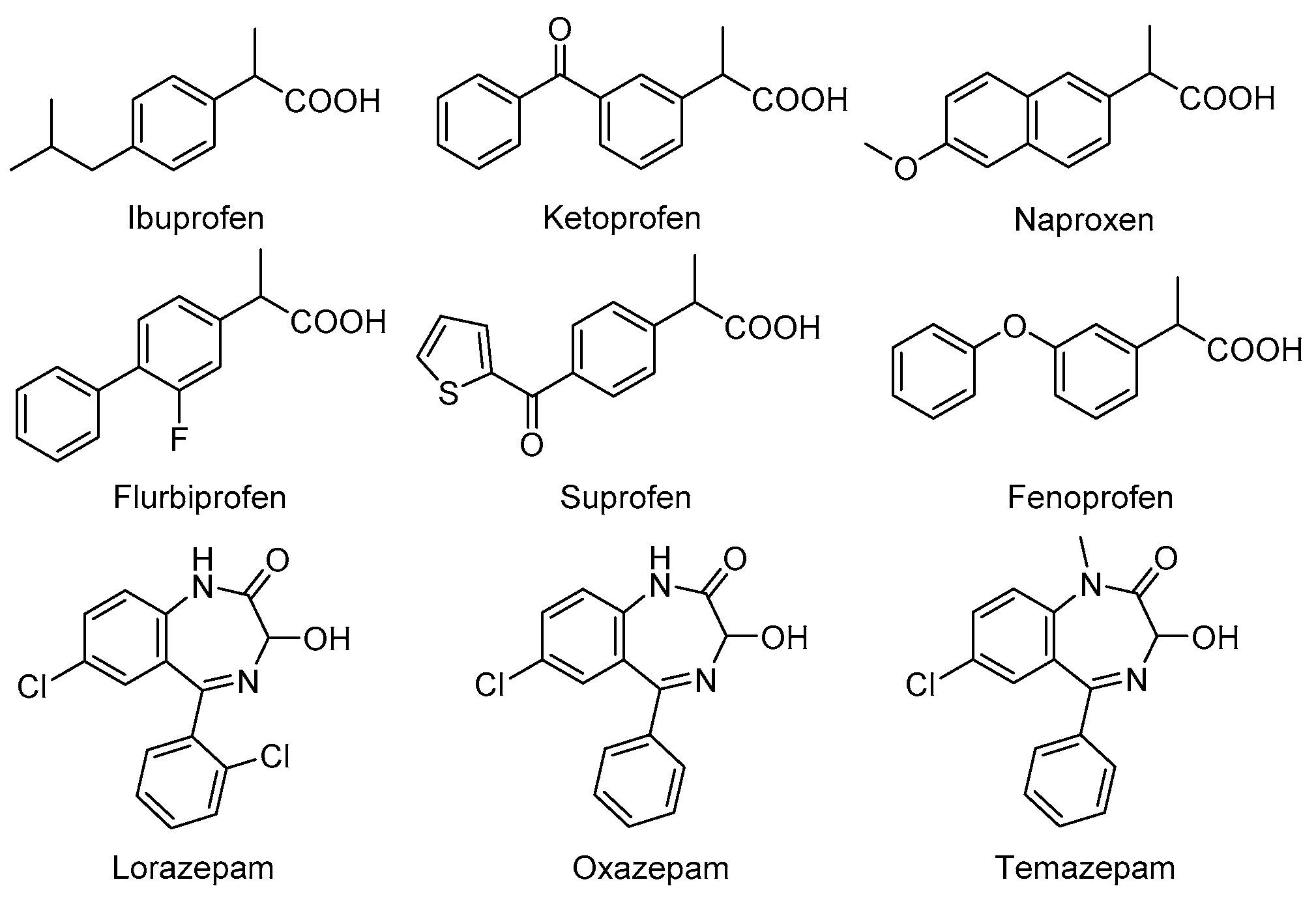
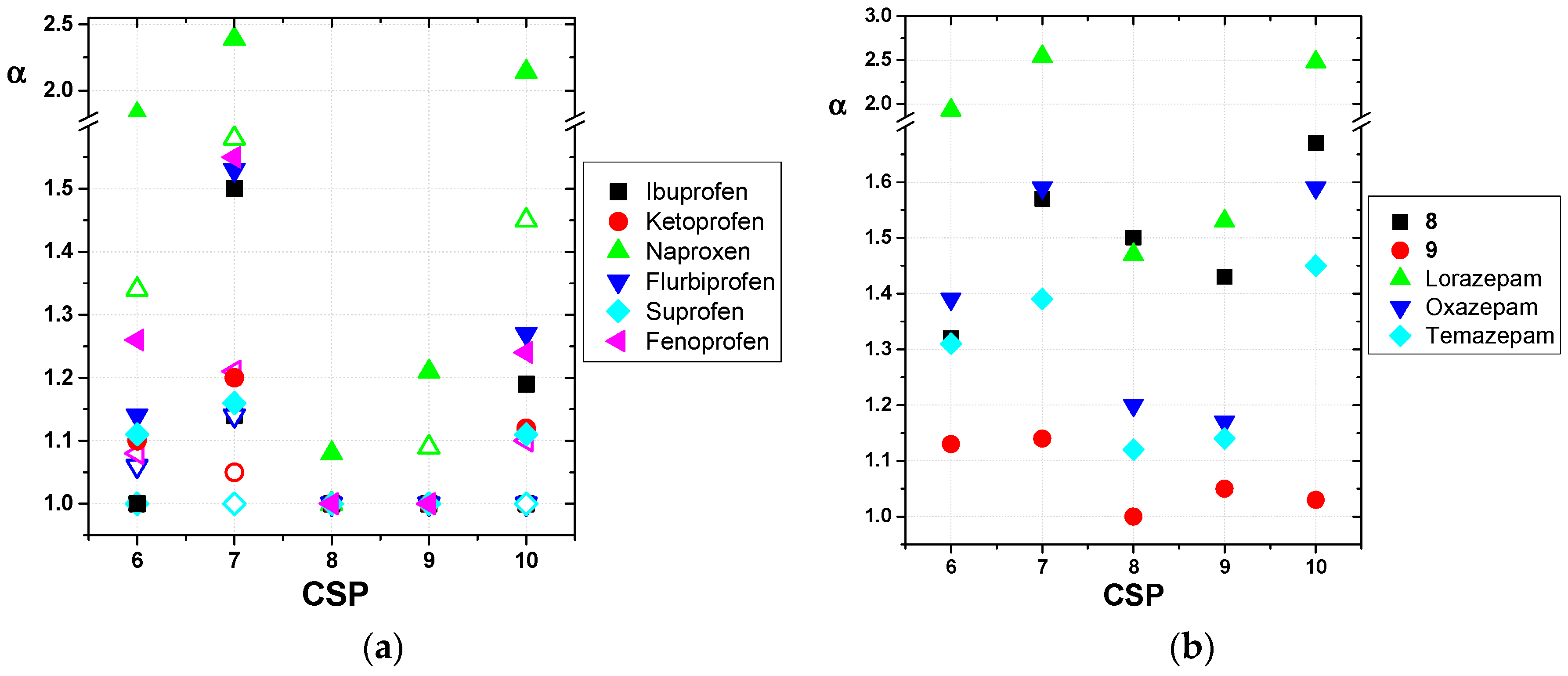
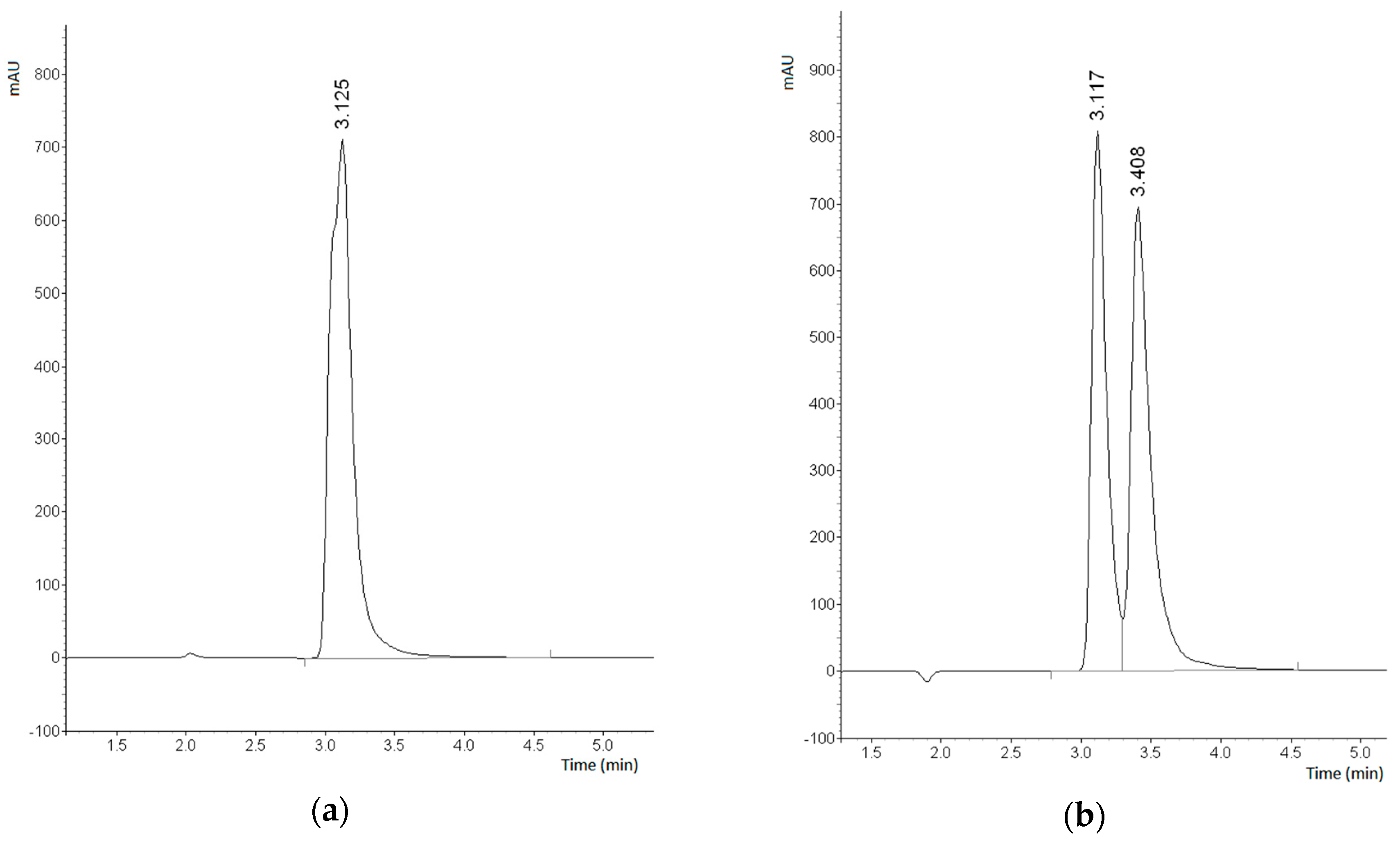
2.3. Enantioseparation of Pharmaceutical Drugs on Prepared CSPs
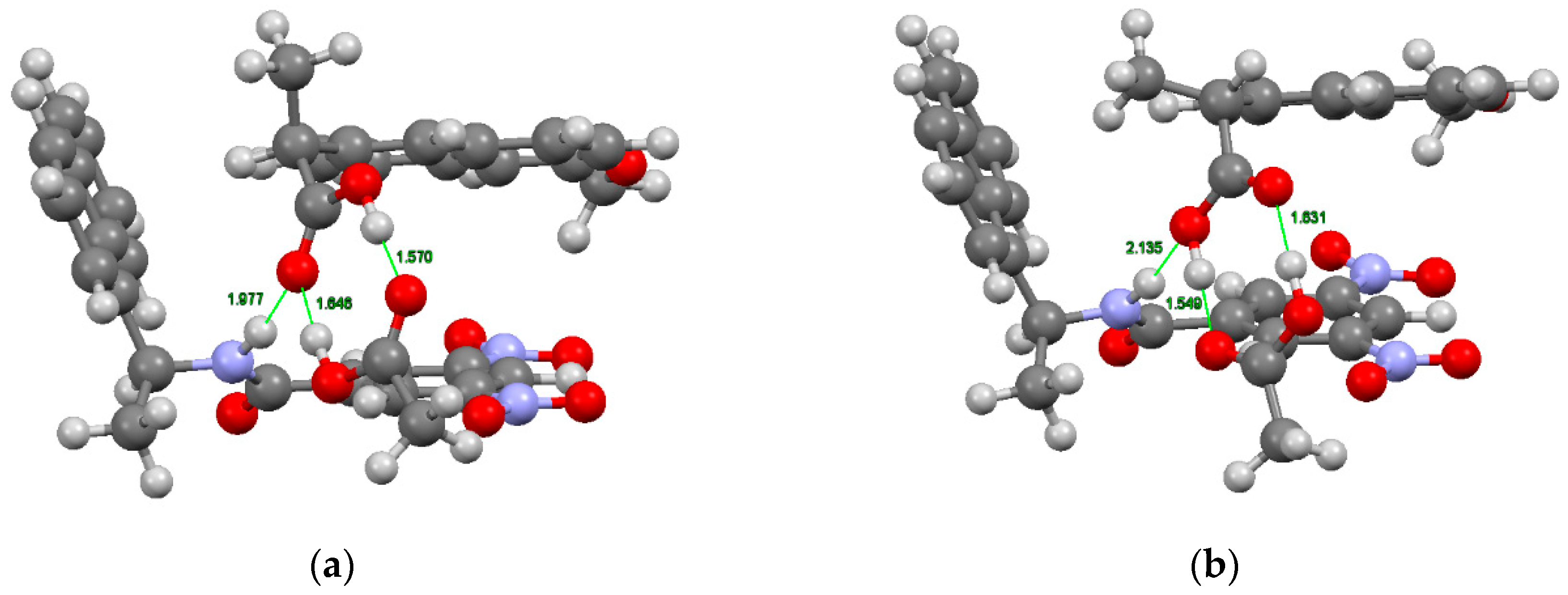
2.4. Analysis of Interactions Responsible for Chiral Recognition between Naproxen and CSP-6
2.5. Influence of Acetic Acid as the Mobile Phase Additive on The Enantioseparation of Naproxen on CSP-6
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Preparation of CSP-OHs
3.3. End-Capping and Packing Procedure
3.4. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FDA’S policy statement for the development of new stereoisomeric drugs. Chirality 1992, 4, 338–340. [CrossRef] [PubMed]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Rio, A.D.; Pierrot-Sanders, J.; Piras, P.; Vanthuyne, N. Chiral liquid chromatography contribution to the determination of the absolute configuration of enantiomers. J. Chromatogr. A 2004, 1037, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.; Seidel-Morgenstern, A. Processes to Separate Enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. Chiral recognition in separation science—An update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.F.; Wimalasinghe, R.M.; Wang, Y.; Barhate, C.L.; Patel, D.C.; Armstrong, D.W. Salient Sub-Second Separations. Anal. Chem. 2016, 88, 8821–8826. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Pasti, L.; Ciogli, A.; Villani, C.; Kocergin, J.; Anderson, S.; Gasparrini, F.; Cavazzini, A.; Catani, M. Pirkle-type chiral stationary phase on core–shell and fully porous particles: Are superficially porous particles always the better choice toward ultrafast high-performance enantioseparations? J. Chromatogr. A 2016, 1466, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zawatzky, K.; Barhate, C.L.; Regalado, E.L.; Mann, B.F.; Marshall, N.; Moore, J.C.; Welch, C.J. Overcoming “speed limits” in high throughput chromatographic analysis. J. Chromatogr. A 2017, 1499, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.J. Are We Approaching a Speed Limit for the Chromatographic Separation of Enantiomers? ACS Cent. Sci. 2017, 3, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Barhate, C.L.; Joyce, L.A.; Makarov, A.A.; Zawatzky, K.; Bernardoni, F.; Schafer, W.A.; Armstrong, D.W.; Welch, C.J.; Regalado, E.L. Ultrafast chiral separations for high throughput enantiopurity analysis. Chem. Commun. 2017, 53, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.; Felletti, S.; Luca, C.; Pasti, L.; Marchetti, N.; Costa, V.; Gasparrini, F.; Cavazzini, A.; Catani, M. The Way to Ultrafast, High-Throughput Enantioseparations of Bioactive Compounds in Liquid and Supercritical Fluid Chromatography. Molecules 2018, 23, 2709. [Google Scholar] [CrossRef] [PubMed]
- Khundadze, N.; Pantsulaia, S.; Fanali, C.; Farkas, T.; Chankvetadze, B. On our way to sub-second separations of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2018, 1572, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Saleem, K.; Hussain, I.; Gaitonde, V.D.; Aboul-Enein, H.Y. Polysaccharides Chiral Stationary Phases in Liquid Chromatography. Sep. Purif. Rev. 2009, 38, 97–147. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Small Molecules as Chromatographic Tools for HPLC Enantiomeric Resolution: Pirkle-Type Chiral Stationary Phases Evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral Stationary Phases Based on Small Molecules: An Update of the Last 17 Years. Sep. Purif. Rev. 2018, 47, 89–123. [Google Scholar] [CrossRef]
- Forjan, D.M.; Gazić, I.; Vinković, V. Role of the weak interactions in enantiorecognition of racemic dihydropyrimidinones by novel brush-type chiral stationary phases. Chirality 2007, 19, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Ranogajec, A.; Kontrec, D.; Vinkovic, V.; Sunjic, V. Enantiomer Separation and Molecular Recognition with New Chiral Stationary Phases on 4-Chloro-3,5-dinitrobenzoic Acid Amides of α,β-Aminoalcohols and α-Arylethylamines. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 63–83. [Google Scholar] [CrossRef]
- Kontrec, D.; Vinkovic, V.; Sunjic, V. Preparation and evaluation of chiral stationary phases based on N,N-2,4-(or 4,6)-disubstituted 4,5-(or 2,5)-dichloro-1,3-dicyanobenzene. Chirality 2000, 12, 63–70. [Google Scholar] [CrossRef]
- Knežević, A.; Novak, J.; Pescitelli, G.; Vinković, V. Determination of the Absolute Configuration of (S)-N-(1-Aryl-allyl)-3,5-dinitrobenzamides and Their Elution Order on Brush-Type Chiral Stationary Phases. Eur. J. Org. Chem. 2018, 2018, 3982–3991. [Google Scholar] [CrossRef]
- Ihara, T.; Sugimoto, Y.; Asada, M.; Nakagama, T.; Hobo, T. Influence of the method of preparation of chiral stationary phases on enantiomer separations in high-performance liquid chromatography. J. Chromatogr. A 1995, 694, 49–56. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Readnour, R.S. The influence of end-capping on the enantioselectivity of a chiral phase. Chromatographia 1991, 31, 129–132. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development; John Wiley & Sons: New York, NY, USA, 2012; ISBN 978-1-118-59151-2. [Google Scholar]
- Chiral Handbook for HPLC & SFC Separations. Available online: http://www.registech.com/literature-library/technical-resources/chiral-handbook-for-hplc-sfc-separations (accessed on 26 September 2018).
- Wahab, M.F.; Patel, D.C.; Wimalasinghe, R.M.; Armstrong, D.W. Fundamental and Practical Insights on the Packing of Modern High-Efficiency Analytical and Capillary Columns. Anal. Chem. 2017, 89, 8177–8191. [Google Scholar] [CrossRef] [PubMed]
- Unger, K.K.; Skudas, R.; Schulte, M.M. Particle packed columns and monolithic columns in high-performance liquid chromatography-comparison and critical appraisal. J. Chromatogr. A 2008, 1184, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, W.H.; Welch, C.J.; Lamm, B. Design, synthesis, and evaluation of an improved enantioselective naproxen selector. J. Org. Chem. 1992, 57, 3854–3860. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Welch, C.J. An Improved Chiral Stationary Phase for the Chromatographic Separation of Underivatized Naproxen Enantiomers. J. Liq. Chromatogr. 1992, 15, 1947–1955. [Google Scholar] [CrossRef]
- Knežević, A.; Landek, G.; Dokli, I.; Vinković, V. An efficient enzymatic approach to (S)-1-aryl-allylamines. Tetrahedron Asymmetry 2011, 22, 936–941. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Welch, J. Chromatographic and 1H NMR support for a proposed recognition model. J. Chromtogr. A 1994, 683, 347–353. [Google Scholar] [CrossRef]
- Zhao, C.F.; Diemert, S.; Cann, N.M. Rational optimization of the Whelk-O1 chiral stationary phase using molecular dynamics simulations. J. Chromatogr. A 2009, 1216, 5968–5978. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, A.; Hayes, J.M.; Stein, M.; Piras, P.; Roussel, C. Theoretical reassessment of Whelk-O1 as an enantioselective receptor for 1-(4-halogeno-phenyl)-1-ethylamine derivatives. Chirality 2004, 16, S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, W.H.; Welch’, C.J. Use of Simultaneous Face to Face and Face to Edge π-π Interactions to Facilitate Chiral Recognition. Tetrahedron Asymmetry 1994, 5, 777–780. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191. [Google Scholar] [CrossRef]
- Zhao, C.F.; Cann, N.M. Molecular Dynamics Study of Chiral Recognition for the Whelk-O1 Chiral Stationary Phase. Anal. Chem. 2008, 80, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.K.; Stringham, R.W. Effect of mobile phase acidic additives on enantioselectivity for phenylalanine analogs. J. Chromatogr. A 2001, 927, 47–52. [Google Scholar] [CrossRef]
- Ye, Y.K.; Stringham, R.W. The effect of acidic and basic additives on the enantioseparation of basic drugs using polysaccharide-based chiral stationary phases. Chirality 2006, 18, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Jibuti, G.; Mskhiladze, A.; Takaishvili, N.; Karchkhadze, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. HPLC separation of dihydropyridine derivatives enantiomers with emphasis on elution order using polysaccharide-based chiral columns: Liquid Chromatography. J. Sep. Sci. 2012, 35, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Mosiashvili, L.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. On the effect of basic and acidic additives on the separation of the enantiomers of some basic drugs with polysaccharide-based chiral selectors and polar organic mobile phases. J. Chromatogr. A 2013, 1317, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gogaladze, K.; Chankvetadze, L.; Tsintsadze, M.; Farkas, T.; Chankvetadze, B. Effect of Basic and Acidic Additives on the Separation of Some Basic Drug Enantiomers on Polysaccharide-Based Chiral Columns with Acetonitrile as Mobile Phase: SEPARATION OF SOME BASIC DRUG ENANTIOMERS. Chirality 2015, 27, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Yamada, H.; Mizuta, M. Self-association of acetic acid in some organic solvents. J. Phys. Chem. 1988, 92, 6768–6772. [Google Scholar] [CrossRef]
- Chocholoušová, J.; Vacek, J.; Hobza, P. Acetic Acid Dimer in the Gas Phase, Nonpolar Solvent, Microhydrated Environment, and Dilute and Concentrated Acetic Acid: Ab Initio Quantum Chemical and Molecular Dynamics Simulations. J. Phys. Chem. A 2003, 107, 3086–3092. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E.; III, T.A.; Darden, R.E.; Duke, T.J.; Giese, H.; Gohlke, A.W.; Goetz, N.; et al. AMBER; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]
- TURBOMOLE 7.0.1; University of Karlsruhe and Forschungszentrum: Karlsruhe, Germany, 2015.
Sample Availability: Samples of the compounds DNB-1–DNB-10 and CSP-1–CSP-10 are available from the authors. |


| Compound | Whelk-O1 | CSP-6 | CSP-7 | CSP-10 |
|---|---|---|---|---|
| Ibuprofen | 1.71 | 1.00 | 1.50 | 1.19 |
| Ketoprofen | 1.30 | 1.10 | 1.20 | 1.12 |
| Naproxen | 1.48 | 1.82 | 2.39 | 2.14 |
| Flurbiprofen | 1.68 | 1.14 | 1.53 | 1.27 |
| Suprofen | 1.27 | 1.11 | 1.16 | 1.11 |
| Fenoprofen | 1.60 | 1.26 | 1.55 | 1.24 |
| Lorazepam | 2.29 | 1.93 | 2.54 | 2.48 |
| Oxazepam | 1.97 | 1.39 | 1.59 | 1.59 |
| Temazepam | 1.38 | 1.31 | 1.39 | 1.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knežević, A.; Novak, J.; Vinković, V. New Brush-Type Chiral Stationary Phases for Enantioseparation of Pharmaceutical Drugs. Molecules 2019, 24, 823. https://doi.org/10.3390/molecules24040823
Knežević A, Novak J, Vinković V. New Brush-Type Chiral Stationary Phases for Enantioseparation of Pharmaceutical Drugs. Molecules. 2019; 24(4):823. https://doi.org/10.3390/molecules24040823
Chicago/Turabian StyleKnežević, Anamarija, Jurica Novak, and Vladimir Vinković. 2019. "New Brush-Type Chiral Stationary Phases for Enantioseparation of Pharmaceutical Drugs" Molecules 24, no. 4: 823. https://doi.org/10.3390/molecules24040823
APA StyleKnežević, A., Novak, J., & Vinković, V. (2019). New Brush-Type Chiral Stationary Phases for Enantioseparation of Pharmaceutical Drugs. Molecules, 24(4), 823. https://doi.org/10.3390/molecules24040823